The Role of Population and Half-Sib Family on Driving Suitable Functional Traits for Quercus suber L. Forest Restoration
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design and Measurements
2.3. Data Analyses
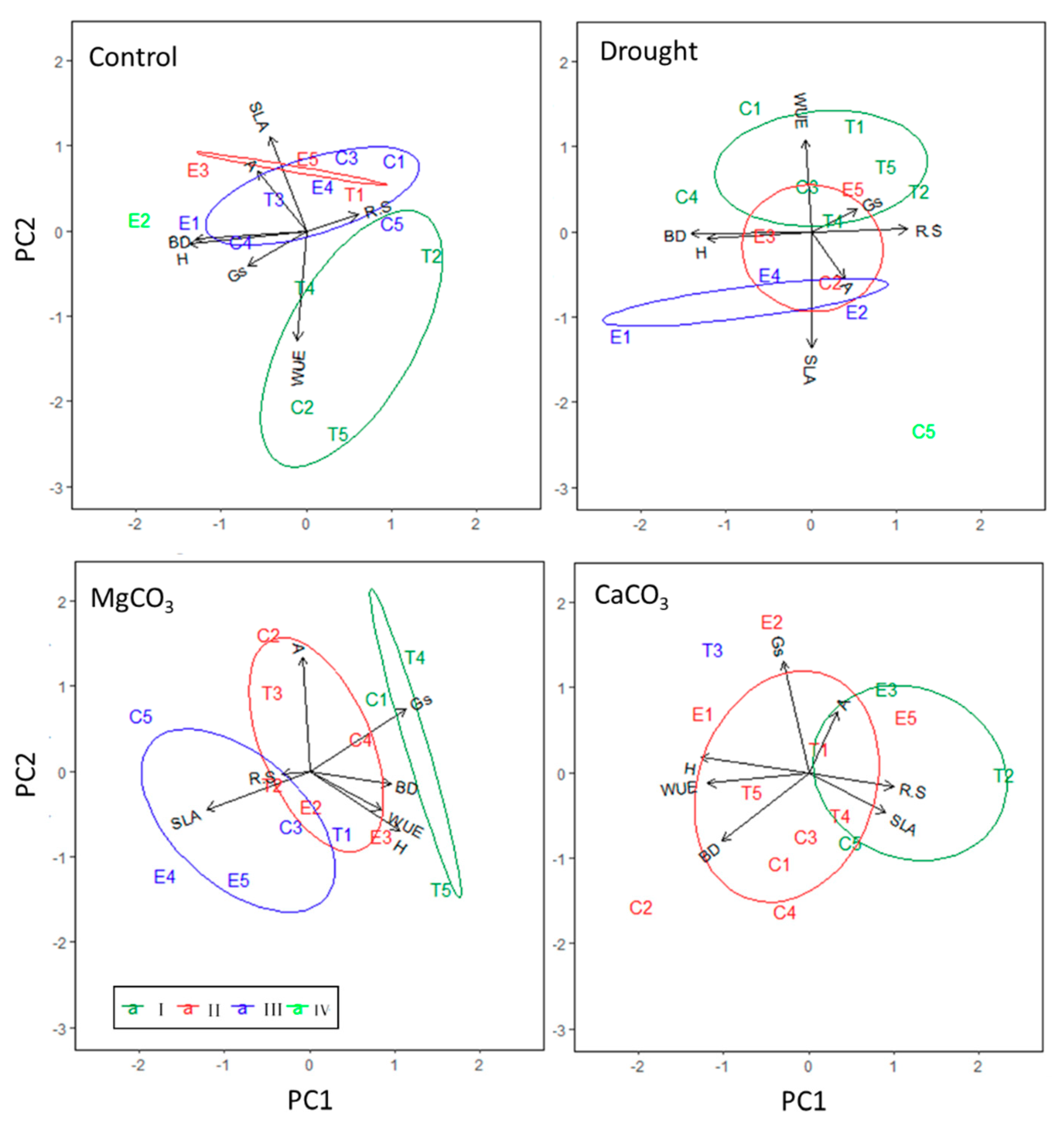
3. Results
3.1. Seedling Response to Drought and Soil Chemistry at the Population Level
3.2. Seedling Response to Drought and Soil Chemistry at the Half-Sib Family Level
4. Discussion
4.1. The Role of Population in Cork Oak Seedling Performance
4.2. The Role of the Half-Sib Family in the Cork Oak Seedling Response to Drought and Soil Chemistry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Reynolds, J.F.; Smith, D.M.S.; Lambin, E.F.; Turner, B.L.; Mortimore, M.; Batterbury, S.P.; Downing, T.E.; Dowlatabadi, H.; Fernández, R.J.; Herrick, J.E.; et al. Global desertification: Building a science for dryland development. Science 2007, 316, 847–851. [Google Scholar] [CrossRef]
- Peñuelas, J.; Lloret, F.; Montoya, R. Severe drought effects on Mediterranean woody flora in Spain. For. Sci. 2001, 47, 214–218. [Google Scholar] [CrossRef]
- Nardini, A.; Ghirardelli, L.; Salleo, S. Vulnerability to freeze stress of seedlings of Quercus ilex L.: An ecological interpretation. Ann. For. Sci. 1998, 55, 553–565. [Google Scholar] [CrossRef]
- Olarieta, J.R.; Rodríguez-Ochoa, R.; Ascaso, E. Soil gypsum and increased penetration resistance restrict early growth of Quercus ilex plantations. Arid Land Res. Manag. 2012, 26, 250–260. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Ibáñez, B.; Serrano, M.S.; De Vita, P.; Ávila, J.M.; Pérez-Ramos, I.M.; García, L.V.; Sánchez, M.E.; Marañón, T. Spatial patterns of soil pathogens in declining Mediterranean forests: Implications for tree species regeneration. New Phytol. 2012, 194, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Morcillo, L.; Gallego, D.; González, E.; Vilagrosa, A. Forest decline triggered by phloem parasitism-related biotic factors in Aleppo pine (Pinus halepensis). Forests 2019, 10, 608. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Quiring, S.M.; Peña-Gallardo, M.; Yuan, S.; Domínguez-Castro, F. A review of environmental droughts: Increased risk under global warming? Earth-Sci. Rev. 2020, 201, 102953. [Google Scholar] [CrossRef]
- Correia, R.A.; Bugalho, M.N.; Franco, A.M.A.; Palmeirim, J.M. Contribution of spatially explicit models to climate change adaptation and mitigation plans for a priority forest habitat. Mitig. Adapt. Strat. Glob. Chang. 2018, 23, 371–386. [Google Scholar] [CrossRef]
- López-Tirado, J.; Vessella, F.; Schirone, B.; Hidalgo, P.J. Trends in evergreen oak suitability from assembled species distribution models: Assessing climate change in south-western Europe. New For. 2018, 49, 471–487. [Google Scholar] [CrossRef]
- Acácio, V.; Dias, F.S.; Catry, F.X.; Rocha, M.; Moreira, F. Landscape dynamics in Mediterranean oak forests under global change: Understanding the role of anthropogenic and environmental drivers across forest types. Glob. Chang. Biol. 2017, 23, 1199–1217. [Google Scholar] [CrossRef] [PubMed]
- Vessella, F.; López-Tirado, J.; Simeone, M.C.; Schirone, B.; Hidalgo, P.J. A tree species range in the face of climate change: Cork oak as a study case for the Mediterranean biome. Eur. J. For. Res. 2017, 136, 555–569. [Google Scholar] [CrossRef]
- Montero, G.; Cañellas, I. Selvicultura de los alcornocales en España. Silva Lusit. 2003, 11, 1–19. [Google Scholar]
- Olea, L.; San Miguel-Ayanz, M.A. The Spanish dehesa. A traditional Mediterranean silvopastoral system linking production and nature conservation. Grassl. Sci. Eur. 2006, 11, 3–13. [Google Scholar]
- Pausas, J.G. Resprouting of Quercus suber in NE Spain after fire. J. Veg. Sci. 1997, 8, 703–706. [Google Scholar] [CrossRef]
- Camilo-Alves, C.S.P.; Da Clara, M.I.E.; Ribeiro, N.M.D.A. Decline of Mediterranean oak trees and its association with Phytophthora cinnamomi: A review. Eur. J. For. Res. 2013, 132, 411–432. [Google Scholar] [CrossRef]
- Ibáñez, B.; Gómez-Aparicio, L.; Ávila, J.M.; Pérez-Ramos, I.M.; Marañón, T. Effects of Quercus suber decline on woody plant regeneration: Potential implications for successional dynamics in Mediterranean forests. Ecosystems 2017, 20, 630–644. [Google Scholar] [CrossRef]
- Camilo-Alves, C.S.P.; Vaz, M.; Da Clara, M.I.E.; Ribeiro, N.M.D.A. Chronic cork oak decline and water status: New insights. New For. 2017, 48, 753–772. [Google Scholar] [CrossRef]
- Sánchez, M.E.; Caetano, P.; Romero, M.A.; Navarro, R.M.; Trapero, A. Phytophthora root rot as the main factor of oak decline in southern Spain. In Progress in Research on phytophthora Diseases of Forest Trees; Brasier, C., Jung, T., Oßwald, W., Eds.; Forest Research: Farnham, UK, 2006; pp. 149–154. [Google Scholar]
- Hufford, K.M.; Mazer, S.J. Plant ecotypes: Genetic differentiation in the age of ecological restoration. Trends Ecol. Evol. 2003, 18, 147–155. [Google Scholar] [CrossRef]
- Chazdon, R.L. Beyond deforestation: Restoring forests and ecosystem services on degraded lands. Science 2008, 320, 1458–1460. [Google Scholar] [CrossRef]
- Bakkenes, M.; Alkemade, J.R.M.; Ihle, F.; Leemans, R.; Latour, J.B. Assessing effects of forecasted climate change on the diversity and distribution of European higher plants for 2050. Glob. Chang. Biol. 2002, 8, 390–407. [Google Scholar] [CrossRef]
- Hällfors, M.H.; Aikio, S.; Schulman, L.E. Quantifying the need and potential of assisted migration. Biol. Conserv. 2017, 205, 34–41. [Google Scholar] [CrossRef]
- Prober, S.M.; Byrne, M.; McLean, E.H.; Steane, D.A.; Potts, B.M.; Vaillancourt, R.E.; Stock, W.D. Climate-adjusted provenancing: A strategy for climate-resilient ecological restoration. Front. Ecol. Evol. 2005, 3, 65. [Google Scholar] [CrossRef]
- Mediavilla, S.; Escudero, A. Stomatal responses to drought at a Mediterranean site: A comparative study of co-occurring woody species differing in leaf longevity. Tree Physiol. 2003, 23, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Corcuera, L.; Morales, F.; Abadía, A.; Gil-Pelegrín, E. Seasonal changes in photosynthesis and photoprotection in a Quercus ilex subsp. ballota woodland located in its upper altitudinal extreme in the Iberian Peninsula. Tree Physiol. 2005, 25, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Serrasolses, I.; Pérez-Devesa, M.; Vilagrosa, A.; Pausas, J.G.; Sauras, T.; Cortina, J.; Vallejo, V.R. Soil Properties Constraining Cork Oak Distribution in Cork Oak Woodlands on the Edge; Aronson, J., Pereira, J.S., Pausas, J.G., Eds.; Island Press: Washington, DC, USA, 2009; pp. 89–101. [Google Scholar]
- Eriksson, G.; Namkoong, G.; Roberds, J.H. Dynamic gene conservation for uncertain futures. For. Ecol. Manag. 1993, 62, 15–37. [Google Scholar] [CrossRef]
- Krichen, K.; Vilagrosa, A.; Chaieb, M. Divergence of functional traits at early stages of development in Stipa tenacissima populations distributed along an environmental gradient of the Mediterranean. Plant Ecol. 2019, 220, 995–1008. [Google Scholar] [CrossRef]
- Leimu, R.; Fischer, M. A meta-analysis of local adaptation. Plants. PLoS ONE 2008, 3, e4010. [Google Scholar] [CrossRef]
- Lampei-Bucharová, A.; Michalski, S.G.; Hermann, J.M.; Heveling, K.; Durka, W.; Hölzel, N.; Kollmann, J.; Bossdorf, O. Genetic differentiation and regional adaptation among seed origins used for grassland restoration: Lessons from a multi-species transplant experiment. J. Appl. Ecol. 2017, 54, 127136. [Google Scholar] [CrossRef]
- Bischoff, A.; Steinger, T.; Müller-Schärer, H. The importance of plant provenance and genotypic diversity of seed material used for ecological restoration. Restor. Ecol. 2008, 18, 338–348. [Google Scholar] [CrossRef]
- Pastor, E.; Soliveres, S.; Vilagrosa, A.; Bonet, A. Intraspecific leaf shape at local scale determines offspring characteristics. J. Arid Environ. 2018, 153, 18–23. [Google Scholar] [CrossRef]
- Lumaret, R.; Jabbour-Zahab, R. Ancient and current gene flow between two distantly related Mediterranean oak species, Quercus suber and Q. ilex. Ann. Bot. 2009, 104, 725–736. [Google Scholar] [CrossRef]
- Cronn, R.; Wendel, J. Cryptic trysts, genome mergers, and plant speciation. New Phytol. 2004, 161, 133–142. [Google Scholar] [CrossRef]
- Petit, R.J.; Bodénès, C.; Ducousso, A.; Roussel, G.; Kremer, A. Hybridization as a mechanism of invasion in oaks. New Phytol. 2004, 161, 151–164. [Google Scholar] [CrossRef]
- Mallet, J. Hybrid speciation. Nature 2007, 446, 279–283. [Google Scholar] [CrossRef]
- López de Heredia, U.; Carrion, J.S.; Jimenez, P.; Collada, C.; Gil, L. Molecular and palaeoecological evidence for multiple glacial refugia for evergreen oaks on the Iberian Peninsula. J. Biogr. 2007, 34, 1505–1517. [Google Scholar] [CrossRef]
- Soto, A.; López de Heredia, U.; Robledo-Arnuncio, J.J.; Gil, L. Simulation analysis of Quercus Ilex—Quercus Suber chloroplast introgression. In Suberwood: New Challenges for the Integration of Cork Oak Forests and Products; Vázquez-Piqué, J., Pereira, H., González Pérez, M.M., Eds.; Universidad de Huelva: Madrid, Spain, 2008; pp. 91–97. [Google Scholar]
- Belahbib, N.; Pemonge, M.H.; Ouassou, A.; Sbay, H.; Kremer, A.; Petit, R.J. Frequent cytoplasmic exchanges between oak species that are not closely related: Quercus suber and Q. ilex in Morocco. Mol. Ecol. 2001, 10, 2003–2012. [Google Scholar] [CrossRef]
- Magri, D.; Fineschi, S.; Bellarosa, R.; Buonamici, A.; Sebastiani, F.; Schirone, B.; Simeone, M.C.; Vendramin, G.G. The distribution of Quercus suber chloroplast haplotypes matches the palaeogeographical history of the western Mediterranean. Mol. Ecol. 2007, 16, 5259–5266. [Google Scholar] [CrossRef]
- Mir, C.; Jarne, P.; Sarda, V.; Bonin, A.; Lumaret, R. Contrasting nuclear and cytoplasmic exchanges between phylogenetically distant oak species (Quercus suber L. and Q. ilex L.) in Southern France: Inferring crosses and dynamics. Plant Biol. 2009, 11, 213–226. [Google Scholar] [CrossRef]
- Jiménez, P.; Agúndez, D.; Alía, R.; Gil, L. Genetic variation in central and marginal populations of Quercus suber L. Silvae Genet. 1999, 48, 278–284. [Google Scholar]
- De Martonne, E. Géographie Physique (Physical Geography), 3rd ed.; Armand Colin: Paris, France, 1920. [Google Scholar]
- González-Martinez, S.C.; Burczyk, J.; Nathan, R.; Nanos, N.; Gil, L.; Alía, R. Effective gene dispersal and female reproductive success in Mediterranean maritime pine (Pinus pinaster Aiton). Mol. Ecol. 2006, 15, 4577–4588. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 29 February 2020).
- Suzuki, R.; Hidetoshi, S. Pvclust: Hierarchical Clustering with P-Values via Multiscale Bootstrap Resampling. 2019. Available online: https://CRAN.R-project.org/package=pvclust (accessed on 3 April 2020).
- Osnas, J.L.; Lichstein, J.W.; Reich, P.B.; Pacala, S.W. Global leaf trait relationships: Mass, area, and the leaf economics spectrum. Science 2013, 340, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Bongarten, B.C.; Hanover, J.W. Provenance variation in blue spruce (Picea pungens) at eight locations in the northern Unites States and Canada. Silvae Genet. 1986, 35, 2–3. [Google Scholar]
- Strandby, U.; Prado, J.P.; Bräuner, U.; Kollman, J. Provenance variation in germination growth of Abies guatemalensis Reher. For. Ecol. Manag. 2008, 255, 1831–1840. [Google Scholar] [CrossRef]
- Gratani, L.; Meneghini, M.; Pesoli, P.; Crescente, M.F. Structural and functional plasticity of Quercus ilex seedlings of different provenances in Italy. Trees Struct. Funct. 2003, 17, 515–521. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sancho-Knapik, D.; Barrón, E.; Camarero, J.J.; Vilagrosa, A.; Gil-Pelegrín, E. Morphological and physiological divergences within Quercus ilex support the existence of different ecotypes depending on climatic dryness. Ann. Bot. 2014, 114, 301–313. [Google Scholar] [CrossRef]
- Aranda, I.; Castro, L.; Alía, R.; Pardos, J.A.; Gil, L. Low temperature during winter elicits differential responses among populations of the Mediterranean evergreen cork oak (Quercus suber). Tree Physiol. 2005, 25, 1085–1090. [Google Scholar] [CrossRef]
- Gandour, M.; Khouja, M.L.; Toumi, L.; Triki, S. Morphological evaluation of cork oak (Quercus suber): Mediterranean provenance variability in Tunisia. Ann. For. Sci. 2007, 64, 549–555. [Google Scholar] [CrossRef]
- Ramírez-Valiente, J.A.; Sánchez-Gómez, D.; Aranda, I.; Valladares, F. Phenotypic plasticity and local adaptation in leaf ecophysiological traits of 13 contrasting cork oak populations under different water availabilities. Tree Physiol. 2010, 30, 618–627. [Google Scholar] [CrossRef]
- Sampaio, T.; Branco, M.; Guichoux, E.; Petit, R.J.; Pereira, J.S.; Varela, M.C.; Almeida, M.H. Does the geography of cork oak origin influence budburst and leaf pest damage? For. Ecol. Manag. 2016, 373, 33–43. [Google Scholar] [CrossRef]
- Ramirez-Valiente, J.A.; Alia, R.; Aranda, I. Geographical variation in growth form traits in Quercus suber and its relation to population evolutionary history. Evol. Ecol. 2014, 28, 55–68. [Google Scholar] [CrossRef]
- Svenning, J.C.; Skov, F. The relative roles of environment and history as controls of tree species composition and richness in Europe. J. Biogeogr. 2005, 32, 1019–1033. [Google Scholar] [CrossRef]
- Toumi, L.; Lumaret, R. Allozyme variation in cork oak (Quercus suber L.): The role of phylogeography and genetic introgression by other Mediterranean oak species and human activities. Theor. Appl. Genet. 1998, 97, 647–656. [Google Scholar] [CrossRef]
- Staudt, M.; Mir, C.; Joffre, R.; Rambal, S.; Bonin, A.; Landais, D.; Lumaret, R. Isoprenoid emissions of Quercus spp. (Q.suber and Q.ilex) in mixed stands contrasting in interspecific genetic introgression. New Phytol. 2004, 163, 573–584. [Google Scholar] [CrossRef]
- Bellarosa, R.; Simeone, M.C.; Papini, A.; Schirone, B. Utility of ITS sequence data for phylogenetic reconstruction of Italian Quercus spp. Mol. Phylogenetics Evol. 2005, 34, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Harzé, M.; Mahy, G.; Monty, A. Functional traits are more variable at the intra-than inter-population level: A study of four calcareous dry-grassland plant species. Tuexenia 2016, 36, 321–336. [Google Scholar]
- Vastag, E.; Cocozza, C.; Orlović, S.; Kesić, L.; Kresoja, M.; Stojnić, S. Half-sib lines of pedunculate oak (Quercus robur L.) respond differently to drought through biometrical, anatomical and physiological traits. Forests 2020, 11, 153. [Google Scholar] [CrossRef]
- Ramírez-Valiente, J.A.; Valladares, F.; Huertas, A.D.; Granados, S.; Aranda, I. Factors affecting cork oak growth under dry conditions: Local adaptation and contrasting additive genetic variance within populations. Tree Genet. Genomes 2011, 7, 285–295. [Google Scholar] [CrossRef]
- Givnish, T.J. Ecology of plant speciation. Taxon 2010, 59, 1326–1366. [Google Scholar] [CrossRef]
- Zeng, Y.F.; Liao, W.J.; Petit, R.J.; Zhang, D.Y. Geographic variation in the structure of oak hybrid zones provides insights into the dynamics of speciation. Mol. Ecol. 2011, 20, 4995–5011. [Google Scholar] [CrossRef]
- Hewitt, G.M. Genetic consequences of climatic changes in the Quaternary. Philos. Trans. R. Soc. Lond. B 2004, 359, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Valiente, J.A.; Valladares, F.; Gil, L.; Aranda, I. Population differences in juvenile survival under increasing drought are mediated by seed size in cork oak (Quercus suber L.). For. Ecol. Manag. 2009, 257, 1676–1683. [Google Scholar] [CrossRef]
- Besson, C.K.; Lobo do Vale, R.; Rodrigues, M.L.; Almeida, P.; Herd, A.; Grant, O.M.; Soares-David, T.; Schmidt, M.; Otieno, D.; Keenan, T.F.; et al. Cork oak physiological responses to manipulated water availability in a Mediterranean woodland. Agric. For. Meteorol. 2014, 184, 230–242. [Google Scholar] [CrossRef]
- Ferrio, J.P.; Florit, A.; Vega, A.; Serrano, L.; Voltas, J. δ13C and tree-ring width reflect different drought responses in Quercus ilex and Pinus halepensis. Oecologia 2003, 442, 512–518. [Google Scholar] [CrossRef]
- Donovan, L.A.; Dudley, S.A.; Rosenthal, D.M.; Ludwig, F. Phenotypic selection on leaf water use efficiency and related ecophysiological traits for natural populations of desert sunflowers. Oecologia 2007, 152, 13–25. [Google Scholar] [CrossRef]
- Leakey, A.D.; Ferguson, J.N.; Pignon, C.P.; Wu, A.; Jin, Z.; Hammer, G.L.; Lobell, D.B. Water use efficiency as a constraint and target for improving the resilience and productivity of C3 and C4 crops. Annu. Rev. Plant Biol. 2019, 70, 781–808. [Google Scholar] [CrossRef]
- Grünzweig, J.M.; Carmel, Y.; Riov, J.; Sever, N.; McCreary, D.D.; Flather, C.H. Growth, resource storage, and adaptation to drought in California and eastern Mediterranean oak seedlings. Can. J. For. Res. 2008, 38, 331–342. [Google Scholar] [CrossRef][Green Version]
- Dudley, S.A. Differing selection on plant physiological traits in response to environmental water availability: A test of adaptive hypotheses. Evolution 1996, 50, 92–102. [Google Scholar] [CrossRef]
- Otieno, D.O.; Besson, C.K.; Liu, J.; Schmidt, M.W.T.; Lobo do Vale, R.; David, T.S.; Siegwolf, R.; Pereira, J.S.; Tenhunenet, J.D. Seasonal variations in soil and plant water status in a Quercus suber L. Stand: Roots as determinants of tree productivity and survival in the Mediterranean-type ecosystem. Plant Soil 2006, 283, 119–135. [Google Scholar] [CrossRef]
- Nepstad, D.C.; de Carvalho, C.R.; Davidson, E.A.; Jipp, P.H.; Lefebvre, P.A.; Negreiros, G.H.; da Silva, E.D.; Stone, T.A.; Trumbore, S.E.; Vieira, S. The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 1994, 372, 666–669. [Google Scholar] [CrossRef]
- Ryan, C.M.; Williams, M.; Grace, J. Above-and belowground carbon stocks in a miombo woodland landscape of Mozambique. Biotropica 2011, 43, 423–432. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Heredia, N.; Millard, P. Remobilization of acorn nitrogen for seedling growth in holm oak (Quercus ilex), cultivated with contrasting nutrient availability. Tree Physiol. 2010, 30, 257–263. [Google Scholar] [CrossRef]
- Collet, C.; Manso, R.; Barbeito, I. Coexistence, association and competitive ability of Quercus petraea and Quercus robur seedlings in naturally regenerated mixed stands. For. Ecol. Manag. 2017, 390, 36–46. [Google Scholar] [CrossRef]
- Padilla, F.M.; Pugnaire, F.I. Rooting depth and soil moisture control Mediterranean woody seedling survival during drought. Funct. Ecol. 2007, 21, 489–495. [Google Scholar] [CrossRef]
- Trubat, R.; Cortina, J.; Vilagrosa, A. Nutrient deprivation improves field performance of woody seedlings in a degraded semi-arid shrubland. Ecol. Eng. 2011, 37, 1164–1173. [Google Scholar] [CrossRef]
| H | BD | R:S | SLA | gs | Tr | A | WUEi | ||
|---|---|---|---|---|---|---|---|---|---|
| Toledo | Co | 13.2 ± 0.5 | 3.7 ± 0.1 | 6.5 ± 0.2 | 69.0 ± 1.4 | 0.112 ± 0.008 | 2.25 ± 0.83 | 6.06 ± 0.94 | 3.72 ± 0.52 |
| D | 12.5 ± 0.3 | 3.5 ± 0.1 | 6.2 ± 0.2 | 67.6 ± 1.1 | 0.118 ± 0.009 | 1.42 ± 0.05 | 5.92 ± 0.63 | 4.35 ± 0.38 | |
| Mg | 12.8 ± 0.4 | 3.6 ± 0.1 | 6.6 ± 0.2 | 71.5 ± 3.2 | 0.112 ± 0.003 | 0.99 ± 0.34 | 3.53 ± 0.46 | 4.87 ± 0.55 | |
| Ca | 13.0 ± 0.4 | 3.7 ± 0.1 | 7.1 ± 0.3 | 68.9 ± 2.2 | 0.140 ± 0.015 | 1.71 ± 0.60 | 4.48 ± 0.69 | 3.87 ± 0.49 | |
| Calderona | Co | 12.8 ± 0.4 | 3.8 ± 0.1 | 5.2 ± 0.6 | 73.9 ± 2.5 | 0.112 ± 0.062 | 1.55 ± 0.63 | 3.10 ± 0.75 | 3.41 ± 0.55 |
| D | 11.9 ± 0.3 | 3.9 ± 0.1 | 4.9 ± 0.2 | 72.7 ± 2.5 | 0.099 ± 0.013 | 2.77 ± 0.88 | 7.55 ± 0.88 | 3.53 ± 0.42 | |
| Mg | 12.0 ± 0.3 | 3.6 ± 0.1 | 5.7 ± 0.3 | 75.7 ± 2.4 | 0.112 ± 0.007 | 2.05 ± 0.63 | 5.71 ± 0.81 | 2.78 ± 0.29 | |
| Ca | 13.9 ± 0.4 | 4.3 ± 0.1 | 6.0 ± 0.3 | 71.9 ± 2.2 | 0.104 ± 0.005 | 0.95 ± 0.19 | 3.56 ± 0.46 | 4.27 ± 1.02 | |
| Espadà | Co | 15.5 ± 0.5 | 4.2 ± 0.1 | 5.2 ± 0.3 | 75.9 ± 2.1 | 0.118 ± 0.014 | 3.61 ± 1.02 | 8.55 ± 1.14 | 2.63 ± 0.31 |
| D | 14.1 ± 0.5 | 4.0 ± 0.1 | 5.1 ± 0.3 | 78.8 ± 2.1 | 0.098 ± 0.006 | 1.69 ± 0.30 | 6.40 ± 0.74 | 3.71 ± 0.34 | |
| Mg | 13.4 ± 0.3 | 3.6 ± 0.1 | 6.1 ± 0.3 | 77.4 ± 1.8 | 0.095 ± 0.011 | 0.82 ± 0.32 | 2.52 ± 0.40 | 3.44 ± 0.53 | |
| Ca | 14.1 ± 0.4 | 3.7 ± 0.1 | 5.9 ± 0.2 | 74.0 ± 1.6 | 0.138 ± 0.020 | 3.57 ± 0.94 | 8.98 ± 1.01 | 2.97 ± 0.26 | |
| Variable | Population (P) | Treatment (T) | P × T |
|---|---|---|---|
| H | 17.45(<0.001) | 5.97(0.001) | 2.37(0.029) |
| BD | 6.55(0.002) | 5.53(0.001) | 5.61(<0.001) |
| R:S | 25.55(<0.001) | 9.51(<0.001) | 0.53(0.789) |
| SLA | 11.15(<0.001) | 1.10(0.351) | 0.45(0.845) |
| gs | 3.74(0.025) | 3.65(0.013) | 2.03(0.062) |
| Tr | 6.99(0.001) | 6.60(<0.001) | 11.03(<0.001) |
| A | 6.00(0.003) | 6.91(<0.001) | 9.03(<0.001) |
| WUEi | 4.96(0.008) | 0.99(0.399) | 1.63(0.139) |
| Variable | Half-Sib Family (F) | Population (P) | Treatment (T) | F × T | P × T | Post Hoc Test |
|---|---|---|---|---|---|---|
| H | 3.91(0.001) | 2.37(0.135) | 2.21(0.105) | 2.88(<0.001) | 0.99(0.442) | nd nd |
| BD | 8.22(<0.001) | 0.69(0.518) | 3.09(0.040) | 1.64(0.014) | 4.25(0.003) | nd Co(a); Ca(a); D(a); Mg(b) |
| R:S | 2.39(0.022) | 6.81(0.011) | 5.42(0.004) | 1.83(0.003) | 0.17(0.983) | T(a); E(b); C(b) Ca(a); Mg(a); Co(b); D(b) |
| SLA | 1.62(0.130) | 4.78(0.034) | 0.70(0.557) | 1.60(0.022) | 0.34(0.910) | T(b); C(a); E(a) nd |
| gs | 1.92(0.066) | 1.42(0.278) | 2.65(0.064) | 1.58(0.026) | 1.45(0.224) | nd nd |
| Tr | 0.90(0.554) | 1.65(0.232) | 1.87(0.154) | 17.31(<0.001) | 2.44(0.046) | nd nd |
| A | 2.17(0.039) | 0.87(0.444) | 2.89(0.050) | 6.01(<0.001) | 3.33(0.011) | nd D(a); Co(ab); Ca(b); Mg(c) |
| WUEi | 1.96(0.062) | 0.95(0.412) | 0.33(0.801) | 6.80(<0.001) | 0.58(0.746) | nd nd |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morcillo, L.; Moutahir, H.; Cortina, J.; Vilagrosa, A. The Role of Population and Half-Sib Family on Driving Suitable Functional Traits for Quercus suber L. Forest Restoration. Forests 2020, 11, 680. https://doi.org/10.3390/f11060680
Morcillo L, Moutahir H, Cortina J, Vilagrosa A. The Role of Population and Half-Sib Family on Driving Suitable Functional Traits for Quercus suber L. Forest Restoration. Forests. 2020; 11(6):680. https://doi.org/10.3390/f11060680
Chicago/Turabian StyleMorcillo, Luna, Hassane Moutahir, Jordi Cortina, and Alberto Vilagrosa. 2020. "The Role of Population and Half-Sib Family on Driving Suitable Functional Traits for Quercus suber L. Forest Restoration" Forests 11, no. 6: 680. https://doi.org/10.3390/f11060680
APA StyleMorcillo, L., Moutahir, H., Cortina, J., & Vilagrosa, A. (2020). The Role of Population and Half-Sib Family on Driving Suitable Functional Traits for Quercus suber L. Forest Restoration. Forests, 11(6), 680. https://doi.org/10.3390/f11060680






