Methyl Salicylate and Sesquiterpene Emissions Are Indicative for Aphid Infestation on Scots Pine
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Insect Exposure
2.3. BVOC Sampling and Analysis
2.4. Statistical Analyses
3. Results
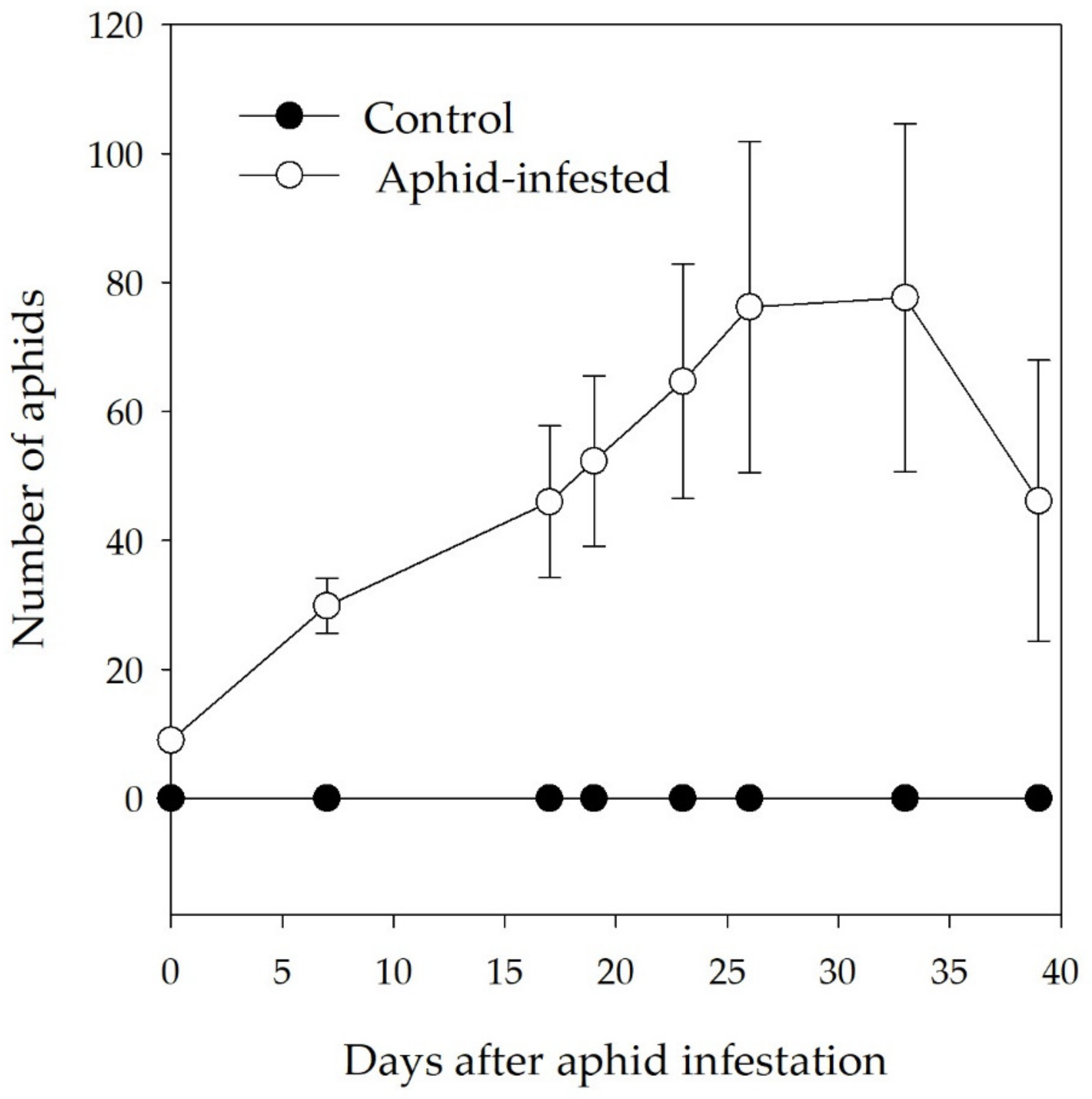
3.1. Aphid Density on Infested Branch Tips
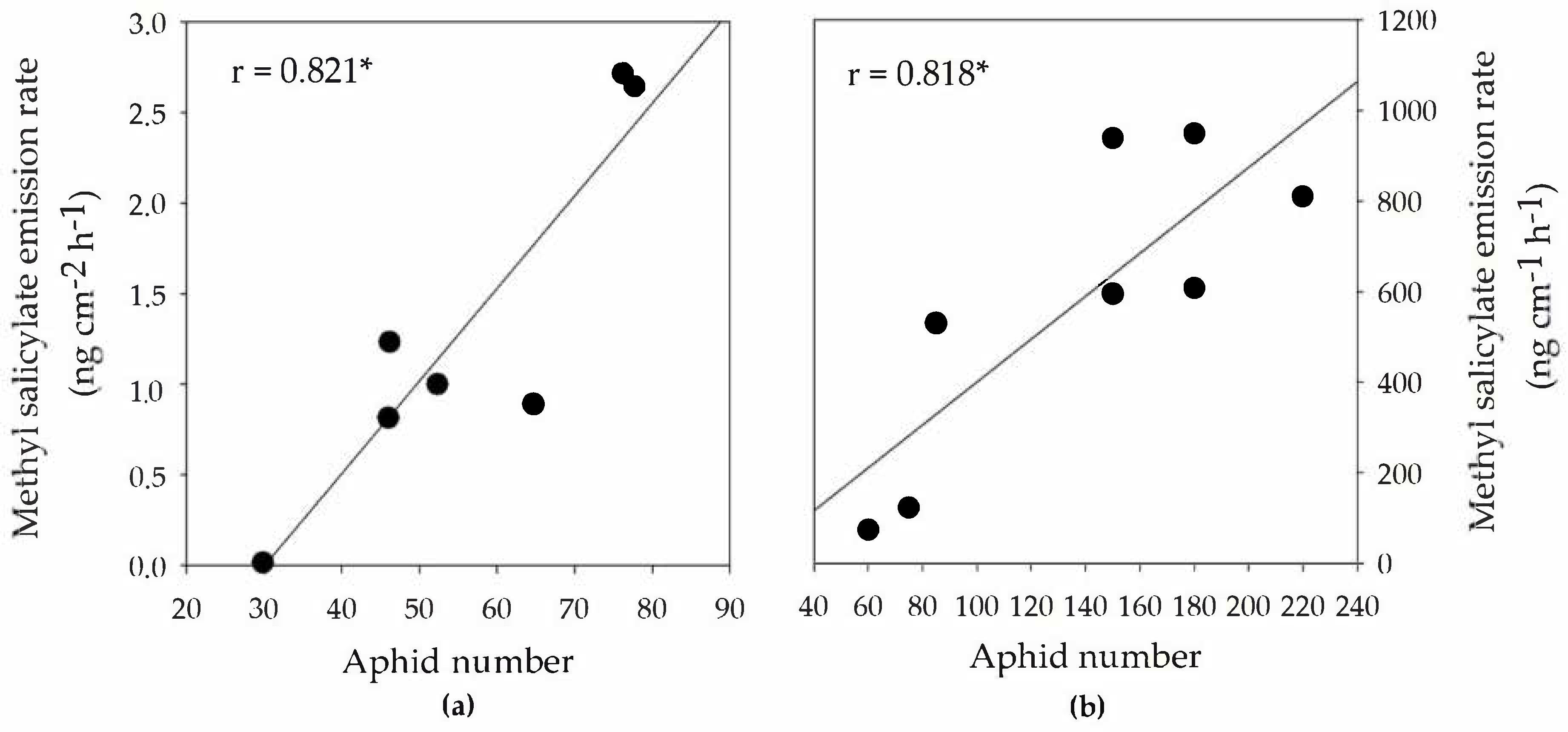
3.2. BVOC Emission Rates from Infested Branch Tips
3.3. BVOC Emissions from Branches Adjacent to Aphid-Infested Branches (Systemic Emissions)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jansen, R.M.C.; Wildt, J.; Kappers, I.F.; Bouwmeester, H.J.; Hofstee, J.W.; van Henten, E.J. Detection of diseased plants by analysis of volatile organic compound emission. Annu. Rev. Phytopathol. 2011, 49, 157–174. [Google Scholar] [CrossRef]
- Li, Z.; Paul, R.; Tis, T.B.; Saville, A.C.; Hansel, J.C.; Yu, T.; Ristaino, J.B.; Wei, Q. Non-invasive plant disease diagnostics enabled by smartphone-based fingerprinting of leaf volatiles. Nat. Plants 2019, 5, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Schnitzler, J.-P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Noe, S.M.; Hueve, K.; Niinemets, U.; Copolovici, L. Seasonal variation in vertical volatile compounds air concentrations within a remote hemiboreal mixed forest. Atmos. Chem. Phys. 2012, 12, 3909–3926. [Google Scholar] [CrossRef]
- Penuelas, J.; Sardans, J.; Filella, I.; Estiarte, M.; Llusia, J.; Ogaya, R.; Carnicer, J.; Bartrons, M.; Rivas-Ubach, A.; Grau, O.; et al. Impacts of global change on mediterranean forests and their services. Forests 2017, 8, 463. [Google Scholar] [CrossRef]
- Simpraga, M.; Ghimire, R.P.; Van Der Straeten, D.; Blande, J.D.; Kasurinen, A.; Sorvari, J.; Holopainen, T.; Adriaenssens, S.; Holopainen, J.K.; Kivimaenpaa, M. Unravelling the functions of biogenic volatiles in boreal and temperate forest ecosystems. Eur. J. For. Res. 2019, 138, 763–787. [Google Scholar] [CrossRef]
- Taipale, D.; Aalto, J.; Schiestl-Aalto, P.; Kulmala, M.; Bäck, J. Emissions of monoterpenes from new Scots pine foliage: Dependency on season, stand age and location and importance for models. Biogeosciences Discuss 2020. Available online: https://www.biogeosciences-discuss.net/bg-2019-502 (accessed on 24 April 2020). [CrossRef]
- Niinemets, U.; Kännaste, A.; Copolovici, L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front Plant Sci. 2013, 4, 262. [Google Scholar] [CrossRef]
- Joutsensaari, J.; Yli-Pirilä, P.; Korhonen, H.; Arola, A.; Blande, J.D.; Heijari, J.; Kivimäenpää, M.; Mikkonen, S.; Hao, L.; Miettinen, P.; et al. Biotic stress accelerates formation of climate-relevant aerosols in boreal forests. Atmos. Chem. Phys. 2015, 15, 12139–12157. [Google Scholar] [CrossRef]
- Faiola, C.L.; Buchholz, A.; Kari, E.; Yli-Pirilä, P.; Holopainen, J.K.; Kivimäenpää, M.; Miettinen, P.; Worsnop, D.R.; Lehtinen, K.E.J.; Guenther, A.B.; et al. Terpene composition complexity controls secondary organic aerosol yields from scots pine volatile emissions. Sci. Rep. 2018, 8, 3053. [Google Scholar] [CrossRef] [PubMed]
- Faiola, C.L.; Pullinen, I.; Buchholz, A.; Khalaj, F.; Ylisirniö, A.; Kari, E.; Miettinen, P.; Holopainen, J.K.; Kivimäenpää, M.; Schobesberger, S.; et al. Secondary organic aerosol formation from healthy and aphid-stressed scots pine emissions. ACS Earth Space Chem. 2019, 3, 1756–1772. [Google Scholar] [CrossRef] [PubMed]
- Faiola, C.; Taipale, D. Impact of insect herbivory on plant stress volatile emissions from trees: A synthesis of quantitative measurements and recommendations for future research. Atmos. Environ X 2020, 5, 100060. [Google Scholar] [CrossRef]
- Zhao, D.F.; Buchholz, A.; Tillmann, R.; Kleist, E.; Wu, C.; Rubach, F.; Kiendler-Scharr, A.; Rudich, Y.; Wildt, J.; Mentel, T.F. Environmental conditions regulate the impact of plants on cloud formation. Nat. Commun. 2017, 8, 14067. [Google Scholar] [CrossRef]
- Tholl, D.; Boland, W.; Hansel, A.; Loreto, F.; Röse, U.S.R.; Schnitzler, J.-P. Practical approaches to plant volatile analysis. Plant J. 2006, 45, 540–560. [Google Scholar] [CrossRef]
- Chang, C.; Wang, J.; Chang, C.; Liang, M.; Lin, M. Development of a multicopter-carried whole air sampling apparatus and its applications in environmental studies. Chemosphere 2016, 144, 484–492. [Google Scholar] [CrossRef]
- McKinney, K.A.; Wang, D.; Ye, J.; de Fouchier, J.; Guimaraes, P.C.; Batista, C.E.; Souza, R.A.F.; Alves, E.G.; Gu, D.; Guenther, A.B.; et al. A sampler for atmospheric volatile organic compounds by copter unmanned aerial vehicles. Atmos. Meas. Tech. 2019, 12, 3123–3135. [Google Scholar] [CrossRef]
- Kefauver, S.C.; Filella, I.; Penuelas, J. Remote sensing of atmospheric biogenic volatile organic compounds (BVOCs) via satellite-based formaldehyde vertical column assessments. Int. J. Remote Sens. 2014, 35, 7519–7542. [Google Scholar] [CrossRef]
- Maja, M.M.; Kasurinen, A.; Yli-Pirilä, P.; Joutsensaari, J.; Klemola, T.; Holopainen, T.; Holopainen, J.K. Contrasting responses of silver birch VOC emissions to short- and long-term herbivory. Tree Physiol. 2014, 34, 241–252. [Google Scholar] [CrossRef]
- Bishop, J.A. Bumble Bees (Bombus hypnorum) Collect Aphid Honeydew on Stone Pine (Pinus pumila) in the Russian Far-East. J. Kans. Entomol. Soc. 1994, 67, 220–222. [Google Scholar]
- Stadler, B.; Michalzik, B. Aphid infested Norway spruce are "hot spots" in throughfall carbon chemistry in coniferous forests. Can. J. For. Res. 1998, 28, 1717–1722. [Google Scholar] [CrossRef]
- Stadler, B.; Dixon, A. Ecology and evolution of aphid-ant interactions. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 345–372. [Google Scholar] [CrossRef]
- Durak, R.; Wegrzyn, E.; Leniowski, K. When a little means a lot—Slight daily cleaning is crucial for obligatory ant-tended aphids. Ethol. Ecol. Evol. 2016, 28, 20–29. [Google Scholar] [CrossRef]
- Wenninger, A.; Hollingsworth, T.; Wagner, D. Predatory hymenopteran assemblages in boreal Alaska: Associations with forest composition and post-fire succession. Ecoscience 2019, 26, 205–220. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Blande, J.D.; Sorvari, J. Functional role of extrafloral nectar in boreal forest ecosystems under climate change. Forests 2020, 11, 67. [Google Scholar] [CrossRef]
- Warrington, S. Relationship between SO2 dose and growth of the pea aphid, Acyrthosiphon Pisum, on peas. Environ. Pollut. 1987, 43, 155–162. [Google Scholar] [CrossRef]
- Bolsinger, M.; Flückiger, W. Ambient air pollution induced changes in amino acid pattern of phloem sap in host plants—Relevance to aphid infestation. Environ. Pollut. 1989, 56, 209–216. [Google Scholar] [CrossRef]
- Warrington, S.; Whittaker, J.B. Interactions between Sitka Spruce, the green spruce aphid, sulfur dioxide pollution and drought. Environ. Pollut. 1990, 65, 363–370. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Kainulainen, P.; Oksanen, J. Effects of gaseous air pollutants on aphid performance on Scots pine and Norway spruce seedlings. Water Air Soil Pollut. 1995, 85, 1431–1436. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Semiz, G.; Blande, J.D. Life-history strategies affect aphid preference for yellowing leaves. Biol. Lett. 2009, 5, 603–605. [Google Scholar] [CrossRef]
- Beetge, L.; Kruger, K. Drought and heat waves associated with climate change affect performance of the potato aphid Macrosiphum euphorbiae. Sci. Rep. 2019, 9, 3645. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Jiang, Y.; Veromann-Jurgenson, L.; Niinemets, U. Petiole gall aphid (Pemphigus spyrothecae) infestation of Populus x petrovskiana leaves alters foliage photosynthetic characteristics and leads to enhanced emissions of both constitutive and stress-induced volatiles. Trees 2019, 33, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Day, K.R.; Ayres, M.P.; Harrington, R.; Kidd, N.A.C. Interannual dynamics of aerial and arboreal green spruce aphid populations. Popul. Ecol. 2010, 52, 317–327. [Google Scholar] [CrossRef]
- Banfield-Zanin, J.A.; Leather, S.R. Frequency and intensity of drought stress alters the population size and dynamics of Elatobium abietinum on Sitka spruce. Ann. Appl. Biol. 2014, 165, 260–269. [Google Scholar] [CrossRef]
- Straw, N.A.; Green, G. Interactions between green spruce aphid (Elatobium abietinum (Walker)) and Norway and Sitka spruce under high and low nutrient conditions. Agric. For. Entomol. 2001, 3, 263–274. [Google Scholar] [CrossRef]
- Kilpeläinen, J.; Finer, L.; Neuvonen, S.; Niemelä, P.; Domisch, T.; Risch, A.C.; Jurgensen, M.F.; Ohashi, M.; Sundström, L. Does the mutualism between wood ants (Formica rufa group) and Cinara aphids affect Norway spruce growth? For. Ecol. Manag. 2009, 257, 238–243. [Google Scholar] [CrossRef]
- Kasal-Slavik, T.; Eschweiler, J.; Kleist, E.; Mumm, R.; Goldbach, H.E.; Schouten, A.; Wildt, J. Early biotic stress detection in tomato (Solanum lycopersicum) by BVOC emissions. Phytochemistry 2017, 144, 180–188. [Google Scholar] [CrossRef]
- Agelopoulos, N.; Birkett, M.A.; Hick, A.J.; Hooper, A.M.; Pickett, J.A.; Pow, E.M.; Smart, L.E.; Smiley, D.; Wadhams, L.J.; Woodcock, C.M. Exploiting semiochemicals in insect control. Pestic. Sci. 1999, 55, 225–235. [Google Scholar] [CrossRef]
- Catola, S.; Centritto, M.; Cascone, P.; Ranieri, A.; Loreto, F.; Calamai, L.; Balestrini, R.; Guerrieri, E. Effects of single or combined water deficit and aphid attack on tomato volatile organic compound (VOC) emission and plant-plant communication. Environ. Exp. Bot. 2018, 153, 54–62. [Google Scholar] [CrossRef]
- Xu, Q.; Hatt, S.; Han, Z.; Francis, F.; Chen, J. Combining E-β-farnesene and methyl salicylate release with wheat-pea intercropping enhances biological control of aphids in North China. Biocontrol. Sci. Technol. 2018, 28, 883–894. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.P.; Tungadi, T.; Donnelly, R.; Bravo-Cazar, A.; Rhee, S.; Watt, L.G.; Mutuku, J.M.; Wamonje, F.O.; Murphy, A.M.; Arinaitwe, W.; et al. Modelling and manipulation of aphid-mediated spread of non-persistently transmitted viruses. Virus Res. 2020, 277, 197845. [Google Scholar] [CrossRef] [PubMed]
- Blande, J.D.; Korjus, M.; Holopainen, J.K. Foliar methyl salicylate emissions indicate prolonged aphid infestation on silver birch and black alder. Tree Physiol. 2010, 30, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Staudt, M.; Jackson, B.; El-Aouni, H.; Buatois, B.; Lacroze, J.; Poessel, J.; Sauge, M. Volatile organic compound emissions induced by the aphid Myzus persicae differ among resistant and susceptible peach cultivars and a wild relative. Tree Physiol. 2010, 30, 1320–1334. [Google Scholar] [CrossRef] [PubMed]
- Joo, E.; Van Langenhove, H.; Simpraga, M.; Steppe, K.; Amelynck, C.; Schoon, N.; Muller, J.-F.; Dewulf, J. Variation in biogenic volatile organic compound emission pattern of Fagus sylvatica L. due to aphid infection. Atmos. Environ. 2010, 44, 227–234. [Google Scholar] [CrossRef]
- Mentel, T.F.; Kleist, E.; Andres, S.; Dal Maso, M.; Hohaus, T.; Kiendler-Scharr, A.; Rudich, Y.; Springer, M.; Tillmann, R.; Uerlings, R.; et al. Secondary aerosol formation from stress-induced biogenic emissions and possible climate feedbacks. Atmos. Chem. Phys. 2013, 13, 8755–8770. [Google Scholar] [CrossRef]
- Bergström, R.; Hallquist, M.; Simpson, D.; Wildt, J.; Mentel, T.F. Biotic stress: A significant contributor to organic aerosol in Europe? Atmos. Chem. Phys. 2014, 14, 13643–13660. [Google Scholar] [CrossRef]
- Pezet, J.; Elkinton, J.; Gomez, S.; Mckenzie, E.A.; Lavine, M.; Preisser, E. Hemlock woolly adelgid and elongate hemlock scale induce changes in foliar and twig volatiles of eastern hemlock. J. Chem. Ecol. 2013, 39, 1090–1100. [Google Scholar] [CrossRef]
- Heie, O.E. The Aphidoidea (Hemiptera) of Fennoscandia and Denmark. VI: Family Aphididae: Part 3 of Tribe Macrosiphini of Subfamily Aphidinae, and Family Lachnidae. Fauna Entomol Scand 31; E.J. Brill: Leiden, The Netherlands, 1995; pp. 148–149. [Google Scholar]
- Manninen, A.M.; Holopainen, T.; Lyytikäinen-Saarenmaa, P.; Holopainen, J.K. The role of low-level ozone exposure and mycorrhizas in chemical quality and insect herbivore performance on Scots pine seedlings. Glob. Chang. Biol. 2000, 6, 111–121. [Google Scholar] [CrossRef]
- Volkl, W. Foraging behaviour and sequential multisensory orientation in the aphid parasitoid, Pauesia picta (Hym., Aphidiidae) at different spatial scales. J. Appl. Entomol. 2000, 124, 307–314. [Google Scholar] [CrossRef]
- Domisch, T.; Finer, L.; Neuvonen, S.; Niemelä, P.; Risch, A.C.; Kilpeläinen, J.; Ohashi, M.; Jurgensen, M.F. Foraging activity and dietary spectrum of wood ants (Formica rufa group) and their role in nutrient fluxes in boreal forests. Ecol. Entomol. 2009, 34, 369–377. [Google Scholar] [CrossRef]
- Baradat, P.; Yazdani, R. Genetic expression for monoterpenes in clones of Pinus sylvestris grown on different sites. Scan. J. For. Res. 1988, 3, 25–36. [Google Scholar] [CrossRef]
- Kivimäenpää, M.; Markkanen, J.M.; Ghimire, R.P.; Holopainen, T.; Vuorinen, M.; Holopainen, J.K. Scots pine provenance affect the emission rate and chemical composition of volatile organic compounds of forest floor. Can. J. For. Res. 2018, 48, 1373–1381. [Google Scholar] [CrossRef]
- Flower-Ellis, J.; Olsson, L. Estimation of volume, total and projected area of Scots pine needles from their regression on length. Studia For. Suec. 1993, 190, 1–19. [Google Scholar]
- Kivimäenpää, M.; Ghimire, R.P.; Sutinen, S.; Häikiö, E.; Kasurinen, A.; Holopainen, T.; Holopainen, J.K. Increases in volatile organic compound emissions of Scots pine in response to elevated ozone and warming are modified by herbivory and soil nitrogen availability. Eur. J. For. Res. 2016, 135, 343–360. [Google Scholar] [CrossRef]
- Kivimäenpää, M.; Magsarjav, N.; Ghimire, R.; Markkanen, J.; Heijari, J.; Vuorinen, M.; Holopainen, J.K. Influence of tree provenance on biogenic VOC emissions of Scots pine (Pinus sylvestris) stumps. Atmos. Environ. 2012, 60, 477–485. [Google Scholar] [CrossRef]
- Ghimire, R.P.; Markkanen, J.M.; Kivimäenpää, M.; Lyytikäinen-Saarenmaa, P.; Holopainen, J.K. Needle removal by sawfly larvae increases branch-level VOC emissions and reduces below-ground emissions of Scots pine. Environ. Sci. Technol. 2013, 47, 4325–4332. [Google Scholar] [CrossRef]
- Pettersson, M.; Unelius, C.R.; Valterova, I.; Borg-Karlson, A. Semiochemicals related to the aphid Cinara pilicornis and its host, Picea abies: A method to assign nepetalactone diastereomers. J. Chromatogr. A 2008, 1180, 165–170. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Li, J.; Hu, H.; Mao, J.; Yu, L.; Stoopen, G.; Wang, M.; Mumm, R.; de Ruijter, N.C.A.; Dicke, M.; Jongsma, M.A.; et al. Defense of pyrethrum flowers: Repelling herbivores and recruiting carnivores by producing aphid alarm pheromone. New Phytol. 2019, 223, 1607–1620. [Google Scholar] [CrossRef]
- Mukrimin, M.; Kovalchuk, A.; Ghimire, R.P.; Kivimäenpää, M.; Sun, H.; Holopainen, J.K.; Asiegbu, F.O. Evaluation of potential genetic and chemical markers for Scots pine tolerance against Heterobasidion annosum infection. Planta 2019, 250, 1881–1895. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dai, H.; Zhang, Y.; Chandrasekar, R.; Luo, L.; Hiromasa, Y.; Sheng, C.; Peng, G.; Chen, S.; Tomich, J.M.; et al. Armet is an effector protein mediating aphid-plant interactions. FASEB J. 2015, 29, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Lu, H.; Wang, T.; Zhang, W.; Kang, L.; Cui, F. Armet, an aphis effector protein, induces pathogen resistance in plants by promoting the eaccumulation of salicylic acid. Philos. Trans. R Soc. Lond B Biol. Sci. 2019, 374, 1767. [Google Scholar] [CrossRef]
- van Bel, A.J.E.; Will, T. Functional Evaluation of Proteins in Watery and Gel Saliva of Aphids. Front Plant Sci. 2016, 7, 1840. [Google Scholar] [CrossRef]
- van Bel, A.J.E.; Furch, A.C.U.; Will, T.; Buxa, S.V.; Musetti, R.; Hafke, J.B. Spread the news: Systemic dissemination and local impact of Ca2+ signals along the phloem pathway. J. Exp. Bot. 2014, 65, 1761–1787. [Google Scholar] [CrossRef]
- Mäntylä, E.; Kleier, S.; Lindstedt, C.; Kipper, S.; Hilker, M. Insectivorous birds are attracted by plant traits induced by insect egg deposition. J. Chem. Ecol. 2018, 44, 1127–1138. [Google Scholar] [CrossRef]
- Heijari, J.; Blande, J.D.; Holopainen, J.K. Feeding of large pine weevil on Scots pine stem triggers localized bark and systemic shoot emission of volatile organic compounds. Environ. Exp. Bot. 2011, 71, 390–398. [Google Scholar] [CrossRef]
- Tieman, D.; Zeigler, M.; Schmelz, E.; Taylor, M.G.; Rushing, S.; Jones, J.B.; Klee, H.J. Functional analysis of a tomato salicylic acid methyl transferase and its role in synthesis of the flavor volatile methyl salicylate. Plant J. 2010, 62, 113–123. [Google Scholar] [CrossRef]
- Mäntylä, E.; Kleier, S.; Kipper, S.; Hilker, M. The attraction of insectivorous tit species to herbivore-damaged Scots pines. J. Ornithol. 2017, 158, 479–491. [Google Scholar] [CrossRef]
- Kovalchuk, A.; Raffaello, T.; Jaber, E.; Kerio, S.; Ghimire, R.; Lorenz, W.W.; Dean, J.F.D.; Holopainen, J.K.; Asiegbu, F.O. Activation of defence pathways in Scots pine bark after feeding by pine weevil (Hylobius abietis). BMC Genom. 2015, 16, 352. [Google Scholar] [CrossRef]
- Kännaste, A.; Nordenhem, H.; Nordlander, G.; Borg-Karlson, A. Volatiles from a mite-infested spruce clone and their effects on pine weevil behavior. J. Chem. Ecol. 2009, 35, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Ament, K.; Kant, M.R.; Sabelis, M.W.; Haring, M.A.; Schuurink, R.C. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 2004, 135, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, E.G.; Kunert, G.; Stephan, C.; David, A.; Rose, U.S.R.; Gershenzon, J.; Boland, W.; Weisser, W.W. Real-time analysis of alarm pheromone emission by the pea aphid (Acyrthosiphon Pisum) under predation. J. Chem. Ecol. 2008, 34, 76–81. [Google Scholar] [CrossRef]
- Kunert, G.; Reinhold, C.; Gershenzon, J. Constitutive emission of the aphid alarm pheromone, (E)-β-farnesene, from plants does not serve as a direct defense against aphids. BMC Ecol. 2010, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, B.; Ristić, M.; Bojović, S.; Marin, P. Variability of the needle essential oils of pinus peuce from different populations in Montenegro and Serbia. Chem. Biodivers. 2008, 5, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Mofikoya, A.O.; Bui, T.N.T.; Kivimäenpää, M.; Holopainen, J.K.; Himanen, S.J.; Blande, J.D. Foliar behaviour of biogenic semi-volatiles: Potential applications in sustainable pest management. Arthropod Plant Interact. 2019, 13, 193–212. [Google Scholar] [CrossRef]
- Goelen, T.; Sobhy, I.S.; Vanderaa, C.; de Boer, J.G.; Delvigne, F.; Francis, F.; Wackers, F.; Rediers, H.; Verstrepen, K.J.; Wenseleers, T.; et al. Volatiles of bacteria associated with parasitoid habitats elicit distinct olfactory responses in an aphid parasitoid and its hyperparasitoid. Funct. Ecol. 2020, 34, 507–520. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Wang, T.; Meng, X.; Chen, T.; Huang, X.; Li, Y.; Hou, B. Methyl salicylate glucosylation regulates plant defense signaling and systemic acquired resistance. Plant Physiol. 2019, 180, 2167–2181. [Google Scholar] [CrossRef]
- Zhu, J.W.; Park, K.C. Methyl salicylate, a soybean aphid-induced plant volatile attractive to the predator Coccinella septempunctata. J. Chem. Ecol. 2005, 31, 1733–1746. [Google Scholar] [CrossRef]
- Mumm, R.; Dicke, M. Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can. J. Zool. 2010, 88, 628–667. [Google Scholar] [CrossRef]

| Compound | Control (n = 4) | Aphid-Infested (n = 8) |
|---|---|---|
| Monoterpenes, MT | ||
| Tricyclene | 2.6 (1.0) | 3.2 (1.0) |
| α-Thujene | 0.6 (0.4) | 1.0 (0.5) |
| α-Pinene | 211.6 (73.0) | 269.9 (89.8) |
| Camphene | 21.5 (5.6) | 35.1 (9.4) |
| Sabinene | 14.9 (2.4) | 18.0 (7.7) |
| β-Pinene | 29.8 (17.7) | 54.1 (20.0) |
| Myrcene | 47.3 (20.7) | 231.5 (184.7) |
| α-Phellandrene | 0.8 (0.6) | 2.3 (1.7) |
| Δ-3-Carene | 245.9 (78.2) | 114.0 (71.2) |
| α-Terpinene | 2.2 (0.5) | 1.8 (0.9) |
| p-Cymene | 2.3 (0.3) | 1.5 (0.8) |
| Limonene | 61.8 (24.5) | 306.1 (197.0) |
| β-Phellandrene | 58.3 (26.4) | 269.5 (201.3) |
| 1,8-Cineole | 16.9 (6.2) | 18.9 (8.8) |
| (E)-β-Ocimene | 4.4 (0.7) | 3.6 (1.8) |
| γ-Terpinene | 3.1 (0.5) | 2.5 (1.3) |
| Terpinolene | 13.2 (4.3) | 10.0 (4.6) |
| Linalool | 0.5 (0.5) | 1.6 (1.0) |
| Camphor | 2.7 (0.9) | 1.8 (0.6) |
| Bornyl acetate | 1.2 (0.6) | 3.5 (1.9) |
| Sesquiterpenes, SQT | ||
| β-Bourbobene | 0.7 (0.4) | 2.8 (2.3) |
| Longifolene | 2.2 (1.0) | 1.3 (0.4) |
| β-Caryophyllene | 1.0 (0.8) | 2.5 (1.4) |
| (E)-β-Farnesene | 3.9 (1.9) | a15.7 (3.6) * |
| α-Farnesene | 6.1 (1.7) | a51.6 (10.5) ** |
| unknown ST1 | 0.2 (0.2) | 4.7 (3.1) |
| (E,E)-α-Farnesene | 11.8 (3.5) | a111.7 (29.8) ** |
| unknown ST2 | 1.7 (0.5) | 4.9 (1.9) |
| γ-Cadinene | 0.7 (0.3) | 1.0 (0.4) |
| δ-Cadinene | 1.4 (0.7) | 3.4 (1.2) |
| cis-α-Bisabolene | 0.2 (0.2) | 1.0 (0.7) |
| Green leaf volatiles, GLV | ||
| cis-3-Hexenyl-acetate | 2.5 (1.5) | 1.6 (1.0) |
| Benzenoids | ||
| Methyl benzoate | 0 (0) | 3.2 (1.4) |
| Benzyl acetate | 0 (0) | 1.0 (0.7) |
| Ethyl benzoate | 0 (0) | 3.4 (2.4) |
| Methyl salicylate | 7.7 (3.6) | b 578.5 (118.5) ** |
| Thymol methyl ether | 0.2 (0.2) | 0.9 (0.8) |
| Anethole | 0 (0) | 2.3 (1.1) |
| trans-Anethole | 0 (0) | 3.1 (1.3) |
| Total emissions | ||
| MTs | 743.9 (187.3) | 1351.4 (665.4) |
| SQTs | 29.6 (7.5) | a 202.0 (41.7) ** |
| GLVs | 2.5 (1.5) | 2.4 (1.8) |
| Benzenoids | 7.9 (3.6) | a 592.3 (122.4) ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kivimäenpää, M.; Babalola, A.B.; Joutsensaari, J.; Holopainen, J.K. Methyl Salicylate and Sesquiterpene Emissions Are Indicative for Aphid Infestation on Scots Pine. Forests 2020, 11, 573. https://doi.org/10.3390/f11050573
Kivimäenpää M, Babalola AB, Joutsensaari J, Holopainen JK. Methyl Salicylate and Sesquiterpene Emissions Are Indicative for Aphid Infestation on Scots Pine. Forests. 2020; 11(5):573. https://doi.org/10.3390/f11050573
Chicago/Turabian StyleKivimäenpää, Minna, Aishat B. Babalola, Jorma Joutsensaari, and Jarmo K. Holopainen. 2020. "Methyl Salicylate and Sesquiterpene Emissions Are Indicative for Aphid Infestation on Scots Pine" Forests 11, no. 5: 573. https://doi.org/10.3390/f11050573
APA StyleKivimäenpää, M., Babalola, A. B., Joutsensaari, J., & Holopainen, J. K. (2020). Methyl Salicylate and Sesquiterpene Emissions Are Indicative for Aphid Infestation on Scots Pine. Forests, 11(5), 573. https://doi.org/10.3390/f11050573






