Non-Thermal Plasma Can Be Used in Disinfection of Scots Pine (Pinus sylvestris L.) Seeds Infected with Fusarium oxysporum
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Fungal Material
2.2. Seed Inoculation
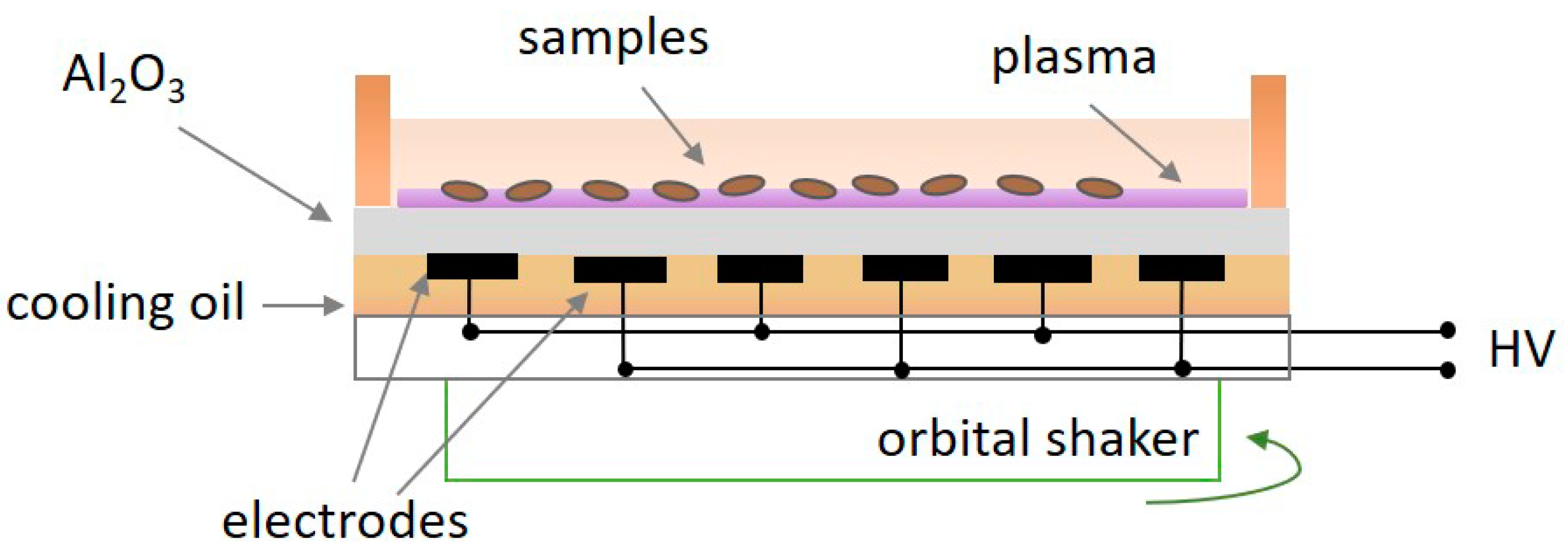
2.3. Description of Diffuse Coplanar Surface Barrier Discharge (DCSBD)
2.4. Plasma Treatment
2.5. Seed Cultivation
- disinfection efficiency (DE): percentage of seed without mold spreading around seeds,
- seed germination (SG): percentage of germinated seeds (germination is positive if a 1 mm radicle occurs),
- germination rate (GR): the percentage ratio of germinated seeds at the beginning (4th day) to germinated seeds at the end (12th day) of the seed germination,
- germination index (GI): the total number of germinated seeds to the respective day (4th, 7th, 9th, and 12th day) of germination.
2.6. Statistical Analysis
2.7. Morphological Observation
3. Results
3.1. Effect of Plasma Treatment on Seed Disinfection and Germination
3.2. Morphological Observation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harrington, C.A. G77-380 Growing Conifers from Seed. In Historical Materials from University of Nebraska-Lincoln Extension; University of Nebraska-Lincoln: Lincoln, NE, USA, 1977; p. 851. Available online: http://digitalcommons.unl.edu/extensionhist/851 (accessed on 11 February 2020).
- Mancini, V.; Romanazzi, G. Seed treatments to control seedborne fungal pathogens of vegetable crops. Pest Manag. Sci. 2014, 70, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, Q.; Xu, B.; Liu, J. Identification of the fungal pathogens of postharvest disease on peach fruits and the control mechanisms of Bacillus subtilis JK-14. Toxins 2019, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Tech. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.J.; Masur, K.; et al. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2019, 400, 39–62. [Google Scholar] [CrossRef]
- Kordas, L.; Pusz, W.; Czapka, T.; Kacprzyk, R. The effect of low-temperature plasma on fungus colonization of winter wheat grain and seed quality. Pol. J. Environ. Stud. 2015, 24, 433–438. [Google Scholar]
- Dubinov, A.E.; Kozhayeva, J.P.; Zuimatch, E.A. Changing germination rate of brown mustard seeds after treatment with plasmas of nanosecond electric discharges. IEEE Trans. Plasma Sci. 2017, 45, 294–300. [Google Scholar] [CrossRef]
- Dubinov, A.E.; Kozhayeva, J.P.; Zuimatch, E.A. Scarification of altaic flax seeds with high-power UV radiation generated by plasma of nanosecond electric discharges. IEEE Trans. Plasma Sci. 2019, 47, 69–75. [Google Scholar] [CrossRef]
- Puac, N.; Gherardi, M.; Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Process Polym. 2018, 15, e1700174. [Google Scholar] [CrossRef]
- Puligundla, P.; Kim, J.W.; Mok, C. Effect of corona discharge plasma jet treatment on decontamination and sprouting of rape seed (Brassica napus L.) seeds. Food Control 2017, 71, 376–382. [Google Scholar] [CrossRef]
- Šerá, B.; Šerý, M.; Gavril, B.; Gajdova, I. Seed germination and early growth responses to seed pre-treatment by non-thermal plasma in hemp cultivars (Cannabis sativa L.). Plasma Chem. Plasma Process. 2017, 37, 207–221. [Google Scholar] [CrossRef]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tueková, Z.; Medvecká, V.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of cold atmospheric pressure plasma on maize seeds: Enhancement of seedlings growth and surface microorganisms inactivation. Plasma Chem. Plasma Process. 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Zhang, J.J.; Jo, J.O.; Huynh, D.L.; Mongre, R.K.; Ghosh, M.; Singh, A.K.; Lee, S.B.; Mok, Y.S.; Hyuk, P.; Jeong, D.K. Growth-inducing effects of argon plasma on soybean sprouts via the regulation of demethylation levels of energy metabolism-related genes. Sci. Rep. 2017, 7, 41917. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Li, Y.F.; Klämpfl, T.G.; Shimizu, T.; Jeon, J.; Morfill, G.E.; Zimmermann, J.L. Inactivation of surface-borne microorganisms and increased germination of seed specimen by cold atmospheric plasma. Food Bioprocess Technol. 2014, 7, 645–653. [Google Scholar] [CrossRef]
- Šerá, B.; Zahoranová, A.; Bujdáková, H.; Šerý, M. Disinfection from pine seeds contaminated with Fusarium circinatum Nirenberg & O’Donnell using non-thermal plasma treatment. Rom. Rep. Phys. 2019, 71, 70. [Google Scholar]
- Gavrilova, O.I.; Pitukhin, A.V.; Zhuravleva, M.V.; Gostev, K.V.; Gostev, V.A. Influence of cold plasma spray on germinating ability of seeds and growth of softwood seedlings. In Proceedings of the International Multidisciplinary Scientific GeoConference-SGEM, 16th International Multidisciplinary Scientific Geoconference (SGEM 2016), Albena, Bulgaria, 30 June 2016; pp. 547–554. [Google Scholar]
- Nelson, S.O.; Krugman, S.L.; Stetson, L.E.; Belcher, E.W.; Works, D.W.; Stone, R.B.; Pettibone, C.A.; Goodenough, J.L. Germination responses of pine seed to radiofrequency, infrared, and gas-plasma-radiation treatments. For. Sci. 1980, 26, 377–388. [Google Scholar] [CrossRef]
- Pauzaite, G.; Malakauskiene, A.; Nauciene, Z.; Zukiene, R.; Filatova, I.; Lyushkevich, V.; Azarko, I.; Mildaziene, V. Changes in Norway spruce germination and growth induced by pre-sowing seed treatment with cold plasma and electromagnetic field: Short-term versus long-term effects. Plasma Process Polym. 2018, 15, e1700068. [Google Scholar] [CrossRef]
- Chao, L.; Walker, D.R. Effect of a magnetic field on the germination of apple, apricot and peach seeds. HortScience 1967, 2, 152–153. [Google Scholar]
- Mildaziene, V.; Pauzaite, G.; Malakauskiene, A.; Zukiene, R.; Nauciene, Z.; Filatova, I.; Azharonok, V.; Lyushkevich, V. Response of perennial woody plants to seed treatment by electromagnetic field and low-temperature plasma. Bioelectromagnetics 2016, 37, 536–548. [Google Scholar] [CrossRef]
- Zivkovic, S.; Puac, N.; Giba, Z.; Grubisic, D.; Petrovic, Z.L. The stimulatory effect of non-equilibrium (low temperature) air plasma pretreatment on light-induced germination of Paulownia tomentosa seeds. Seed Sci. Technol. 2004, 32, 693–701. [Google Scholar] [CrossRef]
- Černák, M.; Černáková, L.I.; Hudec, I.D.; Kováčik, D.A.; Zahoranová, A. Diffuse Coplanar Surface Barrier Discharge and its applications for in-line processing of low-added-value materials. Eur. Phys. J. Appl. Phys. 2009, 47, 22806. [Google Scholar] [CrossRef]
- Stepczyńska, M. Surface modification by low temperature plasma: Sterilization of biodegradable materials. Plasma Process Polym. 2016, 13, 1078–1086. [Google Scholar] [CrossRef]
- Zahoranova, A.; Henselova, M.; Hudecova, D.; Kalinakova, B.; Kovacik, D.; Medvecka, V.; Cernak, M. Effect of cold atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seeds surface. Plasma Chem. Plasma Process. 2016, 36, 397–414. [Google Scholar] [CrossRef]
- Šerý, M.; Zahoranová, A.; Kerdík, A.; Šerá, B. Seed germination of Black pine (Pinus nigra Arnold) after Diffuse Coplanar Surface Barrier Discharge plasma treatment. IEEE Trans. Plasma Sci. 2020, 48, 939–945. [Google Scholar] [CrossRef]
- Machón, P.; Pajares, J.A.; Diez, J.J.; Alves-Santos, F.M. Influence of the ectomycorrhizal fungus Laccaria laccata on pre-emergence, post-emergence and late damping-off by Fusarium oxysporum and F. verticillioides on Stone pine seedlings. Symbiosis 2009, 49, 101–109. [Google Scholar] [CrossRef]
- Martín-Pinto, P.; Pajares, J.; Díez, J. Pathogenicity of Fusarium verticillioides and Fusarium oxysporum on Pinus nigra seedlings in northwest Spain. For. Pathol. 2008, 38, 78–82. [Google Scholar] [CrossRef]
- Anderson, I.C.; Campbell, C.D.; Prosser, J.I. Diversity of fungi in organic soils under a moorland—Scots pine (Pinus sylvestris L.) gradient. Environ. Microbiol. 2003, 5, 1121–1132. [Google Scholar] [CrossRef]
- Redlak, K.; Dahm, H. The effect of ectendomycorrhizal fungi and diazotrophic bacteria on pine seedlings (Pinus sylvestris) in vitro. Sylwan 2001, 3, 81–92. [Google Scholar]
- Evira-Recuenco, M.; Iturritxa, E.; Raposo, R. Impact of seed transmission on the infection and development of Pitch Canker disease in Pinus radiata. Forests 2015, 6, 3353–3368. [Google Scholar] [CrossRef]
- Černák, M.; Rahel, J.; Kovacik, D.; Simor, M.; Brablec, A.; Slavicek, P. Generation of thin surface plasma layers for atmospheric-pressure surface treatments. Contrib. Plasma Phys. 2004, 44, 492–495. [Google Scholar] [CrossRef]
- Kancelista, A.; Piegza, M.; Stolaś, J.; Witkowska, D. Wpływ grzybów rodzaju Trichoderma na wzrost patogennych grzybów strzępkowych w testach biotycznych na nietypowych źródłach węgla. Acta Sci. Pol. Biotechnologia 2009, 8, 3–14. [Google Scholar]
- Karmiłowicz, E. The use of herbicides to regulate weeds in forest nurseries and crops in Poland. Folia For. Pol. Ser. A For. 2019, 61, 222–229. [Google Scholar] [CrossRef][Green Version]
- Okorski, A.; Pszczółkowska, A.; Oszako, T.; Nowakowska, J.A. Current possibilities and prospects of using fungicides in forestry. For. Res. Pap. 2015, 76, 191–206. [Google Scholar] [CrossRef]
- Kumar, R.P.K.; Niharika, S.P.; Hemanth, G. Impact of fungicides on the growth and distribution of soil mycoflora in agriculture fields at Narasannapeta. IJSR 2017, 6, 2337–2347. [Google Scholar] [CrossRef]
- Seipke, R.F.; Kaltenpoth, M.; Hutchings, M.I. Streptomyces as symbionts: An emerging and widespread theme? FEMS Microbiol. Rev. 2012, 36, 862–876. [Google Scholar] [CrossRef]
- Adhikari, B.; Pangomm, K.; Veerana, M.; Mitra, S.; Park, G. Plant Disease Control by Non-Thermal Atmospheric-Pressure Plasma. Front. Plant Sci. 2020, 14. [Google Scholar] [CrossRef]
- Laskowska, M.; Bogusławska-Wąs, E.; Kowal, P.; Hołub, M.; Dąbrowski, W. Skuteczność wykorzystania niskotemperaturowej plazmy w mikrobiologii i medycynie. Post. Mikrobiol. 2016, 55, 172–181. [Google Scholar]
- Terrier, O.; Essere, B.; Yver, M.; Barthélémy, M.; Bouscambert-Duchamp, M.; Kurtz, P.; VanMechelen, D.; Morfin, F.; Billaud, G.; Ferraris, O.; et al. Cold oxygen plasma technology efficiency against different airborne respiratory viruses. J. Clin. Virol. 2009, 45, 119–124. [Google Scholar] [CrossRef]
- Klämpfl, T.G.; Isbary, G.; Shimizu, T.; Li, Y.F.; Zimmermann, J.L.; Stolz, W.; Schlegel, J.; Morfill, G.E.; Schmidt, H.U. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl. Environ. Microbiol. 2012, 78, 5077–5082. [Google Scholar] [CrossRef]
- Ryu, Y.H.; Kim, Y.H.; Lee, J.Y.; Shim, G.B.; Uhm, H.S.; Park, G.; Choi, E.H. Effects of background fluid on the efficiency of inactivating yeast with non-thermal atmospheric pressure plasma. PLoS ONE 2013, 8, e66231. [Google Scholar] [CrossRef]
- Tyczkowska-Sieroń, E.; Markiewicz, J. Inaktywacja grzybów z rodzaju Candida przy użyciu zimnej plazmy atmosferycznej-na drodze ku nowej metodzie eradykacji grzybic powierzchniowych. Med. Dośw. Mikrobiol. 2014, 66, 121–129. [Google Scholar]
- Pignata, C.; D’Angelo, D.; Fea, E.; Gilli, G. A review on microbiological decontamination of fresh produce with nonthermal plasma. J. Appl. Microbiol. 2017, 122, 1438–1455. [Google Scholar] [CrossRef] [PubMed]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and sterilization using plasma technology: Fundamentals and future perspectives for biological applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef] [PubMed]
- Selcuk, M.; Oksuz, L.; Basaran, P. Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresour. Technol. 2008, 99, 5104–5109. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.H.; Pengkit, A.; Choi, K.; Jeon, S.S.; Choi, H.W.; Shin, D.B.; Choi, E.H.; Uhm, H.S.; Park, G. Differential inactivation of fungal spores in water and on seeds by ozone and arc discharge plasma. PLoS ONE 2015, 10, e0139263. [Google Scholar] [CrossRef]
- Ochi, A.; Konishi, H.; Ando, S.; Sato, K.; Yokoyama, K.; Tsushima, S.; Yoshida, S.; Morikawa, T.; Kaneko, T.; Takahashi, H. Management of bakanae and bacterial seedling blight diseases in nurseries by irradiating rice seeds with atmospheric plasma. Plant Pathol. 2017, 66, 67–76. [Google Scholar] [CrossRef]
- Basaran, P.; Basaran-Akgul, N.; Oksuz, L. Elimination of Aspergillus parasiticus from nut surface with low pressure cold plasma (LPCP) treatment. Food Microbiol. 2008, 25, 626–632. [Google Scholar] [CrossRef]
- Jiang, J.; He, X.; Li, L.; Li, J.; Shao, H.; Xu, Q.; Ye, R.; Dong, Y. Effect of cold plasma treatment on seed germination and growth of wheat. Plasma Sci. Technol. 2014, 16, 54. [Google Scholar] [CrossRef]
- Go, S.M.; Park, M.R.; Kim, Y.S.; Choi, W.S.; Jeong, R.D. Antifungal effect of non-thermal atmospheric plasma and its application for control of postharvest Fusarium oxysporum decay of paprika. Food Control 2019, 98, 245–252. [Google Scholar] [CrossRef]
- Avramidis, G.; Stuwe, B.; Wascher, R.; Bellmann, M.; Wieneke, S.; von Tiedemann, A. Fungicidal effects of an atmospheric pressure gas discharge and degradation mechanisms. Surf. Coat. Technol. 2010, 205, S405–S408. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Moreau, S.; Pelletier, J.; Tabrizian, M.; Yahia, L.H. Low-temperature sterilization using gas plasmas: A review of the experiments and an analysis of the inactivation mechanisms. Int. J. Pharm. 2001, 226, 1–21. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Jamróz, P.; Nowak, P. Sterylizacja za pomocą niskotemperaturowej plazmy, generowanej w warunkach ciśnienia osmotycznego. Post. Mikrobiol. 2015, 54, 195–200. [Google Scholar]



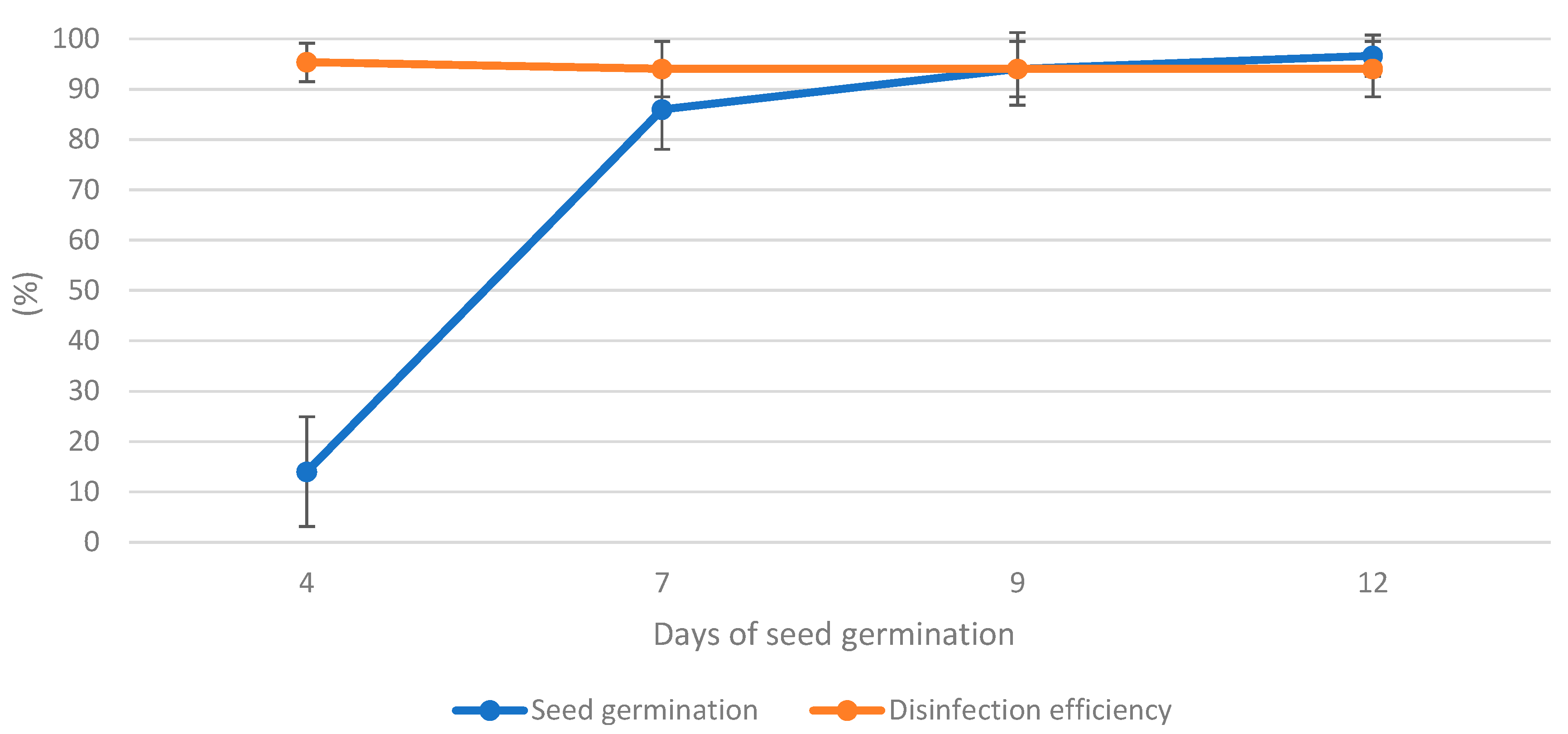
| Seed Germination (%) | Germination Rate (%) | Germination Index | Disinfection Efficiency (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | HSD | Mean | SD | HSD | Mean | SD | HSD | Mean | SD | HSD | |
| control 1 | 76.00 | 13.00 | ad | 65.78 | 12.80 | a | 5.11 | 1.31 | ae | 100.00 | 0.00 | a |
| control 2 | 100.00 | 0.00 | a | 100.00 | 0.00 | a | 10.09 | 0.34 | b | 10.67 | 6.83 | b |
| control 3 | 95.33 | 3.80 | ad | 95.78 | 2.96 | a | 11.34 | 1.54 | b | 100.00 | 0.00 | a |
| 1 s | 94.00 | 1.49 | ad | 97.14 | 5.87 | a | 9.27 | 0.25 | bc | 87.33 | 2.79 | c |
| 3 s | 96.67 | 4.08 | ad | 97.14 | 3.92 | a | 10.29 | 1.38 | bd | 94.00 | 5.48 | ac |
| 5 s | 90.67 | 2.79 | ad | 91.05 | 6.36 | a | 7.52 | 0.82 | acd | 96.67 | 4.08 | a |
| 10 s | 90.67 | 4.35 | ad | 97.69 | 3.44 | a | 8.95 | 0.55 | bcd | 88.00 | 3.80 | c |
| 15 s | 70.00 | 29.91 | bd | 70.91 | 15.58 | a | 4.60 | 1.98 | e | 97.33 | 2.79 | a |
| 20 s | 43.33 | 30.09 | b | 73.15 | 20.64 | a | 2.40 | 1.70 | f | 98.67 | 1.83 | a |
| 30 s | 6.00 | 4.94 | c | 30.00 | 44.72 | b | 0.30 | 0.27 | f | 100.00 | 0.00 | a |
| 60 s | 2.00 | 1.83 | c | 0.00 | 0.00 | b | 0.05 | 0.05 | f | 99.33 | 1.49 | a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świecimska, M.; Tulik, M.; Šerá, B.; Golińska, P.; Tomeková, J.; Medvecká, V.; Bujdáková, H.; Oszako, T.; Zahoranová, A.; Šerý, M. Non-Thermal Plasma Can Be Used in Disinfection of Scots Pine (Pinus sylvestris L.) Seeds Infected with Fusarium oxysporum. Forests 2020, 11, 837. https://doi.org/10.3390/f11080837
Świecimska M, Tulik M, Šerá B, Golińska P, Tomeková J, Medvecká V, Bujdáková H, Oszako T, Zahoranová A, Šerý M. Non-Thermal Plasma Can Be Used in Disinfection of Scots Pine (Pinus sylvestris L.) Seeds Infected with Fusarium oxysporum. Forests. 2020; 11(8):837. https://doi.org/10.3390/f11080837
Chicago/Turabian StyleŚwiecimska, Magdalena, Mirela Tulik, Božena Šerá, Patrycja Golińska, Juliána Tomeková, Veronika Medvecká, Helena Bujdáková, Tomasz Oszako, Anna Zahoranová, and Michal Šerý. 2020. "Non-Thermal Plasma Can Be Used in Disinfection of Scots Pine (Pinus sylvestris L.) Seeds Infected with Fusarium oxysporum" Forests 11, no. 8: 837. https://doi.org/10.3390/f11080837
APA StyleŚwiecimska, M., Tulik, M., Šerá, B., Golińska, P., Tomeková, J., Medvecká, V., Bujdáková, H., Oszako, T., Zahoranová, A., & Šerý, M. (2020). Non-Thermal Plasma Can Be Used in Disinfection of Scots Pine (Pinus sylvestris L.) Seeds Infected with Fusarium oxysporum. Forests, 11(8), 837. https://doi.org/10.3390/f11080837









