Is Soil Contributing to Climate Change Mitigation during Woody Encroachment? A Case Study on the Italian Alps
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Sampling Strategy and Soil Analyses
2.4. Statistics
3. Results
3.1. Soil Physical and Chemical Parameters
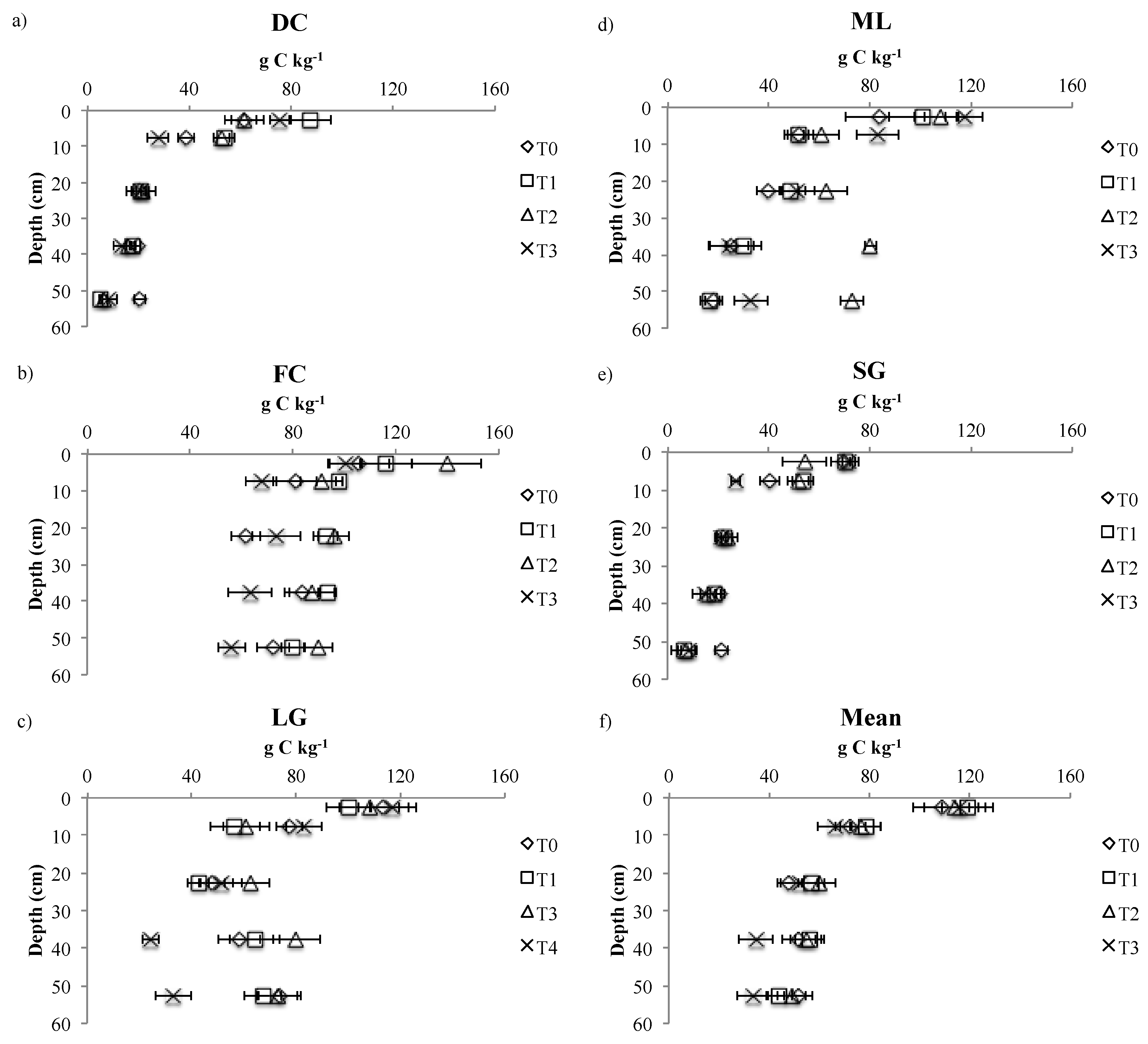
3.2. SOC Stocks Changes
4. Discussion
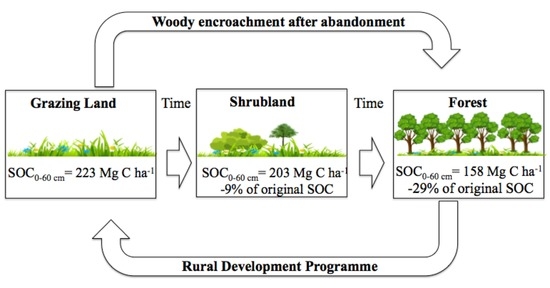
4.1. Impact of Land Abandonment on the SOC Pool
4.2. Implication for Rural Development Programmes and Climate Policies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Successional Stages | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | T0 | T1 | T2 | T3 | ||||||||
| DC | Vegetation * | Age | Area m2 | Vegetation * | Age | Area m2 | Vegetation * | Age | Area m2 | Vegetation * | Age | Area m2 |
| H = Arrhenatherum elatius (L.) P.Beauv. ex J.Presl & C.Presl., Dactylis glomerata L. | Stage | H = Arrhenatherum elatius (L.) P.Beauv. ex J.Presl & C.Presl., Dactylis glomerata L.; S = Sorbus aria (L.) Crantz, Juniperus communis L. | Stage | H = Dactylis glomerata L.; S = Sorbus aria (L.) Crantz, Juniperus communis L.; T = Picea abies (L.) H.Karst., Larix decidua Mill. | Stage | S = Juniperus communis L.; T = Picea abies (L.) H.Karst., Fagus sylvatica L. | Stage | |||||
| 0 | 4976 | ~15 | 29,830 | ~35 | 5203 | ~70 | 5053 | |||||
| plot | plot | plot | Plot | |||||||||
| ~150 | ~625 | ~70 | ~200 | |||||||||
| Successional Stages | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | T0 | T1 | T2 | T3 | ||||||||
| Vegetation * | Age | Area m2 | Vegetation * | Age | Area m2 | Vegetation * | Age | Area m2 | Vegetation * | Age | Area m2 | |
| FC | H = Arrhenatherum elatius (L.) P.Beauv. ex J.Presl & C.Presl., Dactylis glomerata L. | Stage | H = Arrhenatherum elatius (L.) P.Beauv. ex J.Presl & C.Presl., Nardus stricta L., Arctium lappa L.; S = Rhododendron ferrugineum L., Vaccinium spp. | Stage | H = Nardus stricta L.; S = Juniperus communis L.; T = Corylus avellana L. | Stage | S = Juniperus communis L.; T = Picea abies (L.) H.Karst., Fagus sylvatica L. | Stage | ||||
| 0 | 5390 | ~15 | 4669 | ~35 | 4231 | ~70 | 5192 | |||||
| Plot | Plot | plot | Plot | |||||||||
| ~70 | ~100 | ~140 | ~85 | |||||||||
| Successional Stages | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | T0 | T1 | T2 | T3 | ||||||||
| Vegetation * | Age | Area m2 | Vegetation * | Age | Area m2 | Vegetation * | Age | Area m2 | Vegetation * | Age | Area m2 | |
| LG | H = Arrhenatherum elatius (L.) P.Beauv. ex J.Presl & C.Presl., Dactylis glomerata L. | Stage | H = Arrhenatherum elatius (L.) P.Beauv. ex J.Presl & C.Presl., Nardus stricta L., Arctium lappa L.; S = Vaccinium spp. | Stage | H = Nardus stricta L.; S = Vaccinium spp.; T = Picea abies (L.) H.Karst., Fagus sylvatica L. | Stage | S = Vaccinium myrtillus L.; T = Picea abies (L.) H.Karst. | Stage | ||||
| 0 | 233,046 | ~15 | 16,986 | ~35 | 5748 | ~70 | 22,749 | |||||
| Plot | Plot | Plot | Plot | |||||||||
| ~2500 | ~570 | ~55 | ~625 | |||||||||
| Successional Stages | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | T0 | T1 | T2 | T3 | ||||||||
| Vegetation * | Age | Area m2 | Vegetation * | Age | Area m2 | Vegetation * | Age | Area m2 | Vegetation * | Age | Area m2 | |
| ML | H = Cynosurus cristatus L., Scorzoneroides autumnalis (L.) Moench, Lolium perenne L. | Stage | H = Cynosurus cristatus L., Scorzoneroides autumnalis (L.) Moench, Lolium perenne L.; S = Rhododendron ferrugineum L., Vaccinium spp. | Stage | T = Picea abies (L.) H.Karst. | Stage | S = Vaccinum myrtillus L.-T = Picea abies (L.) H.Karst | Stage | ||||
| 0 | 33,273 | 5 | 33220 | ~35 | 10,400 | >62 | 47,122 | |||||
| Plot | Plot | Plot | Plot | |||||||||
| 1110 | 860 | ~260 | ~340 | |||||||||
| Successional Stages | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | T0 | T1 | T2 | T3 | ||||||||
| Vegetation * | Age | Area m2 | Vegetation * | Age | Area m2 | Vegetation * | Age | Area m2 | Vegetation * | Age | Area m2 | |
| SG | H = Festuca alpestris Roem. & Schult., Festuca varia Haenke | Stage | H = Festuca alpestris Roem. & Schult., Festuca varia Haenke; S = Rosa canina L., Sorbus spp., Juniperus communis L. | Stage | H = Festuca alpestris Roem. & Schult., Festuca varia Haenke; S = Sorbus spp., Juniperus communis L.; T = Picea abies (L.) H.Karst., Larix decidua Mill. | Stage | S = Sorbus spp., Juniperus communis L.; T = Picea abies (L.) H.Karst., Larix decidua Mill. | Stage | ||||
| 0 | ~5000 | ~15 | 45,018 | ~35 | 15,035 | ~70 | 10,006 | |||||
| Plot | Plot | plot | Plot | |||||||||
| ~150 | ~860 | ~280 | ~210 | |||||||||
| Successionale Stages | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | ||||||||||||||
| Area | Depth | Sa | Si | Cl | pH | Sa | Si | Cl | pH | Sa | Si | Cl | pH | Sa | Si | Cl | pH |
| cm | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | |||||
| DC | 0–5 | 490 ± 24 | 315 ± 31 | 195 ± 23 | 5.8 ± 0.2 | 475 ± 27 | 301 ± 25 | 224 ± 27 | 6.2 ± 0.2 | 399 ± 29 | 270 ± 32 | 331 ± 31 | 6.3 ± 0.2 | 441 ± 27 | 240 ± 28 | 319 ± 31 | 5.9 ± 0.2 |
| 5–15 | 470 ± 21 | 330 ± 28 | 200 ± 29 | 6.0 ± 0.2 | 474 ± 29 | 321 ± 32 | 205 ± 23 | 6.2 ± 0.3 | 403 ± 27 | 245 ± 27 | 352 ± 27 | 6.5 ± 0.3 | 436 ± 32 | 251 ± 19 | 313 ± 33 | 5.8 ± 0.3 | |
| 15–30 | 486 ± 18 | 315 ± 29 | 199 ± 31 | 6.1 ± 0.1 | 489 ± 31 | 305 ± 29 | 206 ± 33 | 6.4 ± 0.3 | 412 ± 19 | 256 ± 32 | 332 ± 29 | 6.7 ± 0.3 | 423 ±25 | 239 ± 24 | 338 ± 24 | 6.1 ± 0.2 | |
| 30–45 | 490 ± 26 | 290 ± 23 | 220 ± 35 | 6.4 ± 0.2 | 490 ± 30 | 281 ± 25 | 229 ± 31 | 6.7 ± 0.3 | 424 ± 28 | 244 ±35 | 332 ± 32 | 6.7 ± 0.3 | 429 ± 28 | 241 ± 32 | 330 ± 29 | 6.4 ± 0.2 | |
| 45–60 | 495 ± 28 | 298 ± 26 | 207 ± 27 | 6.4 ± 0.1 | 465 ± 28 | 288 ± 23 | 247 ± 21 | 6.9 ± 0.3 | 413 ± 29 | 251 ± 28 | 336 ± 24 | 7.0 ± 0.3 | 410 ± 23 | 263 ± 31 | 327 ± 25 | 6.5 ± 0.3 | |
| FC | 0–5 | 356 ± 33 | 375 ± 32 | 269 ± 33 | 6.1 ± 0.1 | 389 ± 32 | 301 ± 27 | 310 ± 32 | 6.5 ± 0.2 | 387 ± 22 | 298 ± 19 | 315 ± 28 | 5.5 ± 0.2 | 406 ± 19 | 298 ± 21 | 296 ± 23 | 6.7 ± 0.3 |
| 5–15 | 338 ± 27 | 345 ± 28 | 317 ± 25 | 6.3 ± 0.2 | 378 ± 23 | 311 ± 22 | 311 ± 35 | 6.3 ± 0.3 | 393 ± 22 | 302 ± 27 | 305 ± 23 | 5.7 ± 0.2 | 416 ± 23 | 267 ± 25 | 317 ± 21 | 6.5 ± 0.3 | |
| 15–30 | 346 ± 26 | 361 ± 28 | 293 ± 23 | 6.4 ± 0.2 | 381 ± 28 | 319 ± 27 | 300 ± 31 | 6.7 ± 0.3 | 401 ± 22 | 296 ± 29 | 303 ± 29 | 5.9 ± 0.2 | 401 ± 27 | 303 ± 27 | 296 ± 19 | 6.7 ± 0.3 | |
| 30–45 | 332 ± 27 | 342 ± 24 | 326 ± 19 | 6.4 ± 0.2 | 390 ±25 | 321 ± 19 | 289 ± 29 | 6.7 ± 0.3 | 384 ± 23 | 279 ± 31 | 337 ± 31 | 6.3 ± 0.3 | 369 ± 29 | 297 ± 19 | 334 ± 29 | 7.0 ± 0.2 | |
| 45–60 | 374 ± 31 | 299 ± 29 | 327 ± 30 | 6.7 ± 0.2 | 415 ±32 | 297 ± 28 | 288 ± 27 | 6.9 ± 0.3 | 389 ±21 | 258± 26 | 353 ± 27 | 7.0 ± 0.3 | 379 ± 23 | 287 ± 31 | 334 ± 27 | 7.1 ± 0.2 | |
| Successionale Stages | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | ||||||||||||||
| Area | Depth | Sa | Si | Cl | pH | Sa | Si | Cl | pH | Sa | Si | Cl | pH | Sa | Si | Cl | pH |
| cm | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | |||||
| LG | 0–5 | 369 ± 32 | 303 ± 35 | 328 ± 33 | 5.4 ± 0.2 | 401 ± 24 | 299 ± 22 | 300 ± 33 | 5.1 ± 0.2 | 356 ± 23 | 286 ± 32 | 358 ± 22 | 4.9 ± 0.3 | 403 ± 33 | 303 ± 21 | 294 ± 21 | 5.0 ± 0.3 |
| 5–15 | 374 ± 22 | 298 ± 32 | 328 ± 36 | 5.3 ± 0.2 | 415 ± 21 | 301 ± 26 | 284 ± 31 | 5.7 ± 0.3 | 385 ± 29 | 265± 33 | 350 ± 28 | 5.5 ± 0.2 | 399 ± 32 | 287 ± 27 | 314 ± 19 | 5.3 ± 0.2 | |
| 15–30 | 367 ± 29 | 275 ± 35 | 358 ± 29 | 5.9 ± 0.1 | 403 ± 28 | 297 ± 29 | 300 ± 19 | 5.5 ± 0.3 | 398 ± 21 | 257 ± 29 | 345 ± 33 | 5.5 ± 0.3 | 388 ± 36 | 275 ± 26 | 337 ± 29 | 5.5 ± 0.3 | |
| 30–45 | 371 ± 33 | 261 ± 33 | 368 ± 29 | 6.3 ±0.2 | 398 ± 26 | 277 ± 33 | 325 ± 25 | 6.3 ± 0.3 | 401 ± 19 | 243 ± 31 | 356 ± 35 | 5.9 ± 0.2 | 379 ± 29 | 267 ± 21 | 354 ± 31 | 5.5 ± 0.3 | |
| 45–60 | 369 ± 38 | 245 ± 35 | 386 ± 25 | 6.7 ± 0.3 | 381 ± 27 | 275 ± 33 | 344 ± 29 | 6.5 ± 0.2 | 392 ±28 | 252 ± 36 | 356 ± 38 | 6.3 ± 0.3 | 367 ± 29 | 254 ± 22 | 379 ± 33 | 5.9 ± 0.3 | |
| ML | 0–5 | 200 ± 32 | 470 ± 32 | 330 ± 35 | 6.3 ± 0.2 | 180 ± 21 | 470 ± 19 | 350 ± 28 | 6.1 ± 0.3 | 230 ± 21 | 400 ± 32 | 370 ± 23 | 6.0 ± 0.3 | 250 ± 28 | 410 ± 32 | 340 ± 29 | 5.8 ± 0.2 |
| 5–15 | 190 ± 33 | 430 ± 29 | 380 ±29 | 6.3 ± 0.3 | 190 ± 25 | 440 ± 23 | 370 ± 25 | 6.3 ± 0.3 | 250 ± 28 | 380 ± 36 | 370 ± 21 | 6.3 ± 0.3 | 230 ± 29 | 420 ± 33 | 350 ± 31 | 6.0 ± 0.2 | |
| 15–30 | 220 ± 29 | 450 ± 29 | 330 ± 28 | 6.5 ± 0.3 | 190 ± 28 | 420 ± 28 | 390 ± 25 | 6.3 ± 0.3 | 210 ± 29 | 400 ± 33 | 390 ± 29 | 6.5 ± 0.3 | 230 ± 23 | 410 ± 36 | 360 ± 33 | 6.2 ± 0.2 | |
| 30–45 | 200 ± 36 | 450 ± 32 | 350 ± 33 | 6.5 ± 0.3 | 220 ± 23 | 410 ± 25 | 370 ± 29 | 6.5 ± 0.2 | 220 ± 31 | 380 ± 29 | 400 ± 32 | 6.5 ± 0.3 | 240 ± 19 | 370 ± 28 | 390 ± 29 | 6.5 ± 0.2 | |
| 45–60 | 170 ± 37 | 450 ± 33 | 380 ± 29 | 6.7 ± 0.2 | 200 ± 28 | 410 ± 23 | 390 ± 22 | 6.7 ± 0.2 | 230 ± 27 | 370 ± 33 | 400 ± 31 | 6.7 ± 0.2 | 210 ± 22 | 370 ± 29 | 420 ± 27 | 6.5 ± 0.3 | |
| SG | 0–5 | 320 ± 19 | 480 ± 28 | 200 ± 33 | 7.0 ± 0.3 | 360 ± 21 | 410 ± 32 | 230 ± 29 | 7.2 ± 0.3 | 350 ± 23 | 430 ± 23 | 220 ± 33 | 6.9 ± 0.3 | 310 ± 32 | 460 ± 22 | 230 ± 32 | 6.9 ± 0.3 |
| 5–15 | 330 ± 21 | 450 ± 23 | 220 ± 23 | 7.2 ± 0.2 | 350 ± 24 | 420 ± 37 | 230 ± 24 | 7.4 ± 0.2 | 340 ± 28 | 420 ± 32 | 240 ± 35 | 7.0 ± 0.3 | 300 ± 29 | 460 ± 19 | 240 ± 27 | 7.0 ± 0.2 | |
| 15–30 | 300 ± 27 | 460 ± 27 | 240 ± 33 | 7.2 ± 0.2 | 300 ± 27 | 480 ± 35 | 220 ± 23 | 7.5 ± 0.3 | 290 ± 19 | 470 ± 33 | 240 ± 38 | 7.9 ± 0.3 | 270 ± 28 | 490 ± 34 | 240 ± 29 | 7.1 ± 0.3 | |
| 30–45 | 310 ± 23 | 390 ± 22 | 300 ± 31 | 7.7 ± 0.3 | 320 ± 25 | 430 ± 33 | 250 ± 28 | 7.9 ± 0.2 | 290 ± 24 | 450 ± 29 | 260 ± 29 | 7.9 ± 0.3 | 280 ± 33 | 450 ± 28 | 270 ± 27 | 7.8 ± 0.2 | |
| 45–60 | 270 ± 26 | 410 ± 31 | 320 ± 29 | 8.1 ± 0.3 | 300 ± 21 | 420 ± 37 | 280 ± 21 | 8.3 ± 0.3 | 290 ± 19 | 430 ± 35 | 280 ± 28 | 8.4 ± 0.2 | 290 ± 26 | 440 ± 21 | 270 ± 28 | 8.4 ± 0.3 | |
| Successional Stages | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Area | Depth | T0 | T1 | T2 | T3 | ||||
| cm | BD | RF | BD | RF | BD | RF | BD | RF | |
| Mg m−3 | % | Mg m−3 | % | Mg m−3 | % | Mg m−3 | % | ||
| DC | 0–5 | 0.6 ± 0.1 | 0 ± 0 | 0.5 ± 0.1 | 0 ± 0 | 0.6 ± 0.2 | 8 ± 2 | 0.6 ± 0.1 | 1 ± 0 |
| 5–15 | 1.0 ± 0.2 | 0 ± 0 | 0.9 ± 0.2 | 3 ± 1 | 1.3 ± 0.3 | 6 ± 1 | 1.2 ± 0.2 | 6 ± 1 | |
| 15–30 | 1.1 ± 0.2 | 2 ± 1 | 1.4 ± 0.4 | 7 ± 2 | 1.2 ± 0.3 | 5 ± 0.1 | 1.1 ± 0.3 | 12 ± 2 | |
| 30–45 | 1.1 ± 0.3 | 11 ± 5 | 1.2 ± 0.2 | 9 ± 3 | 1.2 ± 0.1 | 10 ± 0.2 | 1.2 ± 0.2 | 16 ± 3 | |
| 45–60 | 1.1 ± 0.2 | 12 ± 4 | 1.2 ± 0.2 | 19 ± 5 | 1.1 ± 0.3 | 11 ± 0.3 | 1.3 ± 0.3 | 11 ± 3 | |
| FC | 0–5 | 1.1 ± 0.1 | 22 ± 8 | 0.8 ± 0.1 | 20 ± 3 | 0.9 ± 0.1 | 28 ± 7 | 0.9 ±0.2 | 20 ± 10 |
| 5–15 | 1.4 ± 0.2 | 23 ± 6 | 1.3 ± 0.2 | 26 ± 8 | 1.1 ± 0.2 | 27 ± 12 | 1.1 ± 0.1 | 22 ± 4 | |
| 15–30 | 1.3 ± 0.1 | 27 ± 4 | 1.3 ± 0.2 | 34 ± 6 | 1.2 ± 0.2 | 31 ±3 | 1.2 ± 0.2 | 25 ± 5 | |
| 30–45 | 1.3 ± 0.3 | 26 ± 3 | 1.4 ± 0.2 | 26 ± 8 | 1.2 ± 0.3 | 32 ± 4 | 1.3 ± 0.3 | 23 ± 3 | |
| 45–60 | 1.3 ± 0.3 | 27 ± 5 | 1.4 ± 0.3 | 26 ± 7 | 1.3 ± 0.2 | 34 ± 6 | 1.3 ± 0.2 | 27 ± 6 | |
| LG | 0–5 | 0.7 ± 0.1 | 11 ± 8 | 0.7 ± 0.1 | 15 ± 5 | 0.6 ± 0.1 | 15 ± 6 | 0.9 ± 0.2 | 43 ± 8 |
| 5–15 | 1.2 ± 0.2 | 37 ± 9 | 1.2 ± 0.2 | 40 ± 6 | 1.0 ± 0.2 | 37 ± 7 | 1.0 ± 0.2 | 52 ± 11 | |
| 15–30 | 1.2 ± 0.3 | 38 ± 12 | 1.2 ± 0.3 | 37 ± 5 | 0.9 ± 0.2 | 46 ± 5 | 1.0 ± 0.1 | 54 ± 4 | |
| 30–45 | 1.1 ± 0.2 | 49 ± 15 | 1.3 ± 0.2 | 38 ± 14 | 1.0 ± 0.3 | 42 ± 8 | 1.1 ± 0.2 | 55 ± 3 | |
| 45–60 | 1.3 ± 0.2 | 46 ± 11 | 1.3 ± 0.2 | 31 ± 4 | 1.0 ± 0.2 | 33 ± 6 | 1.2 ± 0.2 | 53 ± 6 | |
| ML | 0–5 | 0.7 ± 0.2 | 43 ± 15 | 0.8 ± 0.1 | 39 ± 7 | 0.7 ± 0.1 | 27 ± 7 | 0.7 ± 0.1 | 37 ± 13 |
| 5–15 | 1.1 ± 0.1 | 47 ± 8 | 0.9 ± 0.1 | 31 ± 13 | 0.9 ± 0.1 | 42 ± 6 | 1.0 ± 0.2 | 53 ± 12 | |
| 15–30 | 1.1 ± 0.2 | 51 ± 5 | 0.9 ± 0.2 | 28 ± 17 | 1.0 ± 0.2 | 44 ± 7 | 1.0 ± 0.2 | 51 ± 13 | |
| 30–45 | 1.0 ± 0.2 | 0.0 | 1.0 ± 0.2 | 28 ± 15 | 1.0 ± 0.2 | 42 ± 10 | 1.1 ± 0.1 | 7 ± 10 | |
| 45–60 | 1.2 ± 0.3 | 0.0 | 1.0 ± 0.2 | 29 ± 12 | 1.2 ± 0.3 | 47 ± 9 | 1.1 ± 0.2 | 46 ± 9 | |
| SG | 0–5 | 0.9 ± 0.1 | 19 ± 2 | 0.7 ± 0.1 | 19 ± 8 | 0.6 ± 0.1 | 34 ± 5 | 0.7 ± 0.1 | 37 ± 5 |
| 5–15 | 1.0 ± 0.2 | 25 ± 8 | 0.7 ± 0.2 | 22 ± 6 | 0.8 ± 0.2 | 45 ± 4 | 0.8 ± 0.2 | 44 ± 7 | |
| 15–30 | 1.1 ± 0.2 | 17 ± 3 | 0.9 ± 0.2 | 27 ± 3 | 1.2 ± 0.2 | 55 ± 8 | 1.2 ± 0.3 | 49 ± 9 | |
| 30–45 | 1.2 ± 0.2 | 25 ± 5 | 1.2 ± 0.2 | 36 ± 6 | 1.2 ± 0.3 | 62 ± 5 | 1.3 ± 02 | 59 ± 4 | |
| 45–60 | 1.4 ± 0.3 | 31 ± 4 | 1.4 ± 0.3 | 39 ± 5 | 1.3 ± 0.2 | 65 ± 9 | 1.3 ± 0.2 | 61 ± 7 | |
| Successional Stages | |||||
|---|---|---|---|---|---|
| Site | Layer | T0 | T1 | T2 | T3 |
| cm | g C kg−1 ± SD | g C kg−1 ± SD | g C kg−1 ± SD | g C kg−1 ± SD | |
| DC | 0–5 | 61.3 ± 5.0a | 87.6 ± 8.1b | 61.7 ± 7.8a | 75.8 ± 4.2c |
| 5–15 | 38.8 ± 3.4a | 53.8 ± 4.3b | 52.7 ± 3.2b | 27.9 ± 4.0a | |
| 15–30 | 20.7 ± 3.5 | 21.2 ± 5.9 | 22.0 ± 1.0 | 21.3 ± 2.1 | |
| 30–45 | 19.8 ± 1.1 | 18.0 ± 1.0 | 16.3 ± 1.2 | 13.7 ± 3.2 | |
| 45–60 | 20.7 ± 2.1a | 5.5 ± 0.7b | 6.7 ± 1.4b | 8.3 ± 3.6b | |
| FC | 0–5 | 105.5 ± 11.8 | 116.3 ± 9.7 | 139.6 ± 13.6 | 100.2 ± 5.8 |
| 5–15 | 80.8 ± 8.3a | 97.8 ± 1.3a | 91.1 ± 8.2a | 67.7 ± 5.9b | |
| 15–30 | 61.7 ± 5.6a | 92.7 ± 4.6b | 95.8 ± 6.0b | 73.8 ± 9.4a | |
| 30–45 | 83.4 ± 6.6a | 93.7 ± 3.0a | 87.4 ± 8.7a | 63.3 ± 8.5b | |
| 45–60 | 72.2 ± 6.3a | 79.9 ± 4.7a | 89.6 ± 5.6a | 56.2 ± 5.2b | |
| LG | 0–5 | 113.6 ± 9.7 | 100.4 ± 8.4 | 108.3 ± 11.4 | 117.3 ± 8.8 |
| 5–15 | 77.3 ± 5.1a | 56.5 ± 9.6b | 60.9 ± 8.9b | 82.9 ± 7.0a | |
| 15–30 | 48.0 ± 6.0 | 42.8 ± 4.0 | 62.9 ± 7.0 | 51.5 ± 7.9 | |
| 30–45 | 58.1 ± 8.1a | 64.2 ± 9.5a | 80.2 ± 9.0b | 24.3 ± 3.3c | |
| 45–60 | 73.9 ± 7.9a | 67.5 ± 7.1a | 72.9 ± 7.7a | 33.1 ± 6.9b | |
| ML | 0–5 | 83.9 ± 13.7 | 101.1 ± 13.2 | 92.3 ± 6.8 | 99.8 ± 7.3 |
| 5–15 | 51.6 ± 4.1 | 51.9 ± 5.7 | 49.5 ± 6.9 | 59.1 ± 8.4 | |
| 15–30 | 39.9 ± 4.6 | 48.6 ± 3.4 | 35.3 ± 8.4 | 40.2 ± 6.7 | |
| 30–45 | 25.2 ± 8.9 | 30.0 ± 7.0 | 18.9 ± 2.4 | 22.9 ± 7.6 | |
| 45–60 | 18.1 ± 3.5 | 16.7 ± 3.9 | 18.9 ± 4.6 | 27.5 ± 6.5 | |
| SG | 0–5 | 70.0 ± 5.6a | 70.7 ± 1.5a | 54.3 ± 8.6b | 71.7 ± 2.5a |
| 5–15 | 40.7 ± 3.8a | 53.7 ± 4.2a | 51.7 ± 4.2a | 27.0 ± 1.7b | |
| 15–30 | 22.0 ± 1.0 | 22.3 ± 3.1 | 23.7 ± 4.2 | 21.3 ± 2.5 | |
| 30–45 | 20.7 ± 2.1 | 19.0 ± 2.0 | 16.0 ± 2.9 | 14.7 ± 4.6 | |
| 45–60 | 21.3 ± 2.5a | 6.3 ± 4.6b | 7.3 ± 3.5b | 8.3 ± 3.2b | |
| Successional Stages | |||||
|---|---|---|---|---|---|
| Site | Depth | T0 | T1 | T2 | T3 |
| cm | Mg C ha−1 ± SD | Mg C ha−1 ± SD | Mg C ha−1 ± SD | Mg C ha−1 ± SD | |
| DC | Organic H. | 0 | 0 | 0 | 12.8 ± 5.4 |
| 0–30 | 91.1 ± 4.7a | 110.3 ± 11.6ab | 121.0 ± 23.1b | 87.9 ± 1.5a | |
| 30–60 | 58.7 ± 4.4a | 36.6 ± 4.1b | 37.3 ± 3.7b | 35.6 ± 4.3b | |
| 0–60 | 149.8 ± 6.5a | 146.9 ± 12.3a | 158.4 ± 23.4a | 123.5 ± 7.1b | |
| FC | Organic H. | 0 | 0 | 0 | 7.0 ± 1.4 |
| 0–30 | 217.1 ± 11.4 | 256.0 ± 30.0 | 224.1 ± 7.7 | 194.3 ± 26.7 | |
| 30–60 | 233.7 ± 30.9a | 275.4 ± 27.0a | 217.9 ± 8.5a | 164.6 ± 21.6b | |
| 0–60 | 450.8 ± 32.9a | 531.4 ± 40.3b | 441.9 ± 11.5a | 358.9 ± 34.4c | |
| LG | Organic H. | 0 | 0 | 0 | 6.8 ± 3.3 |
| 0–30 | 129.7 ± 21.1 | 116.7 ± 9.1 | 110.6 ± 18.7 | 91.6 ± 25.0 | |
| 30–60 | 115.2 ± 21.0a | 148.4 ± 21.4a | 128.3 ± 9.5a | 44.2 ± 8.0b | |
| 0–60 | 245.0 ± 29.8a | 265.2 ± 23.3a | 238.8 ± 21.0a | 135.8 ± 26.3b | |
| ML | Organic H. | 0 | 0 | 0 | 9.9 ± 3.1 |
| 0–30 | 74.6 ± 10.1 | 84.5 ± 6.7 | 77.8 ± 5.1 | 81.6 ± 14.5 | |
| 30–60 | 65.6 ± 23.4a | 49.9 ± 18.3ab | 31.9 ± 8.3b | 35.4 ± 7.6b | |
| 0–60 | 140.2 ± 25.5 | 134.4 ± 19.5 | 109.7 ± 9.8 | 116.9 ± 16.4 | |
| SG | Organic H. | 0 | 0 | 0 | 20.7 ± 6.3 |
| 0–30 | 87.0 ± 6.1a | 61.4 ± 14.7a | 49.9 ± 2.2b | 41.1 ± 5.7b | |
| 30–60 | 44.7 ± 8.9a | 19.7 ± 2.2b | 16.9 ± 4.4b | 17.9 ± 4.9b | |
| 0–60 | 131.7 ± 10.7a | 71.1 ± 14.8b | 66.8 ± 4.9b | 59.0 ± 7.5b | |
References
- Intergovernmental Panel on Climate Change. Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Intergovernmental Panel on Climate Change: Ginevra, Switzerland, 2019; pp. 1–36. Available online: https://www.ipcc.ch/site/assets/uploads/2019/08/Fullreport-1.pdf (accessed on 14 August 2020).
- Van Leeuwen, C.C.E.; Cammeraat, E.L.H.; De Vent, J.; Boix-Fayos, C. The evolution of soil conservation policies targeting land abandonment and soil erosion in Spain: A review. Land Use Policy 2019, 83, 174–186. [Google Scholar] [CrossRef]
- Nadal-Romero, E.; Cammeraat, L.; Pérez-Cardiel, E.; Lasanta, T. Effects of secondary succession and afforestation practices on soil properties after cropland abandonment in humid Mediterranean mountain areas. Agric. Ecosyst. Environ. 2016, 228, 91–100. [Google Scholar] [CrossRef]
- Romero-Díaz, A.; Ruiz-Sinoga, J.D.; Aymerich, F.R.; Brevik, E.C.; Cerdà, A. Ecosystem responses to land abandonment in Western Mediterranean Mountains. Catena 2017, 149, 824–835. [Google Scholar] [CrossRef]
- Novara, A.; Gristina, L.; Sala, G.; Galati, A.; Crescimanno, M.; Cerdà, A.; Badalamenti, E.; La Mantia, T. Agricultural land abandonment in Mediterranean environment provides ecosystem services via soil carbon sequestration. Sci. Total Environ. 2017, 576, 420–429. [Google Scholar] [CrossRef]
- European Commission. Report from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. The Implementation of the Soil Thematic Strategy and Ongoing Activities; European Commission: Brussels, Belgium, 2012; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52012DC0046&from=NL (accessed on 14 August 2020).
- European Commission. The European Green Deal. COM(2019) 640 Final; European Commission: Brussels, Belgium, 2019; Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:b828d165-1c22-11ea-8c1f-01aa75ed71a1.0002.02/DOC_1&format=PDF (accessed on 12 November 2019).
- European Commission. Regulation (EU) 2018/841 of the European Parliament and of the Council of 30 May 2018 on the inclusion of greenhouse gas emissions and removals from land use, land use change and forestry in the 2030 climate and energy framework, and amending Regulation (EU) No 525/2013 and Decision No 529/2013/EU. Off. J. Eur. Union L 2018, 156, 1–25. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32018R0841&from=IT (accessed on 14 August 2020).
- Ronchi, S.; Salata, S.; Arcidiacono, A.; Piroli, E.; Montanarella, L. Policy instruments for soil protection among the EU member states: A comparative analysis. Land Use Policy 2019, 82, 763–780. [Google Scholar] [CrossRef]
- Diotallevi, F.; Blasi, E.; Franco, S. Greening as compensation to production of environmental public goods: How do common rules have an influence at local level? The case of durum wheat in Italy. Agric. Food Econ. 2015, 3, 1–14. [Google Scholar] [CrossRef][Green Version]
- Borrelli, P.; Paustian, K.; Panagos, P.; Jones, A.; Schütt, B.; Lugato, E. Effect of Good Agricultural and Environmental Conditions on erosion and soil organic carbon balance: A national case study. Land Use Policy 2016, 50, 408–421. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) No 1305/2013 of the European Parliament and of the Council of 17 December 2013 on support for rural development by the European Agricultural Fund for Rural Development (EAFRD) and repealing Council Regulation (EC) No 1698/2005. 2013. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32013R1305 (accessed on 14 August 2020).
- Piussi, P. Expansion of European mountain forests. In Forests in Sustainable Mountain Development: A State of Knowledge Report for 2000; Price, M., Butt, N., Eds.; CAB International: Wallingford, CT, USA, 2000; pp. 19–25. [Google Scholar] [CrossRef]
- Nadal-Romero, E.; Otal-Laín, I.; Lasanta, T.; Sánchez-Navarrete, P.; Errea, P.; Cammeraat, E. Woody encroachment and soil carbon stocks in subalpine areas in the Central Spanish Pyrenees. Sci. Total Environ. 2018, 636, 727–736. [Google Scholar] [CrossRef]
- González Díaz, J.A.; Celaya, R.; Fernández-García, F.; Osoro, K.; García, R.R. Dynamics of rural landscapes in marginal areas of northern Spain. Past, present and future. Land Degrad. Dev. 2019, 30, 141–150. [Google Scholar] [CrossRef]
- Fuchs, R.; Schulp, C.J.; Hengeveld, G.M.; Verburg, P.H.; Clevers, J.G.; Schelhaas, M.J.; Herold, M. Assessing the influence of historic net and gross land changes on the carbon fluxes of Europe. Glob. Chang. Biol. 2016, 22, 2526–2539. [Google Scholar] [CrossRef] [PubMed]
- Alberti, G.; Peressotti, A.; Piussi, P.; Zerbi, G. Forest ecosystem carbon accumulation during a secondary succession on Eastern Prealps (Italy). Forestry 2008, 81, 1–11. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Pellis, G.; Chiti, T.; Rey, A.; Curiel Yuste, J.; Trotta, C.; Papale, D. The ecosystem carbon sink implications of mountain forest expansion into abandoned grazing land: The role of subsoil and climatic factors. Sci. Total Environ. 2019, 672, 106–120. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A.; Vesterdal, L.; Leifeld, J.; Van Wesemael, B.; Schumacher, J.; Gensior, A. Temporal dynamics of soil organic carbon after land-use change in the temperate zone – carbon response functions as a model approach. Glob. Chang. Biol. 2011, 17, 2415–2427. [Google Scholar] [CrossRef]
- Jackson, R.B.; Banner, J.L.; Jobbágy, E.G.; Pockman, W.T.; Wall, D.H. Ecosystem carbon loss with woody plant invasion of grasslands. Nature 2002, 418, 623–626. [Google Scholar] [CrossRef]
- Guidi, C.; Vesterdal, L.; Gianelle, D.; Rodeghiero, M. Changes in soil organic carbon and nitrogen following forest expansion on grassland in the Southern. Alps. For. Ecol. Manag. 2014, 328, 103–116. [Google Scholar] [CrossRef]
- Seeber, J.; Seeber, G.U.H. Effects of land-use changes on humus forms on alpine pastureland (Central Alps, Tyrol). Geoderma 2005, 124, 215–222. [Google Scholar] [CrossRef]
- Seeber, J.; Seeber, G.U.H.; Kossler, W.; Langel, R.; Scheu, S.; Meyer, E. Abundance and trophic structure ofmacro-decomposers on alpine pastureland (Central Apls, Tyrol): Effects of abandonment of pasturing. Pedobiologia 2005, 49, 221–228. [Google Scholar] [CrossRef]
- Hooker, T.D.; Compton, J.E. Forest Ecosystem Carbon and Nitrogen Accumulation During the First Century after Agricultural Abandonment. Ecol. Appl. 2003, 13, 299–313. [Google Scholar] [CrossRef]
- Thuille, A.; Schulze, E.D. Carbon dynamics in successional and afforested spruce stands in Thuringia and the Alps. Glob. Chang. Biol. 2006, 12, 325–342. [Google Scholar] [CrossRef]
- Risch, A.C.; Jurgensen, M.F.; Page-Dumroese, D.S.; Wildi, O.; Schütz, M. Long-term development of above- and below-ground carbon stocks following land-use change in subalpine ecosystems of the Swiss National Park. Can. J. For. Res. 2008, 38, 1590–1602. [Google Scholar] [CrossRef]
- Chiti, T.; Blasi, E.; Pellis, G.; Perugini, L.; Chiriacò, M.V.; Valentini, R. Soil organic carbon pool’s contribution to climate change mitigation on marginal land of a Mediterranean montane area in Italy. J. Environ. Manag. 2018, 218, 593–601. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World soil resources reports; IUSS Working Group WRB: Rome, Italy, 2015; Volume 106. [Google Scholar]
- ARPA Veneto. IL SUOLO–Formazione, Proprietà E Funzioni. 2014. Available online: https://www.arpa.veneto.it/temi-ambientali/suolo (accessed on 5 March 2020).
- Walker, L.R.; Wardle, D.A.; Bardgett, R.D.; Clarkson, B.D. The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 2010, 98, 725–736. [Google Scholar] [CrossRef]
- Veneto Region. Programma Di Sviluppo Rurale Per Il Veneto 2007–2013. Available online: https://www.regione.veneto.it/web/agricoltura-e-foreste/psr-2007-2013 (accessed on 14 August 2020).
- Stolbovoy, V.; Montanarella, L.; Filippi, N.; Jones, A.; Gallego, J.; Grassi, G. Soil Sampling Protocol to Certify the Changes of Organic Carbon Stock in Mineral Soil of the European Union; Version 2. EUR 21576 EN/2; Office for Official Publications of the European Communities: Luxembourg, 2007; p. 56. ISBN 978-92-79-05379-5. [Google Scholar]
- Penman, J.; Gytarsky, M.; Hiraishi, T.; Krug, T.; Kruger, D.; Pipatti, R.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K.; et al. Good Practice Guidance for Land Use, Land-Use Change and Forestry; IPCC National Greenhouse Gas Inventories Programme and Institute for Global Environmental Strategies: Kanagawa, Japan, 2003. [Google Scholar]
- ISO. Soil Quality–Sampling–Part 1: Guidance on the Design of Sampling Programmes; International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- Blake, G.R. Bulk density in methods of soil analyses. Agronomy 1965, 9, 374–390. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; SSSA Book Series; Soil Science Society of America: Madison, WI, USA; American Society of Agronomy: Madison, WI, USA, 1986; ISBN 978-0-89118-811-7. [Google Scholar]
- Poeplau, C.; Vos, C.; Don, A. Soil organic carbon stocks are systematically overestimated by misuse of the parameters bulk density and rock fragment content. Soil 2017, 3, 61–66. [Google Scholar] [CrossRef]
- Hobley, E.U.; Murphy, B.; Simmons, A. Comment on “soil organic stocks are systematically overestimated by misuse of the parameters bulk density and rock fragment content” by Poeplau et al. (2017). Soil 2018, 4, 169–171. [Google Scholar] [CrossRef]
- Adams, W.A. The effect of organic matter on the bulk and true densities of some uncultivated podzolic soils. Eur. J. Soil Sci. 1973, 24, 10–17. [Google Scholar] [CrossRef]
- Chiti, T.; Díaz-Pinés, E.; Rubio, A. Soil organic carbon stock of conifers, broadleaf and evergreen broadleaf forests of Spain. Biol. Fertil. Soils 2012, 48, 817–826. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Guidelines for National Greenhouse Gas Inventories, Prepared by the National Greenhouse Gas Inventories Programme; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; IGES: Kanagawa, Japan, 2006; Volume 4, Chapter 2; pp. 2–29. [Google Scholar]
- Stephens, M.A. Asymptotic results for goodness-of-fit statistics with unknown parameters. Ann. Stat. 1976, 4, 357–369. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 14 August 2020).
- Alberti, G.; Leronni, V.; Piazzi, M.; Petrella, F.; Mairota, P.; Peressotti, A.; Piussi, P.; Valentini, R.; Gristina, l.; La Mantia, T.; et al. Impact of woody encroachment on soil organic carbon and nitrogen in abandoned agricultural lands along a rainfall gradient in Italy. Reg. Environ. Chang. 2011, 11, 917–924. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Adams, D.E.; Wild, A. Model estimates ofCO2 emissions from soil in response to global warming. Nature 1991, 351, 304–306. [Google Scholar] [CrossRef]
- Vesterdal, L.; Schmidt, I.K.; Callesen, I.; Nilsson, L.O.; Gundersen, P. Carbon and nitro- gen in forest floor and mineral soil under six common European tree species. For. Ecol. Manag. 2008, 255, 35–48. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Sensitivity of soil organic carbon stocks and fractions to differ- ent land-use change across Europe. Geoderma 2013, 192, 189–201. [Google Scholar] [CrossRef]
- Vesterdal, L.; Ritter, E.; Gundersen, P. Change in soil organic carbon following afforestation of former arable land. For. Ecol. Mang. 2002, 169, 137–147. [Google Scholar] [CrossRef]
- Muys, B.; Lust, N.; Granval, P. Effects of grassland afforestation with different tree species on earthworm communities, litter decomposition and nutrient status. Soil Biol. Biochem. 1992, 24, 1459–1466. [Google Scholar] [CrossRef]
- Puhe, J. Growth and development of the root systern of Norway spruce (Picea abies) in forest stands-a review. For. Ecol. Manag. 2003, 175, 253–273. [Google Scholar] [CrossRef]
- Thuille, A.; Buchmann, N.; Schulze, E.-D. Carbon stocks and soil respiration rates during deforestation, grassland use and subsequent Norway spruce afforestation in the Southern Alps. Italy. Tree Physiol. 2000, 20, 849–857. [Google Scholar] [CrossRef]
- Montané, F.; Rovira, P.; Casals, P. Shrub encroachment into mesic mountain grasslands in the Iberian peninsula: Effects of plant quality and temperature on soil C and N stocks. Glob. Biogeochem. Cycles 2007, 21, 4016. [Google Scholar] [CrossRef]
- Panagos, P.; Van Liedekerke, M.; Jones, A.; Montanarella, L. European Soil Data Centre: Response to European policy support and public data requirements. Land Use Policy 2012, 29, 329–338. [Google Scholar] [CrossRef]
- Renwick, A.; Jansson, T.; Verburg, P.H.; Revoredo-Giha, C.; Britz, W.; Gochte, A.; McCracken, D. Policy reform and agricultural land abandonment in the EU. Land Use Policy 2013, 30, 446–457. [Google Scholar] [CrossRef]
- Navarro, L.M.; Pereira, H.M. Rewilding Abandoned Landscapes in Europe 2012. Ecosystems 2012, 15, 900–912. [Google Scholar] [CrossRef]
- Laiolo, F.; Dondero, F.; Ciliento, E.; Rolando, A. Consequences of pastoral abandonment for the structure and diversity of the alpine avifauna. J. Appl. Ecol. 2004, 41, 294–304. [Google Scholar] [CrossRef]
- Cocca, G.; Sturaro, E.; Gallo, L.; Ramanzin, M. Is the abandonment of traditional livestock farming systems the main driver of mountain landscape change in Alpine areas? Land Use Policy 2012, 29, 878–886. [Google Scholar] [CrossRef]
- Fino, E. Analisi Degli Stock Di Carbonio Organico Nel Suolo Di Ambienti Agropastorali Alpini Soggetti a Cambiamenti Di Uso E Copertura Del Suolo Nell’ambito Della Politica Di Sviluppo Rurale Del Veneto. Ph.D. Thesis, University of Tuscia, Viterbo, Italy, 13 July 2015; p. 166. [Google Scholar]
- Veneto Region. Deliberazione Della Giunta Regionale N. 178 Del 21 Febbraio 2017 Programma Di Sviluppo Rurale Per Il Veneto 2014–2020; Veneto Region: Veneto, Italy, 2017; Available online: https://bur.regione.veneto.it/BurvServices/pubblica/DettaglioDgr.aspx?id=340551 (accessed on 14 August 2020).
- Shackelford, G.E.; Kelsey, R.; Sutherland, W.J.; Kennedy, C.M.; Wood, S.A.; Gennet, S.; Karp, D.S.; Kremen, C.; Seavy, N.E.; Jedlicka, J.A.; et al. Evidence Synthesis as the Basis for Decision Analysis: A Method of Selecting the Best Agricultural Practices for Multiple Ecosystem Services. Front. Sustain. Food Syst. 2019, 3, 83. [Google Scholar] [CrossRef]
- European Commission. Decision No 529/2013/eu of the European Parliament and of the council of 21 May 2013 on Accounting Rules on Greenhouse Gas Emissions and Removals Resulting from Activities Relating to Land Use, Landuse Change and Forestry and on Information Concerning Actions Relating to Those Activities; European Commission: Brussels, Belgium, 2013; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32013D0529 (accessed on 14 August 2020).
- Savaresi, A.; Perugini, L. The Land Sector in the 2030 EU Climate Change Policy Framework: A Look at the Future. J. Eur. Environ. Plan. Law 2019, 16, 148–164. [Google Scholar] [CrossRef]
- European Environmental Agency. Annual European Union Greenhouse Gas Inventory 1990–2018 and Inventory Report 2020; Submission to the UNFCCC Secretariat: Copenhagen, Denmark, 27 May 2020; Available online: https://www.eea.europa.eu/publications/european-union-greenhouse-gas-inventory-2020 (accessed on 14 August 2020).
- National Inventory Report (NIR). Italian Greenhouse Gas Inventory 1990–2017; Institute for Environmental Protection and Research (ISPRA): Rome, Italy, 2019; p. 568. [Google Scholar]
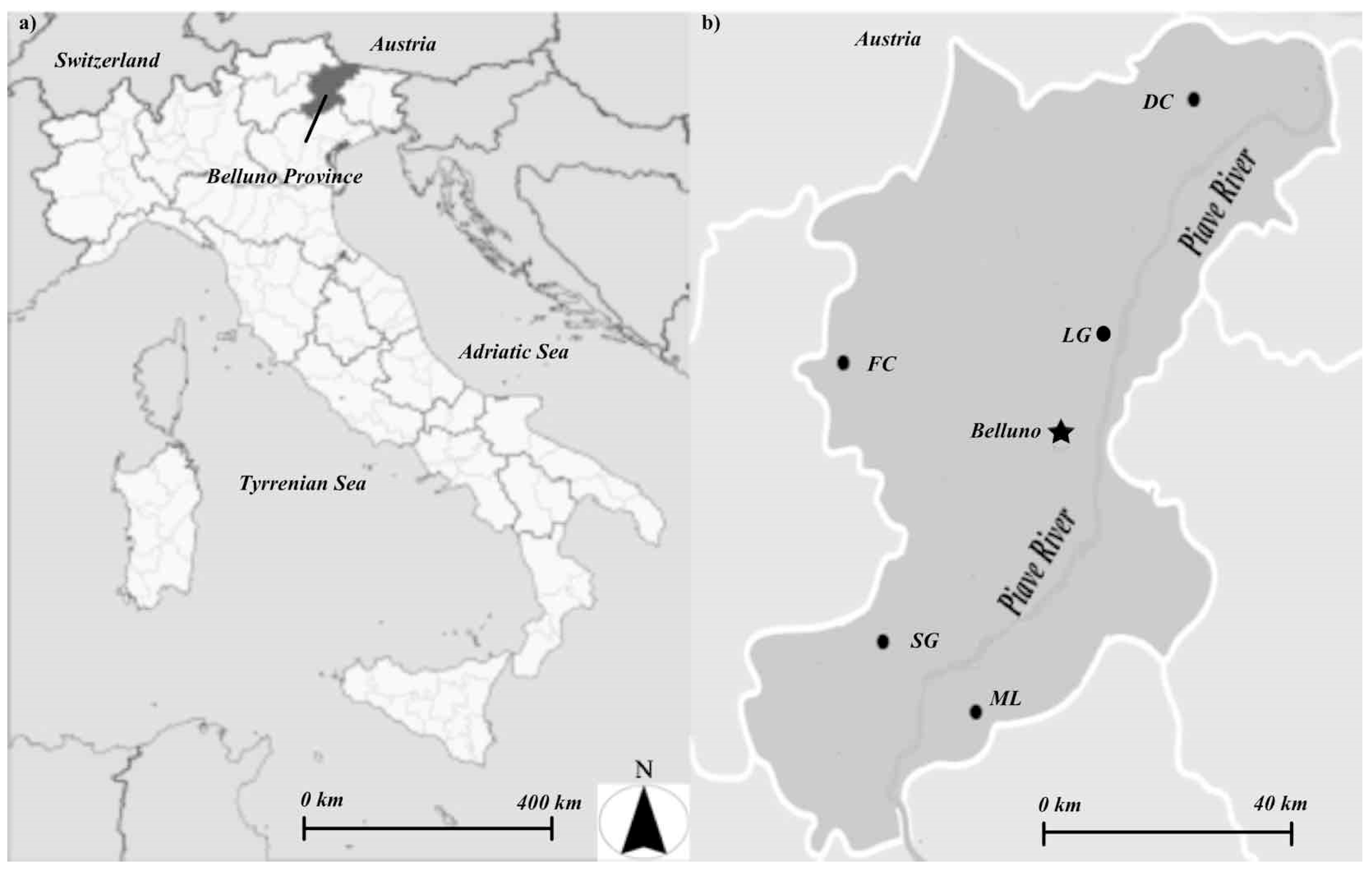

| Site | Elevation | MAT ∞ | MAP § | Latitude † | Longitude † | Exposition Ω | Slope | Soil Type * |
|---|---|---|---|---|---|---|---|---|
| m a.s.l. | °C | mm | N | E | % | |||
| DC | 1483 | 3.5 | 1043 | 46.5683 | 12.5122 | SE | 16 | Cambisol |
| FC | 1289 | 1.5 | 1228 | 46.3577 | 11.8736 | S | 40 | Phaeozem |
| LG | 1278 | 6.1 | 1337 | 46.2724 | 12.3006 | SE | 15 | Phaeozem |
| ML | 1288 | 6.5 | 1521 | 46.0618 | 12.0789 | NW | 10 | Phaeozem |
| SG | 1224 | 8.3 | 1559 | 46.0864 | 12.0442 | SE | 50 | Phaeozem |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fino, E.; Blasi, E.; Perugini, L.; Pellis, G.; Valentini, R.; Chiti, T. Is Soil Contributing to Climate Change Mitigation during Woody Encroachment? A Case Study on the Italian Alps. Forests 2020, 11, 887. https://doi.org/10.3390/f11080887
Fino E, Blasi E, Perugini L, Pellis G, Valentini R, Chiti T. Is Soil Contributing to Climate Change Mitigation during Woody Encroachment? A Case Study on the Italian Alps. Forests. 2020; 11(8):887. https://doi.org/10.3390/f11080887
Chicago/Turabian StyleFino, Ernesto, Emanuele Blasi, Lucia Perugini, Guido Pellis, Riccardo Valentini, and Tommaso Chiti. 2020. "Is Soil Contributing to Climate Change Mitigation during Woody Encroachment? A Case Study on the Italian Alps" Forests 11, no. 8: 887. https://doi.org/10.3390/f11080887
APA StyleFino, E., Blasi, E., Perugini, L., Pellis, G., Valentini, R., & Chiti, T. (2020). Is Soil Contributing to Climate Change Mitigation during Woody Encroachment? A Case Study on the Italian Alps. Forests, 11(8), 887. https://doi.org/10.3390/f11080887