Leaf Habit and Stem Hydraulic Traits Determine Functional Segregation of Multiple Oak Species along a Water Availability Gradient
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Leaf and Stem Trait Measurements
2.3. Leaf Life Span
2.4. Climatic Variables
2.5. Soil Characterization
2.6. Data Analysis
3. Results
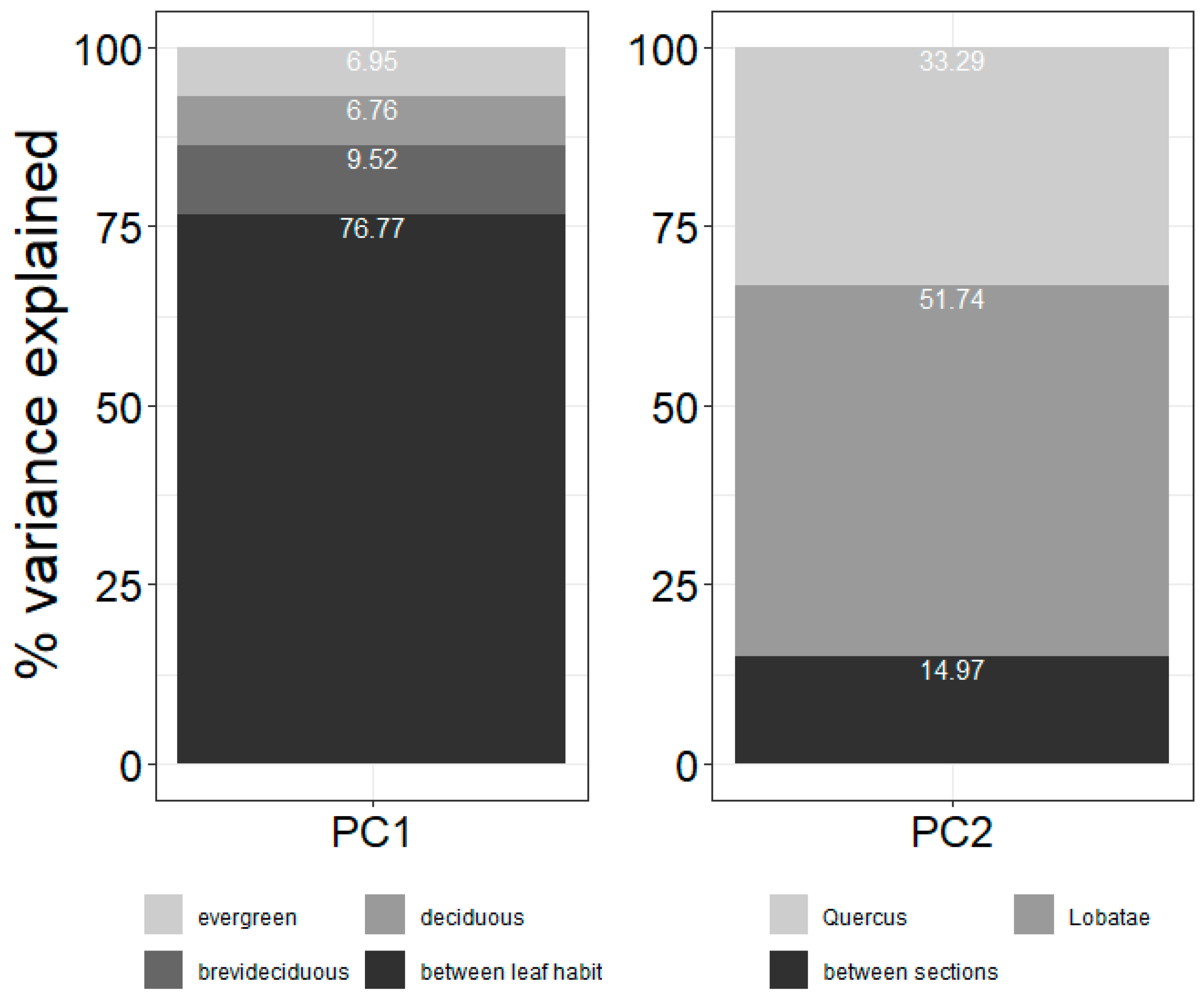
3.1. Variance Partitioning of Functional Traits
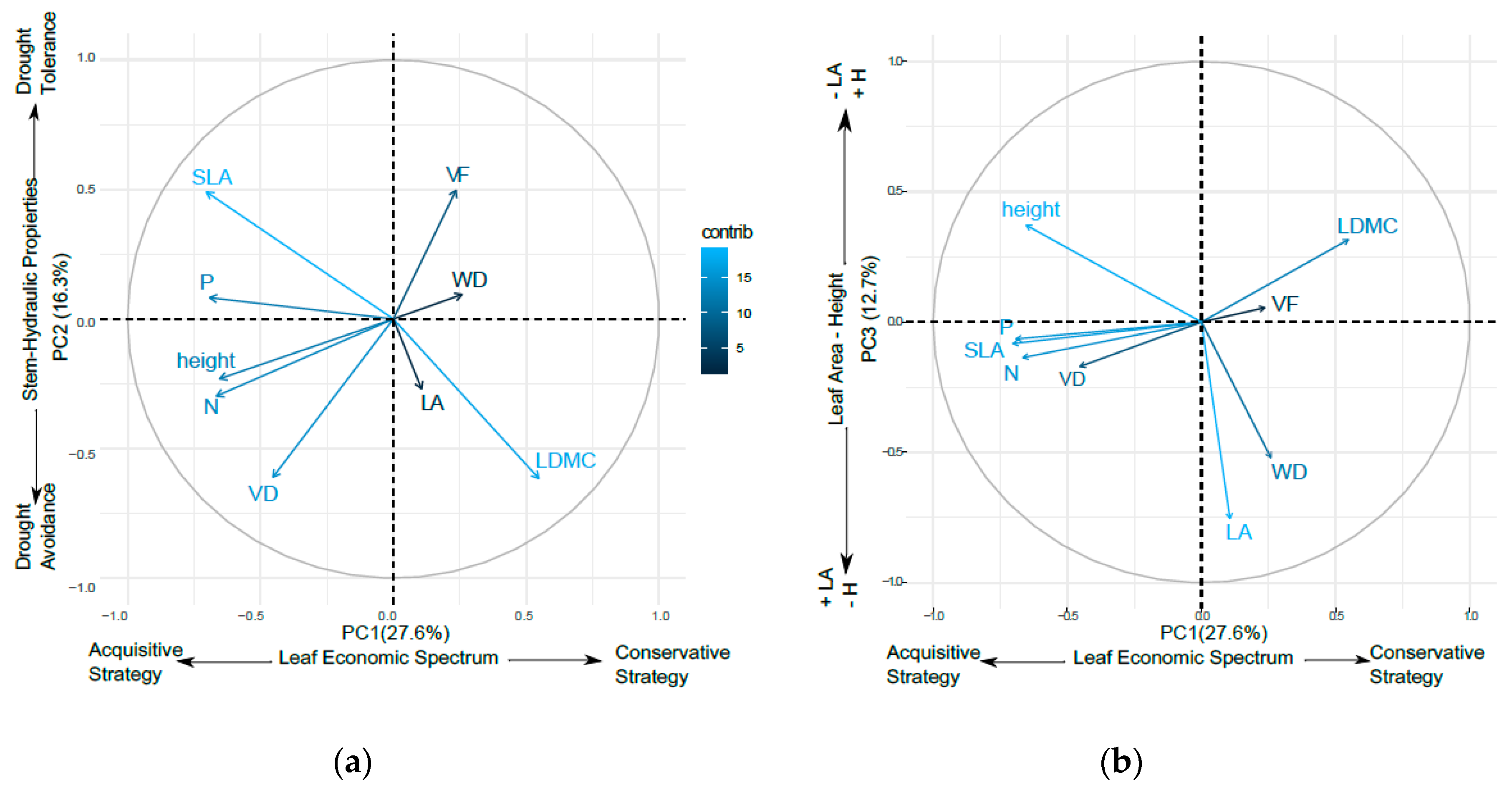
3.2. Oak Functional Strategies
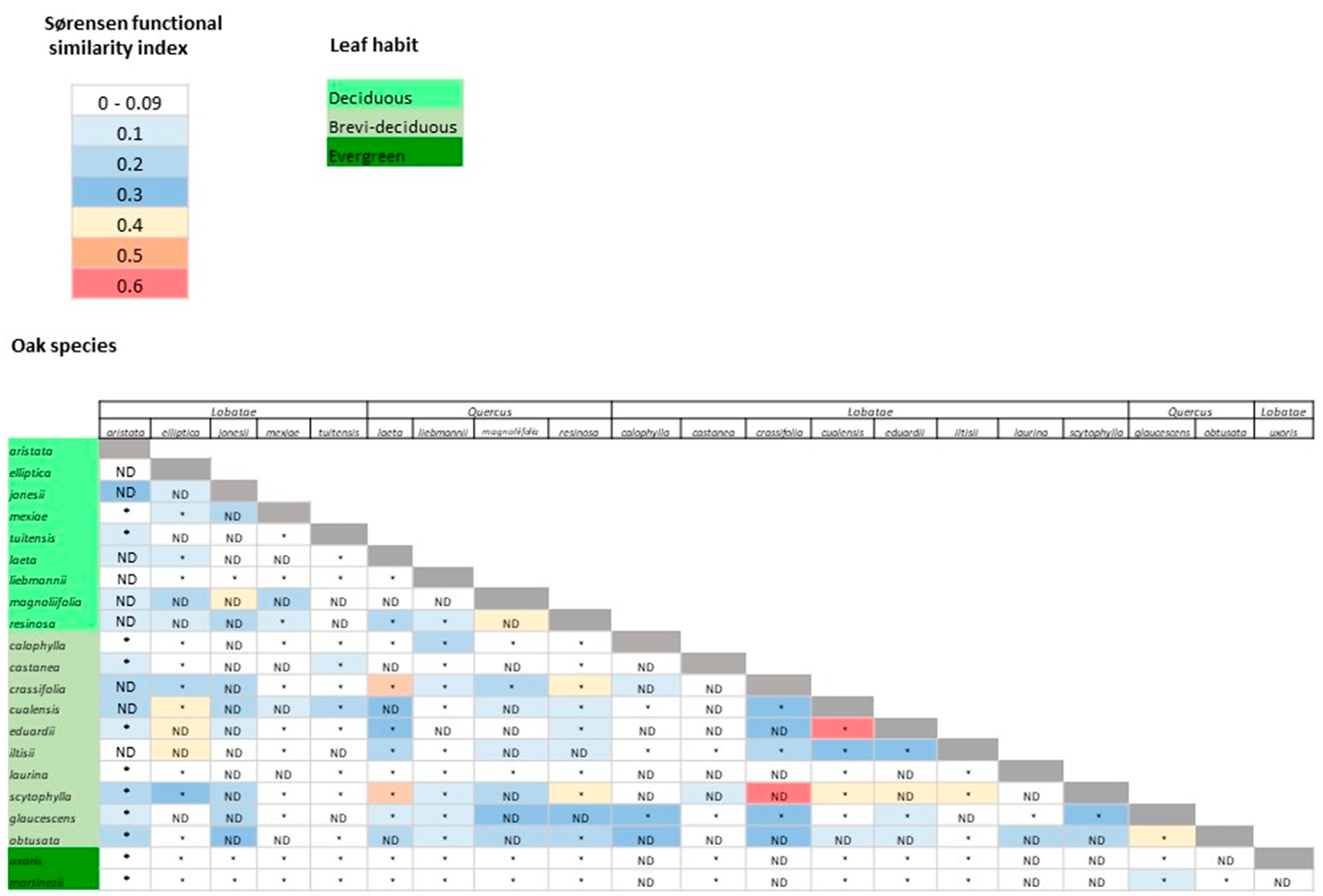
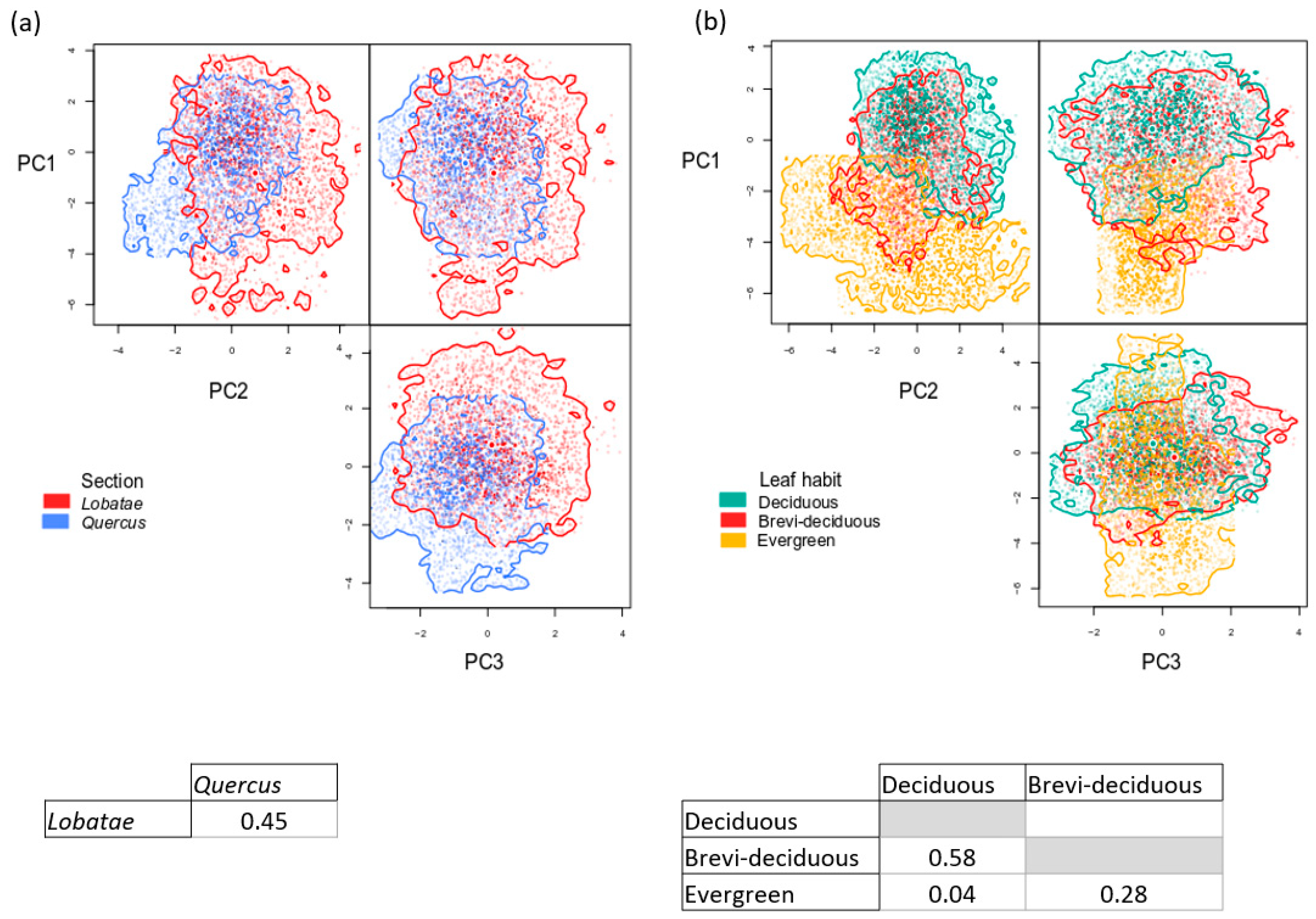
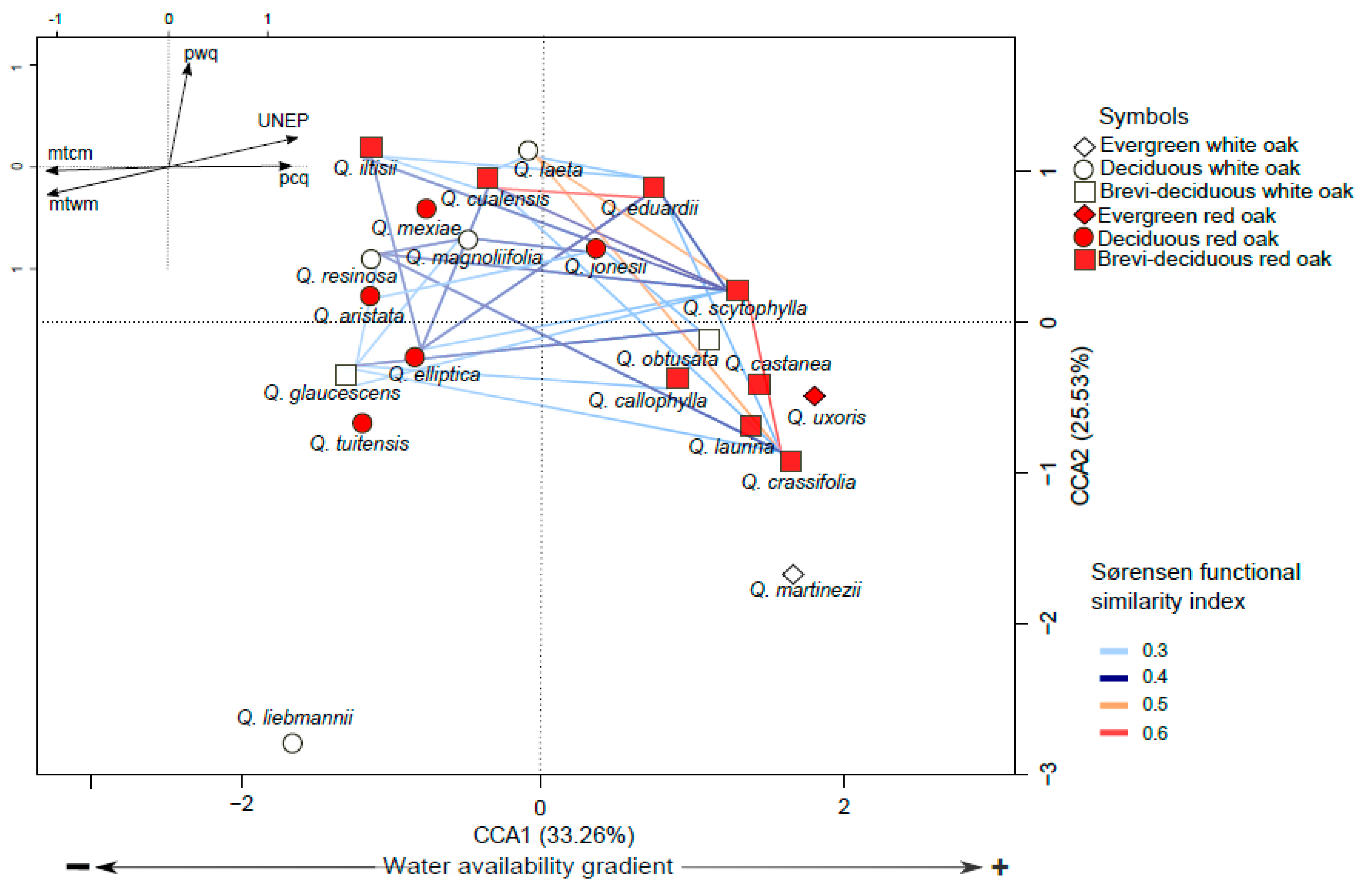
3.3. Functional Space and Overlap
4. Discussion
4.1. Oaks Functional Strategies for Water Use
4.1.1. Leaf Economic Spectrum and Leaf Life Span Strategies
4.1.2. Stem Hydraulic Strategies
4.1.3. Leaf Area and Plant Height
4.2. Variation in Functional Traits and Niche Segregation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Leaf Chemical Analysis
Appendix B. Leaf Habit Category
Appendix C. Potential Evapotranspiration and Aridity Index Calculations
- ET0 = potential evapotranspiration by mm/day
- Ci = constant 0.0023
- Tmed = average temperature in °C
- Tmax = maximum temperature in °C
- Tmin = minimum temperature in °C
- Evap eq = equivalent evaporation in mm/day
Appendix D
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| LDMC | 0.347 | −0.507 | 0.296 |
| SLA | −0.446 | 0.404 | −0.077 |
| LA | 0.067 | −0.222 | −0.706 |
| N | −0.422 | −0.245 | −0.127 |
| P | −0.438 | 0.067 | −0.060 |
| WD | 0.164 | 0.078 | −0.487 |
| VD | −0.287 | −0.503 | −0.159 |
| VF | 0.149 | 0.410 | 0.051 |
| Height | −0.414 | −0.190 | 0.348 |
Appendix E
References
- Markesteijn, L.; Poorter, L.; Bongers, F.; Paz, H.; Sack, L. Hydraulics and life history of tropical dry forest tree species: Coordination of species’ drought and shade tolerance. New Phytol. 2011, 191, 480–495. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Colin Prentice, I.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Garnier, E.; Navas, M.-L. A trait-based approach to comparative functional plant ecology: Concepts, methods and applications for agroecology. A review. Agron. Sustain. Dev. 2012, 32, 365–399. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide “fast-slow” plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Sterck, F.; Markesteijn, L.; Schieving, F.; Poorter, L. Functional traits determine trade-offs and niches in a tropical forest community. Proc. Natl. Acad. Sci. USA 2011, 108, 20627–20632. [Google Scholar] [CrossRef] [PubMed]
- de la Riva, E.G.; Marañón, T.; Violle, C.; Villar, R.; Pérez-Ramos, I.M. Biogeochemical and Ecomorphological Niche Segregation of Mediterranean Woody Species along a Local Gradient. Front. Plant Sci. 2017, 8, 1242. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramos, I.M.; Matías, L.; Gómez-Aparicio, L.; Godoy, Ó. Functional traits and phenotypic plasticity modulate species coexistence across contrasting climatic conditions. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cavender-Bares, J.; Kitajima, K.; Bazzaz, F.A. Multiple trait associations in relation to habitat differentiation among 17 Floridian oak species. Ecol. Monogr. 2004, 74, 635–662. [Google Scholar] [CrossRef]
- Ramírez-Valiente, J.A.; Cavender-Bares, J. Evolutionary trade-offs between drought resistance mechanisms across a precipitation gradient in a seasonally dry tropical oak (Quercus oleoides). Tree Physiol. 2017, 37, 889–901. [Google Scholar] [CrossRef]
- Van Ommen Kloeke, A.E.E.; Douma, J.C.; Ordoñez, J.C.; Reich, P.B.; Van Bodegom, P.M. Global quantification of contrasting leaf life span strategies for deciduous and evergreen species in response to environmental conditions. Glob. Ecol. Biogeogr. 2012, 21, 224–235. [Google Scholar] [CrossRef]
- Lusk, C.H.; Jimenez-Castillo, M.; Aragón, R.; Easdale, T.A.; Poorter, L.; Hinojosa, L.F.; Mason, N.W.H. Testing for functional convergence of temperate rainforest tree assemblages in Chile and New Zealand. N. Z. J. Bot. 2016, 54, 175–203. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Z.; Xia, F.; Su, Y. Local adaptation to temperature and precipitation in naturally fragmented populations of Cephalotaxus oliveri, an endangered conifer endemic to China. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Querejeta, J.I.; Prieto, I.; Torres, P.; Campoy, M.; Alguacil, M.M.; Roldán, A. Water-spender strategy is linked to higher leaf nutrient concentrations across plant species colonizing a dry and nutrient-poor epiphytic habitat. Environ. Exp. Bot. 2018, 153, 302–310. [Google Scholar] [CrossRef]
- Sperry, J.S.; Meinzer, F.C.; McCulloh, K.A. Safety and efficiency conflicts in hydraulic architecture: Scaling from tissues to trees. Plant Cell Environ. 2008, 31, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Carlquist, S. Comparative Wood Anatomy, 2nd ed.; Springer: Berlin, Germany, 2001. [Google Scholar]
- Poorter, L.; McDonald, I.; Alarcón, A.; Fichtler, E.; Licona, J.C.; Peña-Claros, M.; Sterck, F.; Villegas, Z.; Sass-Klaassen, U. The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rainforest tree species. New Phytol. 2010, 185, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, F.C.; Johnson, D.M.; Lachenbruch, B.; McCulloh, K.A.; Woodruff, D.R. Xylem hydraulic safety margins in woody plants: Coordination of stomatal control of xylem tension with hydraulic capacitance. Funct. Ecol. 2009, 23, 922–930. [Google Scholar] [CrossRef]
- Méndez-Alonzo, R.; Paz, H.; Zuluaga, R.C.; Rosell, J.A.; Olson, M.E. Coordinated evolution of leaf and stem economics in tropical dry forest trees. Ecology 2012, 93, 2397–2406. [Google Scholar] [CrossRef]
- Poorter, L.; Markesteijn, L. Seedling Traits Determine Drought Tolerance of Tropical Tree Species. Biotropica 2008, 40, 321–331. [Google Scholar] [CrossRef]
- Baraloto, C.; Paine, C.E.T.; Poorter, L.; Beauchene, J.; Bonal, D.; Domenach, A.M.; Hérault, B.; Patiño, S.; Roggy, J.C.; Chave, J. Decoupled leaf and stem economics in rain forest trees. Ecol. Lett. 2010, 13, 1338–1347. [Google Scholar] [CrossRef]
- Li, L.; McCormack, M.L.; Ma, C.; Kong, D.; Zhang, Q.; Chen, X.; Zeng, H.; Niinemets, Ü.; Guo, D. Leaf economics and hydraulic traits are decoupled in five species-rich tropical-subtropical forests. Ecol. Lett. 2015, 18, 899–906. [Google Scholar] [CrossRef]
- Reich, P.B.; Ellsworth, D.S.; Walters, M.B.; Vose, J.M.; Gresham, C.; Volin, J.C.; Bowman, W.D. Generality of leaf trait relationships: A test across six biomes. Ecology 1999, 80, 1955–1969. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornellssen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Givnish, T.J. Adaptive significance of evergreen vs. deciduous leaves: Solving the triple paradox. Silva Fenn. 2002, 36, 703–743. [Google Scholar] [CrossRef]
- Bai, K.; He, C.; Wan, X.; Jiang, D. Leaf economics of evergreen and deciduous tree species along an elevational gradient in a subtropical mountain. AoB Plants 2015, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.; Donovan, L. Evolution of the leaf economics spectrum in herbs: Evidence from environmental divergences in leaf physiology across Helianthus (Asteraceae). Evolution 2015, 69. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.J.; Chatelet, D.S.; Sack, L.; Donoghue, M.J. Leaf life span and the leaf economic spectrum in the context of whole plant architecture. J. Ecol. 2014, 102, 328–336. [Google Scholar] [CrossRef]
- Manos, P.S.; Doyle, J.J.; Nixon, K.C. Phylogeny, biogeography, and processes of molecular differentiation in Quercus subgenus Quercus (Fagaceae). Mol. Phylogenet. Evol. 1999, 12, 333–349. [Google Scholar] [CrossRef]
- Deng, M.; Jiang, X.-L.; Hipp, A.L.; Manos, P.S.; Hahn, M. Phylogeny and biogeography of East Asian evergreen oaks (Quercus section Cyclobalanopsis; Fagaceae): Insights into the Cenozoic history of evergreen broad-leaved forests in subtropical Asia. Mol. Phylogenet. Evol. 2018, 119, 170–181. [Google Scholar] [CrossRef]
- Cavender-Bares, J. Diversification, adaptation, and community assembly of the American oaks (Quercus), a model clade for integrating ecology and evolution. New Phytol. 2018, 221, 669–692. [Google Scholar] [CrossRef]
- de la Riva, E.G.; Arenas Navarro, M.; Villar Montero, R.; García Oliva, F.; Oyama, K. El papel de la evapotranspiración en la distribución funcional foliar de 28 especies del género Quercus de España y México. Ecosistemas 2019, 28, 199–207. [Google Scholar] [CrossRef]
- Bouchenak-Khelladi, Y.; Onstein, R.E.; Xing, Y.; Schwery, O.; Linder, H.P. On the complexity of triggering evolutionary radiations. New Phytol. 2015, 207, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Navarro, M.; García-Oliva, F.; Torres-Miranda, A.; Téllez-Valdés, O.; Oyama, K. Environmental filters determine the distribution of tree species in a threatened biodiversity hotspot in western Mexico. Bot. Sci. 2020, 98, 219–237. [Google Scholar] [CrossRef]
- Valencia-A, S. Diversidad del género Quercus (Fagaceae) en México. Bol. Soc. Bot. Méx. 2004, 75, 33–53. [Google Scholar]
- Nixon, K.C. Global Neotropical distribution and diversity of oak (genus Quercus) and oak forest. In Ecology and Conservation of Neotropical Montane Oak Forests; Kappelle, M., Ed.; Springer: Berlin, Germany, 2006; pp. 3–13. ISBN 978-3-540-28909-8. [Google Scholar]
- Hipp, A.L.; Manos, P.S.; González-Rodríguez, A.; Hahn, M.; Kaproth, M.; McVay, J.D.; Valencia-Ávalos, S.; Cavender-Bares, J. Sympatric parallel diversification of major oak clades in the Americas and the origins of Mexican species diversity. New Phytol. 2018, 217, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Nixon, K.C. Infrageneric classification of Quercus (Fagaceae) and typification of sectional names. Ann. des Sci. For. 1993, 50, 25–34. [Google Scholar] [CrossRef]
- Renninger, H.J.; Carlo, N.; Clark, K.L.; Schafer, K.V.R. Physiological strategies of co-occurring oaks in a water- and nutrient-limited ecosystem. Tree Physiol. 2014, 34, 159–173. [Google Scholar] [CrossRef]
- Aguilar-Romero, R.; Pineda-Garcia, F.; Paz, H.; González-Rodríguez, A.; Oyama, K. Differentiation in the water-use strategies among oak species from central Mexico. Tree Physiol. 2017, 37, 915–925. [Google Scholar] [CrossRef]
- Lobo, A.; Torres-Ruiz, J.M.; Burlett, R.; Lemaire, C.; Parise, C.; Francioni, C.; Truffaut, L.; Tomášková, I.; Hansen, J.K.; Kjær, E.D.; et al. Assessing inter- and intraspecific variability of xylem vulnerability to embolism in oaks. For. Ecol. Manag. 2018, 424, 53–61. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.M.; Kremer, A.; Carins Murphy, M.R.; Brodribb, T.; Lamarque, L.J.; Truffaut, L.; Bonne, F.; Ducousso, A.; Delzon, S. Genetic differentiation in functional traits among European sessile oak populations. Tree Physiol. 2019. [Google Scholar] [CrossRef]
- Servicio Meteorológico Nacional Normales Climatológicas por Estado. Available online: https://smn.conagua.gob.mx/es/informacion-climatologica-por-estado?estado=jal (accessed on 5 December 2017).
- Velázquez-Ruiz, A.; Martínez, L.M.; Carrillo, F.M. Caracterización climática para la región de Bahia de Banderas mediante el sistema de Köppen, modificado por García, y técnicas de sistemas de información geográfica. Invest. Geogr. 2012, 79, 7–19. [Google Scholar] [CrossRef]
- Torres-Miranda, A.; Luna-Vega, I.; Oyama, K. New approaches to the biogeography and areas of endemism of red oaks (Quercus L., Section Lobatae). Syst. Biol. 2013, 62, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Rodriguez, Y.L.; Urbatsch, E.; Karaman-Castro, V.; Figueroa-Rangel, B.L. Acer binzayedii (Sapindaceae), a new maple species from Mexico. Brittonia 2017, 69, 249–252. [Google Scholar] [CrossRef]
- González-Villarreal, L.M. Two new species of oak (Fagaceae, Quercus sect. Lobatae) from the Sierra Madre del Sur, Mexico. Brittonia 2003, 55, 49–60. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.; Craine, J.; Gurvich, D.; et al. New handbook for standardise measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Cuervo-Robayo, A.P.; Téllez-Valdés, O.; Gómez-Albores, M.A.; Venegas-Barrera, C.S.; Manjarrez, J.; Martínez-Meyer, E. An update of high-resolution monthly climate surfaces for Mexico. Int. J. Climatol. 2014, 34, 2427–2437. [Google Scholar] [CrossRef]
- Hutchinson, M.F. Anusplin Version 4.36 User Guide; Centre for Resource and Environmental Studies. The Australian National University: Canberra, Australia, 2006. [Google Scholar]
- United Nations Environment Programme. World Atlas of Desertification, 2nd ed.; Middleton, N., Thomas, D.S.G., Eds.; United Nations Environment Programme/Edward Arnold: London, UK, 1997; ISBN 0340555122. [Google Scholar]
- Huffman, E.W., Jr. Performance of a new automatic carbon dioxide coulometer. Microchem. J. 1977, 22, 567–573. [Google Scholar] [CrossRef]
- Bremmer, J.M. Nitrogen-total. In Methods of Soil Analysis Part 3: Chemical Analysis; Sparks, D., Page, A.L., Helmke, P.A., Loerppert, R.G.H., Soltanpour, P.N., Tabatabai, M.A., Jhonson, C.T., Sumner, M.E., Eds.; Soil Science Society of American and American Society of Agronomy: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Robertson, P.G.; Coleman, D.C.; Bledsoe, C.S.; Sollins, P. Standard Soil Methods for Long-Term Ecological Research (LTER); Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Michelaki, C.; Fyllas, N.M.; Galanidis, A.; Aloupi, M.; Evangelou, E.; Arianoutsou, M.; Dimitrakopoulos, P.G. An integrated phenotypic trait-network in thermo-Mediterranean vegetation describing alternative, coexisting resource-use strategies. Sci. Total Environ. 2019, 672, 583–592. [Google Scholar] [CrossRef]
- de Bello, F.; Lavorel, S.; Albert, C.H.; Thuiller, W.; Grigulis, K.; Dolezal, J.; Janeček, Š.; Lepš, J. Quantifying the relevance of intraspecific trait variability for functional diversity. Methods Ecol. Evol. 2011, 2, 163–174. [Google Scholar] [CrossRef]
- Grueber, C.E.; Nakagawa, S.; Laws, R.J.; Jamieson, I.G. Multimodel inference in ecology and evolution: Challenges and solutions. J. Evol. Biol. 2011, 24, 699–711. [Google Scholar] [CrossRef]
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.; Fisher, D.N.; Goodwin, C.E.D.; Robinson, B.S.; Hodgson, D.J.; Inger, R. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 2018, 2018, e4794. [Google Scholar] [CrossRef] [PubMed]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Blonder, B.; Morrow, C.B.; Maitner, B.; Harris, D.J.; Lamanna, C.; Violle, C.; Enquist, B.J.; Kerkhoff, A.J. New approaches for delineating n-dimensional hypervolumes. Methods Ecol. Evol. 2018, 9, 305–319. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Lamanna, C.; Blonder, B.; Violle, C.; Kraft, N.; Sandel, B.; Šímová, I.; Donoghue, J.; Svenning, J.-C.; Mcgill, B.; Boyle, B.; et al. Functional trait space and the latitudinal diversity gradient. Proc. Natl. Acad. Sci. USA 2014, 111, 13745–13750. [Google Scholar] [CrossRef]
- Mammola, S. Assessing similarity of n- dimensional hypervolumes: Which metric to use? J. Biogeogr. 2019, 46, 2012–2023. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.; B. O’Hara, R.; Simpson, G.; Solymos, P. Vegan: Community Ecology Package. Ordination Methods, Diversity Analysis and Other Functions for Community and Vegetation Ecologists. Version 2.4-0. Available online: https://CRAN.R-project.org/package=vegan (accessed on 24 April 2016).
- Bougeard, S.; Dray, S. Supervised multiblock analysis in R with the ade4 Package. J. Stat. Softw. 2018, 86, 1–17. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1-121. Available online: https://cran.r-project.org/web/packages/nlme (accessed on 1 April 2019).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference; R Packag. version 1.43.17. Available online: https://cran.r-project.org/web/packages/MuMIn (accessed on 15 February 2020).
- Blonder, B.; Lamanna, C.; Violle, C.; Enquist, B.J. The n-dimensional hypervolume. Glob. Ecol. Biogeogr. 2014, 23, 595–609. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://cran.r-project.org (accessed on 26 October 2017).
- Uribe-Salas, D.; Sáenz-Romero, C.; González-Rodríguez, A.; Téllez-Valdéz, O.; Oyama, K. Foliar morphological variation in the white oak Quercus rugosa Née (Fagaceae) along a latitudinal gradient in Mexico: Potential implications for management and conservation. For. Ecol. Manag. 2008, 256, 2121–2126. [Google Scholar] [CrossRef]
- Lara-De La Cruz, L.I.; García-Oliva, F.; Oyama, K.; Rodríguez, A.G. Association of functional trait variation of Quercus castanea with temperature and water availability gradients at the landscape level. Bot. Sci. 2020, 98. [Google Scholar] [CrossRef]
- de la Riva, E.G.; Pérez-Ramos, I.M.; Tosto, A.; Navarro-Fernández, C.M.; Olmo, M.; Marañón, T.; Villar, R. Disentangling the relative importance of species occurrence, abundance and intraspecific variability in community assembly: A trait-based approach at the whole-plant level in Mediterranean forests. Oikos 2016, 125, 354–363. [Google Scholar] [CrossRef]
- Ramirez-Valiente, J.A.; Sanchez-Gomez, D.; Aranda, I.; Valladares, F. Phenotypic plasticity and local adaptation in leaf ecophysiological traits of 13 contrasting cork oak populations under different water availabilities. Tree Physiol. 2010, 30, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Borchert, R.; Robertson, K.; Schwartz, M.D.; Williams-Linera, G. Phenology of temperate trees in tropical climates. Int. J. Biometeorol. 2005, 50, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Read, Q.D.; Moorhead, L.C.; Swenson, N.G.; Bailey, J.K.; Sanders, N.J. Convergent effects of elevation on functional leaf traits within and among species. Funct. Ecol. 2014, 28, 37–45. [Google Scholar] [CrossRef]
- González Villarreal, L.M.; Universidad de Guadalajara, Instituto de Botánica. Contribuciόn al Conocimiento del Género Quercus (Fagaceae) en el Estado de Jalisco; Instituto de Botánica, Universidad de Guadalajara: Guadalajara, Mexico, 1986; ISBN 9688950270. [Google Scholar]
- Zanne, A.E.; Westoby, M.; Falster, D.S.; Ackerly, D.D.; Loarie, S.R.; Arnold, S.E.J.; Coomes, D.A. Angiosperm wood structure: Global patterns in vessel anatomy and their relation to wood density and potential conductivity. Am. J. Bot. 2010, 97, 207–215. [Google Scholar] [CrossRef]
- Ziemińska, K.; Westoby, M.; Wright, I.J. Broad Anatomical variation within a narrow wood density range—A study of twig wood across 69 Australian angiosperms. PLoS ONE 2015, 10, e0124892. [Google Scholar] [CrossRef]
- Fortunel, C.; Ruelle, J.; Beauchêne, J.; Fine, P.V.A.; Baraloto, C. Wood specific gravity and anatomy of branches and roots in 113 Amazonian rainforest tree species across environmental gradients. New Phytol. 2014, 202, 79–94. [Google Scholar] [CrossRef]
- Scholz, A.; Stein, A.; Choat, B.; Jansen, S. How drought and deciduousness shape xylem plasticity in three Costa Rican woody plant species. IAWA J. 2014, 35, 337–355. [Google Scholar] [CrossRef]
- Fisher, J.B.; Goldstein, G.; Jones, T.J.; Cordell, S. Wood vessel diameter is related to elevation and genotype in the Hawaiian tree Metrosideros polymorpha (Myrtaceae). Am. J. Bot. 2007, 94, 709–715. [Google Scholar] [CrossRef]
- Tyree, M.T.; Davis, S.D.; Cochard, H. Biophysical perspectives of xylem evolution: Is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction? IAWA J. 1994, 15, 335–360. [Google Scholar] [CrossRef]
- Jacobsen, A.L.; Ewers, F.W.; Pratt, R.B.; Paddock, W.A.; Davis, S.D. Do xylem fibers affect vessel cavitation resistance? Plant Physiol. 2005, 139, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Li, S.; Zhang, H.; Zhang, S.; Tyree, M.T. Recalcitrant vulnerability curves: Methods of analysis and the concept of fibre bridges for enhanced cavitation resistance. Plant. Cell Environ. 2014, 37, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lens, F.; Espino, S.; Karimi, Z.; Klepsch, M.; Schenk, H.J.; Schmitt, M.; Schuldt, B.; Jansen, S. Intervessel pit membrane thickness as a key determinant of embolism resistance in angiosperm xylem. IAWA J. 2016, 37, 152–171. [Google Scholar] [CrossRef]
- Carlquist, S. Vasicentric tracheids as a drought survival mechanism in the woody flora of southern California and similar regions; review of vasicentric tracheids. Aliso 1985, 11, 37–68. [Google Scholar]
- Fontes, C.G.; Cavender-Bares, J. Toward an integrated view of the ‘elephant’: Unlocking the mysteries of water transport and xylem vulnerability in oaks. Tree Physiol. 2019, 40, 1–4. [Google Scholar] [CrossRef]
- Martin, R.E.; Asner, G.P.; Sack, L. Genetic variation in leaf pigment, optical and photosynthetic function among diverse phenotypes of Metrosideros polymorpha grown in a common garden. Oecologia 2007, 151, 387–400. [Google Scholar] [CrossRef]
- Dunbar-Co, S.; Sporck, M.J.; Sack, L. Leaf trait diversification and design in seven rare taxa of the Hawaiian Plantago radiation. Int. J. Plant Sci. 2009, 170, 61–75. [Google Scholar] [CrossRef]
- Moles, A.; Warton, D.; Warman, L.; Swenson, N.; Laffan, S.; Zanne, A.; Pitman, A.; Hemmings, F.; Leishman, M. Global patterns in plant height. J. Ecol. 2009, 97, 923–932. [Google Scholar] [CrossRef]
- Laughlin, D.C. The intrinsic dimensionality of plant traits and its relevance to community assembly. J. Ecol. 2014, 102, 186–193. [Google Scholar] [CrossRef]
- Russo, S.E.; Kitajima, K. The Ecophysiology of leaf lifespan in tropical forests: Adaptive and plastic responses to environmental heterogeneity. In Tropical Tree Physiology; Goldstein, G., Santiago, L., Eds.; Springer: Cham, Germany, 2016; pp. 357–383. ISBN 978-3-319-27422-5. [Google Scholar]
- Bruelheide, H.; Dengler, J.; Purschke, O.; Lenoir, J.; Jiménez-Alfaro, B.; Hennekens, S.M.; Botta-Dukát, Z.; Chytrý, M.; Field, R.; Jansen, F.; et al. Global trait–environment relationships of plant communities. Nat. Ecol. Evol. 2018, 2, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Godoy, O.; Levine, J.M. Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl. Acad. Sci. USA 2015, 112, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Gleason, S.M.; Butler, D.W.; Waryszak, P. Shifts in leaf and stem hydraulic traits across aridity gradients in Eastern Australia. Int. J. Plant Sci. 2013, 174, 1292–1301. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Ackerly, D.D.; Baum, D.A.; Bazzaz, F.A. Phylogenetic overdispersion in Floridian oak communities. Am. Nat. 2004, 163, 823–843. [Google Scholar] [CrossRef]
- Violle, C.; Jiang, L. Towards a trait-based quantification of species niche. J. Plant Ecol. 2009, 2, 87–93. [Google Scholar] [CrossRef]
- Hargreaves, G.H.; Samani, Z.A. Reference crop evapotranspiration from temperature. Appl. Eng. Agric. 1985, 1, 96–99. [Google Scholar] [CrossRef]
- NASA National Aeronautics and Space Administration. Available online: https://power.larc.nasa.gov/data-access-viewer/ (accessed on 25 December 2017).
- Allen, R.G. Evapotranspiración del Cultivo: Guías Para la Determinación de los Requerimientos de Agua de los Cultivos; Food & Agriculture Org.: Roma, Italy, 2006; Volume 56. [Google Scholar]
| Species | Section | Leaf Habit |
|---|---|---|
| Quercus aristata Hook. & Arn. | Lobatae | deciduous |
| Quercus calophylla Schltdl. & Cham. | Lobatae | brevi-deciduous |
| Quercus castanea Née | Lobatae | brevi-deciduous |
| Quercus crassifolia Bonpl. | Lobatae | brevi-deciduous |
| Quercus cualensis L.M. González | Lobatae | brevi-deciduous |
| Quercus eduardii Trel. | Lobatae | brevi-deciduous |
| Quercus elliptica Née | Lobatae | deciduous |
| Quercus glaucescens Bonpl. | Quercus | brevi-deciduous |
| Quercus iltisii L.M. González | Lobatae | brevi-deciduous |
| Quercus jonesii Trel. | Lobatae | deciduous |
| Quercus laeta Liebm. | Quercus | deciduous |
| Quercus laurina Bonpl. | Lobatae | brevi-deciduous |
| Quercus liebmannii Oerst. ex Trel. | Quercus | deciduous |
| Quercus magnoliifolia Née | Quercus | deciduous |
| Quercus martinezii C.H. Mull. | Quercus | evergreen |
| Quercus mexiae L.M. González | Lobatae | deciduous |
| Quercus obtusata Bonpl. | Quercus | brevi-deciduous |
| Quercus resinosa Liebm. | Quercus | deciduous |
| Quercus scytophylla Liebm. | Lobatae | brevi-deciduous |
| Quercus tuitensis L.M. González | Lobatae | deciduous |
| Quercus aff. uxoris McVaugh | Lobatae | evergreen |
| Traits | Abbreviation | Unit | Mean | Range |
|---|---|---|---|---|
| Leaf traits | ||||
| Leaf area | LA | cm2 | 47.35 | 5.01–270.62 |
| Specific leaf area | SLA | cm2 g−1 | 71.25 | 22.52–174.52 |
| Leaf dry matter content | LDMC | mg g−1 | 0.436 | 0.20–0.64 |
| Nitrogen concentration | N | mg g−1 | 10.59 | 6.74–16.63 |
| Phosphorus concentration | P | mg g−1 | 1.03 | 0.39–2.5 |
| Stem traits | ||||
| Vessel frequency | VF | mm2 | 5.99 | 3.0–14.0 |
| Vessel diameter | VD | µm | 189.75 | 98.40–284.4 |
| Wood density | WD | g/cm3 | 0.66 | 0.36–0.89 |
| Height | H | m | 9.85 | 3.40–28.00 |
| Source of Variance | Proportion of Variance | ||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| Species | 0.32 | 0.40 | 0.50 |
| Sections | - | 0.28 | 0.14 |
| Leaf habit | 0.58 | 0.07 | - |
| Intraspecific | 0.08 | 0.02 | 0.1 |
| Habitat | - | 0.05 | 0.05 |
| Model | R2 Marginal | R2 Conditional | AICc | ||
|---|---|---|---|---|---|
| Leaf economic spectrum | PC1 | logUNEP * log (pwq) | 0.09 | 0.30 | 597.00 |
| 618.00 | |||||
| Hydraulic stem traits | PC2 | logUNEP * log (mtwm) | 0.05 | 0.23 | 642.71 |
| 648.43 | |||||
| Leaf area and height | PC3 | logUNEP * log (pcq) | 0.16 | 0.40 | 562.10 |
| 574.16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arenas-Navarro, M.; García-Oliva, F.; Terrazas, T.; Torres-Miranda, A.; Oyama, K. Leaf Habit and Stem Hydraulic Traits Determine Functional Segregation of Multiple Oak Species along a Water Availability Gradient. Forests 2020, 11, 894. https://doi.org/10.3390/f11080894
Arenas-Navarro M, García-Oliva F, Terrazas T, Torres-Miranda A, Oyama K. Leaf Habit and Stem Hydraulic Traits Determine Functional Segregation of Multiple Oak Species along a Water Availability Gradient. Forests. 2020; 11(8):894. https://doi.org/10.3390/f11080894
Chicago/Turabian StyleArenas-Navarro, Maribel, Felipe García-Oliva, Teresa Terrazas, Andrés Torres-Miranda, and Ken Oyama. 2020. "Leaf Habit and Stem Hydraulic Traits Determine Functional Segregation of Multiple Oak Species along a Water Availability Gradient" Forests 11, no. 8: 894. https://doi.org/10.3390/f11080894
APA StyleArenas-Navarro, M., García-Oliva, F., Terrazas, T., Torres-Miranda, A., & Oyama, K. (2020). Leaf Habit and Stem Hydraulic Traits Determine Functional Segregation of Multiple Oak Species along a Water Availability Gradient. Forests, 11(8), 894. https://doi.org/10.3390/f11080894





