Abstract
The process of post-fire recovery in mixed Siberian spruce–Scots pine forests (Picea obovata Ledeb.-Pinus sylvestris L.), typical for the European North-West, was studied in the Kola peninsula (Russia). We used the spatial–temporal approach to reveal the size structure (diameter at breast height (DBH) distribution) and vital state of Siberian spruce and Scots pine stands, tree regeneration and species structure of the dwarf shrub–herb and lichen–moss layers at different stages of post-fire succession (8–380 years after the fire). It was found that in both forest-forming species, the process of stand stratification results in the allocation of two size groups of trees. In Siberian spruce, these groups persist throughout the succession. In Scots pine, DBH distributions become more homogeneous at the middle of succession (150–200 years after the fire) due to the extinction of small-size individuals. Siberian spruce stands are dominated by moderately and strongly weakened trees at all succession stages. The vitality status of Scots pine stands is higher compared to Siberian spruce up to 150 years after a fire. The dynamics of regeneration activity is similar in both species, with a minimum at the middle of the restoration period. The results indicate that in Siberian spruce–Scots pine forests, the stand structure and regeneration activity differs substantially in the first half of succession (up to 200 years after the fire) and become similar in the late-succession community. The study of lower layers revealed that the cover of moss–lichen and dwarf shrub–herb layers stabilize 150 years after a fire. Changes in species structure in both layers are observed until the late stage of succession. The originality of the structure and dynamics of mixed Siberian spruce–Scots pine forests is revealed based on a comparison with pure Siberian spruce forests in the same region.
1. Introduction
Over the past century, against the backdrop of an ever-increasing intensity of forest exploitation and management and the expansion of industrial pollution zones, we have seen a sharp decrease in the area of natural boreal forests [1,2,3]. Forests of the boreal zone are a mosaic of communities at different stages of recovery after logging and fires; undisturbed and intact communities are rather fragmentary [4,5]. They are preserved mainly in the northern part of the taiga. This is explained by their inaccessibility and low productivity, and by the longer intervals between fires in forested wetlands typical of the northern taiga zone [6,7,8,9].
In boreal forests, among natural and anthropogenic factors of disturbance, resulting in the formation of a mosaic of disturbed and undisturbed forest communities, fires continue to be one of the most important agents [2,8,10,11,12,13]. An analysis of peat deposits in the territory of Fennoscandia has shown that forest fires resulting from natural factors occur no more than once every thousand years, especially in forested wetlands typical of the Northern taiga [8,9,10]. However, in recent centuries, as a result of anthropogenic activity, the fire cycle in the boreal forest decreased to 50–100 years and rarely exceeds 300 years [5,6,8,11].
Forest communities recovering after one-time disturbances and periodically disturbed forests that are under management differ from subclimax and climax ones by their species structure transformation [14,15,16,17,18]. Taking into account the high proportion of disturbed forest communities, it is important to determine both the ways and speed of their recovery after disturbances and the basic structural characteristics of undisturbed forests. In Northern Europe, mainly the most widespread spruce (Picea abies (L.) Karst., P. obovata Ledeb.) and Scots pine (Pinus sylvestris L.) forests [12,18,19,20,21,22,23,24,25,26,27,28,29] have been studied. The information on the structure and dynamics of more rare types of forest communities is still very limited. Mixed Siberian spruce–Scots pine forest in the north-west of Europe is of particular interest [22,30,31,32,33]. First, the study of these communities enables us to complement the information on the ecosystem diversity and vegetation originality of this region, second, to identify various aspects of the interaction of two jointly growing forest-forming species. The second problem cannot be solved without a comparative analysis of the structure and dynamics of their populations at different succession stages. Whereas the study of the age and size structure of stands is traditional, the vitality structure is analyzed less frequently [34,35,36], although this parameter is important for identifying the current state and competitive relationships of forest-forming species.
Our goal was to answer the following questions: (1) How does the structure of Scots pine and Siberian spruce stands change during post-fire succession? (2) What are the post-fire dynamics of Scots pine and Siberian spruce natural regeneration? (3) How does the presence of Scots pine in a forest community affect the stand structure and renewal of Siberian spruce? (4) What are the post-fire dynamics and recovery rate of the main parameters of the lower layers in Siberian spruce–Scots pine forest.
2. Materials and Methods
2.1. Study Site
The studies were carried out in the Kola peninsula, in the middle reaches of the Liva river (67°49′–67°51′ N, 31°17′–31°22′ E) in the dwarf shrub–green moss Siberian spruce–Scots pine forests (Picea obovata Ledeb.–Pinus sylvestris L.). In the territory studied, which had an area ca 100 km2, the presence of natural barriers (swamps and rivers) resulted in the formation of mosaics of forest communities with different fire histories. Six permanent sample sites (SS) of 0.10–0.50 ha size were arranged in the communities formed after the fires that happened 8, 15, 80, 150, 200 and 380 years ago. The similarity of the genesis, texture and composition of parent rocks, as well as the uniformity of soil types, allowed combining the communities into one spatial–temporal series when considering problems of their post-fire dynamics. To reveal the characteristic features of the structure and dynamics of mixed Siberian spruce–Scots pine forests, the data obtained in the dwarf shrub–green moss Siberian spruce forests located in the same region and in two other regions of the Kola peninsula (68°08′–68°09′ N, 33°56′–33°57′ E; 67°31′–67°32′ N, 33°58′–34°11′ E; 67°38′–67°39′ N, 34°35′–34°40′ E) were used. In Siberian spruce communities (11SS), the time elapsed since the last fire varied from 55 to 500 years.
The studied Siberian Spruce–Scots pine and Siberian spruce forests occupy flat areas and gentle slopes composed of sandy and sandy loamy moraine deposits with boulders. The groundwater table was at a depth of more than 1.5 m in the Siberian spruce–Scots pine forests and 1.0–1.2 m in the Siberian spruce ones. In these conditions, illuvial-humus podzols are formed under the spruce–pine forests, having the O-E-BH-(BF)-C profile. These are unsaturated, shallow, illuvial-high humus, sandy-loamy sandy and medium-skeletal soils. According to the World Reference Base (WRB) classification, they are identified as Albic Carbic Podzols (Arenic) [37]. The illuvial-humus podzols are characterized by a small thickness (up to 60–70 cm) and distinct morphological and chemical differences between the genetic horizons. The profile differentiation is due to the redistribution of chemical elements with the formation of an illuvial horizon, which is characterized by the accumulation of oxalate-soluble Al and Fe compounds, as well as of fulvate humus, with content of not less than 4–5% [38].
The time since the last fire in the communities studied was determined from core simples that were taken from living trees with fire damage to their trunks (at least 5 trees) within 50–100 m of sample sites. In the community where a fire occurred more than 300 years ago, this was assessed based on the length of the continuous-age series (with steps of 10 years), composed of individuals of all tree species. The continuity of the age series indicated the absence of fire in the intervening time, because small conifers die in fires. In addition, the age of the trees that grew on the decomposed logs without traces of fire may be used. We summarized the age of a living tree, the approximate age of a fallen tree (using its size parameters), the time required for the decay of a fallen trunk and seedlings’ appearance to assess the period without fire. The time of the fallen log’s decomposition was estimated by [39].
In the northern taiga Siberian spruce–Scots pine forests, the tree layer was formed by Picea obovata, Pinus sylvestris and Betula pubescens Ehrh. The stands were characterized by low average values of height, diameter and basal area (Table 1). The main dominants of the dwarf shrub–herb layer in the northern taiga communities are Vaccinium myrtillus L., V. vitis-idaea L. and Empetrum hermaphroditum Hagerup. The moss–lichen layer is dominated by Pleurozium schreberi (Brid.) Mitt.

Table 1.
Forest stands (diameter at breast height (DBH) >4 cm) data from the investigated simple plots.
2.2. Stand and Lower Layers Sampling
For the measurements, sample sites were divided into quadrats of 5 m × 5 m each. All individuals of woody plants with a height of more than 0.1 m were counted. Smaller individuals of woody plants (over 1 year) were counted at 40–100 sample plots 1 m × 1 m in size, evenly spaced within the sample site. The number of sample plots depended on the size of the SS and the density of seedlings. In the populations of woody plants, two groups of individuals were analyzed: (1) trees (individuals >1.3 m high) and (2) seedlings (individuals <1.3 m high). The following main parameters were determined: diameter at breast height (DBH), height and vitality state.
The projected coverage and species number of ground vegetation were estimated in 20–70 sample plots of 1 m × 1 m size organized along parallel transect lines within each sample site.
2.3. Tree Vitality Classification
The vitality state of Scots pine and Siberian spruce trees was determined based on the comparison of crown density with that of a reference individual assigned a value of 100% [40,41]. The reference individual is represented by an unsuppressed tree growing in an open area or in a large canopy gap. The crown density was estimated visually. The final grade was the average of estimates from two observers. The tree vitality state was evaluated using 5 classes: I—healthy (i.e., unsuppressed or slightly suppressed) trees with a relative crown density (CD) of >75–100%; II—moderately weakened (i.e., moderately suppressed) trees with a CD of >50–75%; III—strongly weakened (i.e., strongly suppressed) trees with a CD of >25–50%; IV—dying trees with a CD of >0–25%; V—dry trees.
2.4. Stand Vitality Index
Based on the obtained vitality spectra, the vitality state index (Ln) of the trees and seedlings of Scots pine and Siberian spruce was calculated using the equation [40,41]:
where ki is the needles’ mass coefficient, determined on the basis of the average relative density of the crown and equal to 1.0, 0.71, 0.43, 0.14 and 0, respectively, for healthy, moderately weakened, strongly weakened, dying and dry individuals; fi is the proportion of healthy, moderately weakened, strongly weakened, drying and dry individuals, calculated by their number. The maximum index value is 1.0.
2.5. Pielou’s Evenness Index for the Ground Vegetation
Species structure of the dwarf shrub and herb layer as well as of the moss–lichen layer was estimated by Pielou’s index [42]:
where H is Shannon index, N is the species number over an area of 1 m2, si is the relative coverage of species, i.
2.6. Statistical Analysis
The size structure of Picea obovata and Pinus sylvestris trees (individuals of more than 1.3 m in height) was examined using statistical parameters of DBH distributions, the value of the bimodality coefficient (BC) and approximations by Weibull distributions with two and three parameters [43,44,45,46]. A bimodality coefficient has the following equation:
where n is the number of trees, S is the skewness, K is the kurtosis. If BC was >0.5556, the distribution was considered bimodal.
The Weibull probability density function was described as:
where λ is the scale parameter and k is the shape parameter. The reliability of the approximation was defined using the Chi-Squared Test.
To assess the differences in the size distributions of Scots pine and Siberian spruce, some statistical parameters were used as well as the shape and scale parameters of the Weibull distribution. The shape parameter (k) is closely correlated with the variation coefficient and the skewness of the empirical distribution (according to our data, R = −0.77–0.80; p < 0.001). The higher the value of the shape parameter, the more symmetrical the distribution curve and the less variability of the characteristic. The scale parameter (λ) has a close positive relationship with the average value of the characteristic (R = 0.88; p < 0.001).
3. Results
3.1. Structure of Scots Pine and Siberian Spruce Stands
3.1.1. Size Structure
At the initial stage (eight years after a fire) of the post-fire recovery of Siberian spruce–Scots pine forests, the tree layer consisted of a small number of trees of pre-fire origin that survived fire and retained viability. Among them, individuals of Scots pine dominated. They varied substantially in diameter, from 10 to 44 cm (Figure 1a). Siberian spruce was represented by a single individual (Figure 1b).
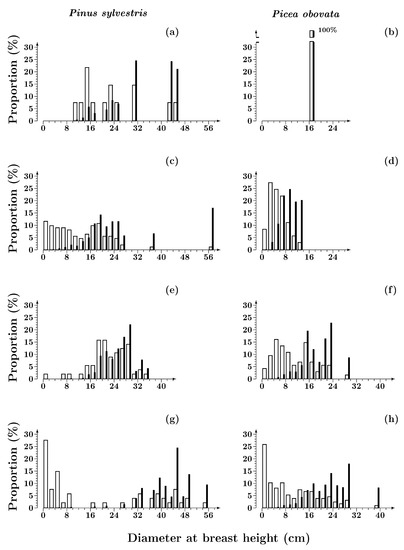
Figure 1.
Diameter class distributions of Pinus sylvestris and Picea obovata trees in Siberian spruce– Scots pine forests with a post-fire period of 8 (a,b), 80 (c,d), 150 (e,f) and 380 (g,h) years. Open bars—by number of individuals; solid bars—by volume.
Eighty years after a fire, Scots pine and Siberian spruce populations were dominated by individuals of post-fire origin, since the majority of trees that had survived the fire were already dead. The pre-fire component in the forest stand was represented by single Scots pine trees (Figure 1c). DBH distributions of the Scots pine and Siberian spruce post-fire generations differed substantially (Figure 1c,d). Scots pine was characterized by a wide DBH range (ca 27 cm); the distribution was nearly symmetrical and had a tendency to bimodality, as evidenced by the value of the coefficient BC (Table 2). The distribution suggested the presence of two groups of individuals, with DBH values below and above 12 cm. In each of these fractions, the diameter distribution curves could be approximated by the Weibull distribution (Table 3). In Siberian spruce, the diameter range was 50% less than in Scots pine (Table 2); it was similar to the range of the small-sized fraction of the Scots pine stand (Figure 1c,d). The distribution curve was unimodal, with a moderate positive asymmetry and, with a high degree of accuracy, could be approximated by the Weibull distribution. The distribution parameters were close to those established for the small-sized fraction of the Scots pine stand (Table 3).

Table 2.
Statistical parameters of diameter class distributions of Pinus sylvestris and Picea obovata trees in Siberian spruce–Scots pine and Siberian spruce forests.

Table 3.
Results of approximation of the DBH distributions by Weibull distribution with two and three parameters.
By 150–200 years after a fire, the range of DBH increased in both species, more so in Siberian spruce (by 130% on average) than in Scots pine (by 35% on average). However, the average DBH of the Siberian spruce individuals (9.2–10.8 cm) remained 50% less than that of Scots pine (Table 2). The DBH distribution of the Scots pine individuals was negative or slightly positively asymmetric due to the predominance of medium- and large-sized individuals. In Siberian spruce, the distribution had a pronounced positive asymmetry; small and medium-sized individuals predominated (Figure 1e,f). In Scots pine, at low DBH values (less than 12 cm) the distribution was discontinuous, and the total proportion of small-sized individuals was only 6–10%, while in Siberian spruce more than half of all individuals was small in size. The distribution curves for both species were adequately approximated by the Weibull distribution with two parameters. The shape and scale parameters in Siberian spruce and Scots pine differed by 30–60% (Table 3). The greatest differences in the parameters values (50–60%) and, accordingly, in the size structure of the two species were revealed 150 years after fire.
From 150 to 380 years after fire, in both species the mean DBH remained unchanged (Table 2). However, in Scots pine the DBH range increased to 50 cm, and in Siberian spruce to nearly 40 cm. In the late-succession community, the proportion of individuals with a DBH of less than 2 cm was remarkably high (40–44%, Figure 1g,h). In both species, the distributions were positively asymmetrical and bimodal (Table 2). In the Scots pine stand, the bimodality was more pronounced due to a very low proportion (<5%) of individuals with diameters from 10 to 26 cm (Figure 1g). The discontinuity of the distribution was inherited from the previous stages of succession (Figure 1e). In the Siberian spruce stand, small- and large-sized groups were separated by a reduced proportion of trees with a diameter of 8 to 12 cm (Figure 1h). The DBH distributions (in Scots pine only for the large-sized group) were approximated by the Weibull distribution (Table 3). The shape and scale parameters in large-sized Siberian spruce trees were 50% lower than in the analogous group of Scots pine individuals.
The comparison of the size structure of Siberian spruce stands in Siberian Spruce–Scots pine (Figure 1) and Siberian spruce (Figure 2) forests revealed substantial differences. In Siberian spruce forests, from 80 to 200 years after a fire, the shape parameter of the DBH distributions increased by 40%, and by the end of succession decreased by 50% (Table 3). In Siberian Spruce–Scots pine forests, from 80 to 380 years after a fire the value of the shape parameter consequentially decreased by 45%. The greatest differences in the parameter (30%) were observed in the middle of succession (200–220 years after a fire) due to a significant decrease in the proportion of small-sized individuals (DBH < 8 cm) in the stand of Siberian spruce forest (Figure 2c), while this phenomenon was absent in Siberian Spruce–Scots pine forests.
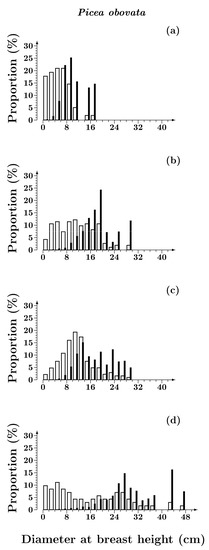
Figure 2.
Diameter class distributions of Picea obovata trees in Siberian spruce forests with post-fire periods of 80 (a), 150 (b), 220 (c) and 380 (d) years. Open bars—by number of individuals; solid bars—by volume.
3.1.2. Vitality Structure
The bulk of the tree layer of the forest communities studied was formed mainly by individuals of the first post-fire generations. Eight years after a fire, the vitality state index of Scots pine seedlings (Ln = 0.53) was noticeably higher than that of Siberian spruce (Ln = 0.33) (Table 4). Only the most viable and fast growing individuals of both species could form the future forest stand.

Table 4.
The vitality state index of trees and seedlings of Pinus sylvestris and Picea obovata in forests with different post-fire periods.
Accordingly, 80 years after a fire, healthy and moderately weakened individuals of both Scots pine and Siberian spruce accounted for a significant proportion of the tree numbers (55–60%) and volume (60–80%) (Figure 3a,b).
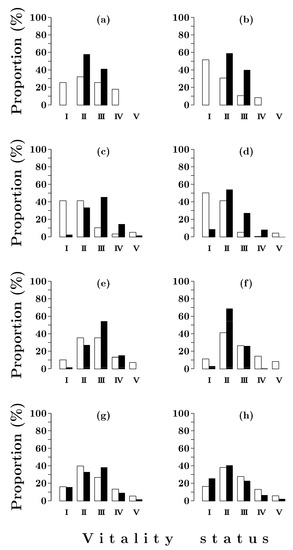
Figure 3.
Vitality class distributions of Pinus sylvestris (open bars) and Picea obovata (solid bars) trees in forests with post-fire periods of 80 (a,b), 150 (c,d), 200 (e,f) and 380 (g,h) years. I–V—vitality status: I—healthy trees; II—moderately weakened trees; III—strongly weakened trees; IV—dying trees; V—dry trees. a, c, e, g—by number of individuals; b, d, f, h—by volume.
The Scots pine trees had a noticeably larger vitality range, from healthy to dying. The Siberian spruce stand consisted only of moderately and strongly weakened trees. When assessed by the ratio of the number of individuals of different vitalities, the general state of the stands of the two species was similar (Ln = 0.60–0.62). At the same time, in Scots pine the vitality state index value calculated from the volume was noticeably higher (0.79, Table 4). This was due to the fact that about half of the total volume of the Scots pine stand was formed by healthy trees, while the proportion of strongly weakened individuals did not exceed 10%. In Siberian spruce stand, strongly weakened trees accounted for the proportion of the volume that was four times higher than in the Scots pine stand.
By 150 years after a fire, the difference in the state of the Scots pine and Siberian spruce stands became most evident. In the Scots pine stand, healthy and moderately weakened trees totally dominated in terms of their numbers and volume (Figure 3c,d). The vitality state index of the Scots pine stand reached the highest values (0.75–0.82) (Table 4). In the Siberian spruce stand, moderately and strongly weakened trees still dominated. Consequently, the vitality state index was lower (0.50–0.60).
From 150 to 200 years after a fire, the vitality state of Scots pine stands worsened significantly, while the vitality of Siberian spruce stands remained unchanged (Table 4). This was due to a sharp (4–5-fold) decrease in the proportion of the number and volume of healthy Scots pine trees and a noticeable increase in the number of strongly weakened and dying individuals (Figure 3f,g). As a result, moderately and strongly weakened trees became predominant in both Scots pine and Siberian spruce stands. The vitality state index in terms of number of trees was similar in the two species; in terms of volume, it was slightly lower in the Scots pine (Table 4) due to the presence of large-sized dying and dead trees of pre-fire origin (Figure 3e,f).
By 380 years after a fire, moderately and strongly weakened trees prevailed in the stands of both species, and accounted for 65–75% of the total number of individuals and volume (Figure 3g,h). The proportion of dying and dead trees was higher in the Scots pine stand. Healthy individuals accounted for the same proportion by the tree numbers in both species, and those of Siberian spruce accounted for a slightly higher proportion by volume compared with Scots pine. The value of the vitality state index calculated on the number of individuals was the same in both species, while the value based on volume was higher in Siberian spruce (Table 4). The reason, apparently, was a higher average age (>306 years) and lower vitality of the old large-sized Scots pine trees.
The comparison of the vitality structure of Siberian spruce stands in Siberian Spruce–Scots pine and Siberian spruce forests shows that in the second case, the proportion of healthy individuals (Figure 4) and the value of the vitality state index (Table 4) are noticeably higher. This is especially true for the first half of succession.
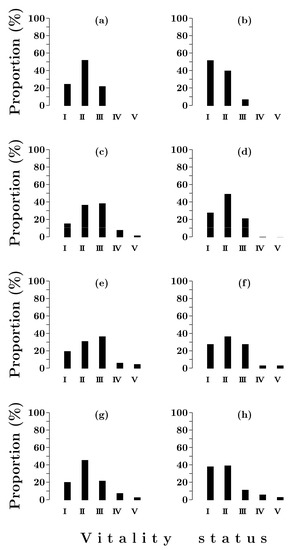
Figure 4.
Vitality class distributions of Picea obovata stands in Siberian spruce forests with post-fire periods of 80 (a,b), 150(c,d), 220 (e,f) and 380 (g,h) years. I–V—vitality status: I—healthy trees; II—moderately weakened trees; III—strongly weakened trees; IV—dying trees; V—dry trees. a, c, e, g—by number of individuals; b, d, f, h—by volume.
3.2. Natural Regeneration of Scots Pine and Siberian Spruce
Eight years after a fire, seedlings of post-fire origin made up 97–99% of all living Scots pine and Siberian spruce individuals (Figure 5a). By 80 years after a fire, the proportion of seedlings in the Scots pine and Siberian spruce populations decreased markedly. In both species, it was approximately the same and ranged from 40% to 50%. From 80 to 150 years after a fire, the proportion of seedlings was changing. In Scots pine, it was decreasing (down to 30%), and in Siberian spruce it was increasing by up to 60% (Figure 5a). From 150 to 380 years after a fire, the trend was the same in both species. By the end of the reviewed period, the proportion of seedlings in Scots pine and Siberian spruce populations increased and reached 90%.
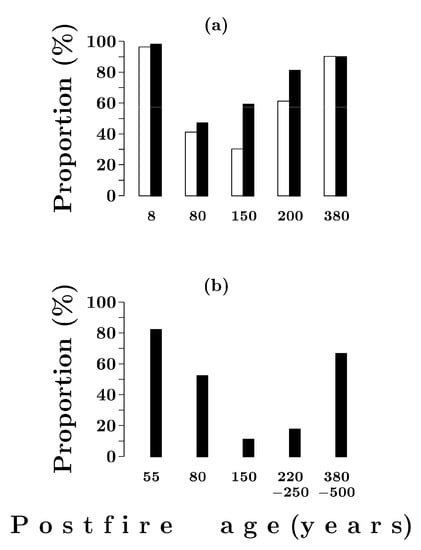
Figure 5.
The proportion of seedlings in Pinus sylvestris (open bars) and Picea obovata (solid bars) populations in Siberian spruce–Scots pine (a) and Siberian spruce (b) forests with different post-fire periods.
The proportion of seedlings in Siberian spruce populations demonstrates similar successional dynamics in mixed Siberian spruce–Scots pine and pure Siberian spruce forests (Figure 5a,b). However, in Siberian spruce forests, the values are noticeably lower. This is especially true for the period 150–250 years after a fire, when they do not exceed 10–20%.
Eight years after a fire in a Siberian spruce–Scots pine community, the vitality state of Scots pine and Siberian spruce seedlings was highly variable (Figure 6a). Healthy, dying and dead individuals were present. In Scots pine, the proportion of strongly weakened individuals was rather high (ca 40%), while the proportions of healthy and dying individuals were both approximately 25%. The vitality structure of Siberian spruce seedlings was characterized by the predominance of dying and dead individuals (Figure 6a). The observed differences were reflected in the values of the vitality state index—0.53 and 0.33, respectively (Table 4).
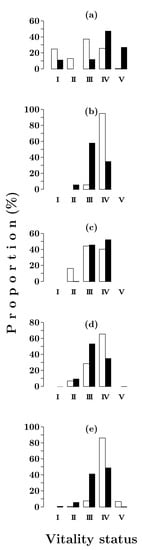
Figure 6.
Vitality class distributions of Pinus sylvestris (open bars) and Picea obovata (solid bars) seedlings in Siberian spruce–Scots pine forests with post-fire periods of 8 (a), 80 (b), 150 (c), 200 (d) and 380 (e) years. I–V—vitality status: I—healthy trees; II—moderately weakened trees; III—strongly weakened trees; IV—dying trees; V—dry trees.
After the formation of the stand, the vitality state of Scots pine seedlings sharply deteriorated. Eighty years after a fire, dying individuals predominated (Figure 6b), and the vitality state index dropped to 0.14. Subsequently, during the succession, noticeable fluctuations in the vitality state of Scots pine seedlings were recorded (Figure 6c–e). They were reflected in a considerable variation in the value of the vitality state index (Table 4).
In contrast, Siberian spruce seedlings had a rather stable vitality structure with a prevalence of strongly weakened and dying individuals (Figure 6b–e), the total proportion of which ranged from 90% to 99%. As a result, the fluctuations in the vitality state index of Siberian spruce seedlings (Table 4) were insignificant (10–20%).
3.3. Species Composition of the Ground Cover
3.3.1. Dwarf Shrub and Herb Layer
Immediately after a fire, the dwarf shrub and herb layer started to develop rapidly, mainly due to the expansion of Vaccinium myrtillus (Figure 7a). Eight years after a fire, the total cover was 31%, and 15 years after a fire, 33%. Eighty years after a fire, the total cover of the dwarf shrub and herb layer reached its maximum (58%); by 150 years, it decreased to 32%; and in the period up to 380 years after a fire, it did not change significantly, averaging 37% (Figure 7a). Thus, from 150 to ca 400 years after a fire, the total cover of the layer was stable.
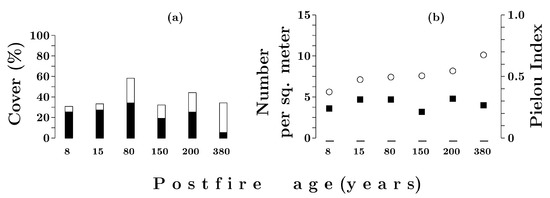
Figure 7.
Total cover (a) of dwarf shrub and herb layer and cover by Vaccinium myrtillus (solid bars). Diversity characteristics (b): solid squares—number of species; open circles—Pielou’s index in forests with different post-fire periods.
In the time interval studied, the number of species in the dwarf shrub and herb layer in an area of 1 m2 varied from 3.2 to 4.8 and averaged 4.0 (Figure 7b). Thus, the recovery of species diversity was already observed by the end of the first decade after a fire. For up to 200 years after a fire, Vaccinium myrtillus dominated the dwarf shrub and herb layer; its cover varied from 20% to 35%, with a maximum at 80 years after a fire. In the late succession community (with a post-fire age of 380 years), its proportion decreased to 6%. In the first 15 years after a fire, the cover of Vaccinium vitis-idaea averaged 2.5% (Table 5). From 80 to 380 years after a fire, it varied from 5% to 10% and averaged 8%. From 8 to 380 years, the cover of Empetrum hermaphroditum—a typical species of boreal forests—gradually increased from 0% to 17%. The cover of herbaceous species (including Avenella flexuosa, Melampyrum pratense) reached its maximum (ca 1.5%) ten years after a fire; after 15 years, it decreased and remained stable at 0.25%. The value of Pielou’s index, which reflects the distribution of species covers, reached the smallest value for the entire period of research (E = 0.4) at a post-fire age of eight years. In the subsequent period, it gradually increased, reaching the maximum value (E = 0.7) at a post-fire age of 380 years.

Table 5.
Cover of dwarf shrub and herb layer species in forests with different post-fire periods.
3.3.2. Moss and Lichen Layer
Eight years after a fire, the total cover of the moss and lichen layer was 9%; the cover of mosses was 7.5%, and lichens, 1.5% (Figure 8a). Pohlia nutans (4%) dominated the layer; lichens were represented mainly by primary thalli (1%) and a crustaceous species Trapeliopsis granulosa (0.3%). Fifteen years after a fire, the total cover was 61%; it was formed mainly by the primary thalli of lichens (37%). The total cover of mosses was 21%. Among bryophytes, Pohlia nutans (12%) still dominated; the total cover of green mosses (Pleurozium schreberi and species of the genus Dicranum) was 4.5%; and the cover of species of the genus Polytrichum was 0.7%. For up to 80 years after a fire, the total coverage of Hepaticae sp. was 0.4% (Table 6).
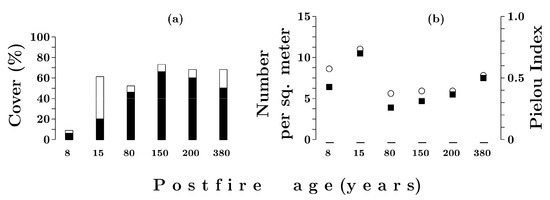
Figure 8.
Total coverage (a) by lichens (open bars) and mosses (solid bars). Diversity characteristics (b): solid squares—number of species; open circles—Pielou’s index in Siberian Spruce–Scots pine forests with different post-fire periods.

Table 6.
Cover of lichens and mosses in Siberian spruce–Scots pine forests with different post-fire periods.
Eighty years after a fire, the cover of the moss and lichen layer decreased to ca 50%. During the period from 150 to 380 years it increased, and then until 380 years after a fire, it remained stable at an average of 70% (Figure 8a).
Eighty years after a fire, Pleurozium schreberi dominated, and its cover reached the maximum (52–55%) at the age post-fire of 150–200 years; in the late succession community, its cover was 38% (Table 6). From 80 to 150 years after a fire, the cover of Hylocomium splendens averaged 7% and then subsequently decreased to 1–2%. From 80 to 380 years, the total cover of species of the genus Dicranum increased from 1% to 7%. The total cover of the Hepaticae species increased from 0.1% to 3%. From 80 to 200 years after a fire, the total cover of lichens was significantly lower than in the previous period, averaging 4% (Figure 8a). Three hundred and eighty years after a fire, their cover increased to 17%. From 80 to 200 years, the cover of lichens of the genus Cladonia was 2.5–3%; in the late succession community, it increased to 16%, mainly due to the expansion of Cladonia rangiferina and C. stellaris (Table 6). The lichen Nephroma arcticum was represented in communities with a post-fire age of 150–380 years and had a cover of 0.5% to 2%.
The average number of epigeous lichens and mosses on an area of 1 m2 at the initial stage of recovery (at the age post-fire of eight years) is 6.4 species, and reaches a maximum of 10.5 species at the post-fire age of 15 years (Figure 8b). At 80–200 years after a fire, the number of species in the moss–lichen layer is reduced to 4.7 and increases in the late-succession forest to 7.5. The distribution of species’ covers at the post-fire age of 15 years has the highest Pielou’s index (E = 0.75), which means a high uniformity of species cover proportions. The moss–lichen cover in forests burned 80–200 years ago is characterized by low Pielou’s index values (E = 0.3–0.4) and the monodominance of green moss Pleurozium schreberi. The index value at the late stage of succession (E = 0.55) indicates a decrease in the Pleurozium schreberi dominance and an increase in the evenness of species coverages.
4. Discussion
The results obtained in the north-taiga Siberian spruce–Scots pine forest revealed differences in the size structure and vital state of the stands of the two main forest-forming species in the first half of succession (up to 200 years after a fire) that smooth out at the late stages of post-fire recovery. Scots pine seedlings appeared on burnt areas 10–20 years earlier than Siberian spruce seedlings and demonstrated a more successful growth and development and a higher vitality in the first decades after a fire. A characteristic feature of Siberian spruce seedlings is a higher sensitivity to the microclimate of open post-fire areas [19,47]; only in rare cases and small numbers do they appear simultaneously with Scots pine [24]. Our study showed that after a fire, the formation of Scots pine stands occurred faster than those of Siberian spruce; around 100 years after a fire, the size range of Scots pine trees was 50% of that in the sub-climax community, while that of Siberian spruce trees was no more than 35%. This caused the competitive suppression of Siberian spruce by Scots pine. The vitality level of post-fire Scots pine stands remained higher throughout the first half of succession. The process of stand differentiation in Scots pine started about 50 years earlier than in Siberian spruce. This led to the appearance of two dimensionally and functionally different (small- and large-sized) groups of trees. The mass extinction of small-sized Scots pine trees in the middle of succession (150–200 years after a fire) and the survival of this fraction among Siberian spruce can be associated not only with a low tolerance to the shading of Scots pine, but also with a more pronounced reaction of this species to root competition [48].
There was a noticeable difference in the dynamics of the size structure of Siberian spruce stands in mixed Siberian spruce–Scots pine and pure Siberian spruce forests. It can be assumed that the low survival rate of small-sized Siberian spruce individuals in pure stands at the middle stage of succession is due to the stronger competitive suppression compared to mixed stands.
An important feature that distinguishes Siberian spruce–Scots pine forests from Siberian spruce forests is the presence of pre-fire Scots pine trees. The most significant consequence is a much earlier (50–100 years after a fire)—compared with Siberian spruce forests—onset of gap dynamics processes (as a result of the death and decay of pre-fire trees). The appearance of small windthrow gaps may be sufficient for the survival of young individuals of a shade-tolerant species such as Siberian spruce, and ensures their ingrowth in the stand [18,26,49,50,51,52]. Many researchers [3,8,21,53,54,55] noted that small- and medium-scale disturbances were a typical and important structure-forming phenomenon, both at the level of individual communities and at the landscape level.
A significant decline in the seed reproduction of coniferous species was recorded in this study in the middle of succession in both forest types. This is probably due to the high coverage of green mosses, exceeding 70%, and the rather thick (from 3.5 to 8 cm) forest litter [56,57], which can inhibit seed germination and the survival of seedlings [49,58]. The seedlings of coniferous species largely depend on the uprooting and decomposed logs created by fallen trees [50,52,56,59,60,61,62] where the green moss layer and forest litter had been disturbed. Spruce seedlings survive in a rather wide range of microhabitats but developed successfully on decaying wood [56,60,62], while Scots pine seedlings are mostly confined to the mineral substrate of uprootings pits and mounts [50]. According to our data on Siberian spruce–Scots pine and Siberian spruce forests 150–200 years after a fire, the number of decaying logs was no more than 7–10 pcs./ha. These ecological features can explain the lower abundance of Scots pine seedlings, which was also noted by other researchers who studied northern forests [61]. The weak regeneration of Scots pine in the middle stages of succession has led many authors to conclude that the natural regeneration of this species depended on periodic fires [48,58,63].
Around 400 years after a fire, the small-sized fraction of trees in the Scots pine stand had been restored and, as in Siberian spruce, had reached 50%. The proportion of seedlings increased to 90% in the populations of both species. A tendency toward a gradual convergence of the diameter distribution curves toward the “reversed J” was clearly exhibited. Intensive gap dynamics led to the appearance of additional microsites favorable for seed germination and seedling growth [3,26,52,53,54,55,60]. In old-growth forests, the windthrow-disturbed area ranges from 4–7% to 10–15% [20,26,59,64]. According to our data, at the late stages of succession in Siberian spruce–Scots pine and Siberian spruce forests, the number of decaying logs was approximately 150–200 pcs./ha, but it could reach 400 pcs./ha [20]. Thus, the conditions for sustainable self-maintenance and a continuous stream of generation of not only Siberian spruce, but also Scots pine, were provided. This indicates the beginning of the formation of the so-called “full-storied” type of structure [65], which reflects the presence in the tree stand of individuals of all dimensions, the absence of pronounced canopies and the numerical predominance of small-sized individuals over large-sized ones. This type of structure is a characteristic feature of undisturbed boreal forest [12,18,21,22,23,25,27,28,66,67,68].
A characteristic feature of the dwarf shrub and herb layer was the rapid (during the first decade after a fire) recovery, mainly due to the vegetative propagation of Vaccinium myrtillus. The highest layer coverage was observed during the period of the maximal proportion of Betula pubescens in the stand (50–80 years after a fire), when, due to the abundance of leaf litter, additional nutrients enriched the upper soil horizons [69,70,71]. Similar dynamics of recovery were observed during the restoration of Siberian spruce forests [72]. The stabilization of the total layer coverage in both mixed Siberian spruce–Scots pine and pure Siberian spruce forests was observed at the same time during the period from 120 to 160 years after a fire. In green moss and green moss–lichen Scots pine forests, on the condition that there was no species change in the stand during succession, the stabilization of the total cover of the dwarf shrub–herb layer occurred earlier than in Siberian spruce–Scots pine and Siberian spruce forests during the period from 60 to 100 years after a fire [72,73].
In the studied post-fire time interval (8–380 years), the number of species in the dwarf shrub and herb layer (3–5 species) did not change much. However, the degree of species coverage evenness changed significantly—it increased and reached its maximum in the late succession community. It is necessary to point out the change in the species structure; 300 years after a fire, there was a decrease in Vaccinium myrtillus dominance and a formation of the cover with the co-domination of three species: Empetrum hermaphroditum, Vaccinium myrtillus and V. vitis-idaea. The polydominant layer structure in Siberian spruce forests, unlike in Siberian spruce–Scots pine forests, was formed immediately after a fire and did not change during the recovery period [72]. Another difference was the increased proportion of herbaceous plants in the initial stage of succession in Siberian spruce forests. This may be explained by the fact that Siberian spruce forests are mainly formed on sandy loams and loams, unlike Siberian spruce–Scots pine forests, which are formed on sands [71]. The final stage of recovery was characterized by a high proportion of Empetrum hermaphroditum in both forest types. The same pattern was also typical for old-growth green moss and green moss–lichen Scots pine forests [72,73].
At the early stage of recovery, the studied forests took up an intermediate place between Siberian spruce and Scots pine forests according to the characteristics of the moss–lichen layer. The mixed forests differed from pure Siberian spruce forests in the increased proportion of lichens, and in a higher proportion of mosses from pure Scots pine forests. Such status of the cover persisted for at least 30 years after a fire [72,73,74]. In the process of succession, the characteristics of the green moss–lichen layer of Siberian spruce–Scots pine forests gradually approached the characteristics of the layer in Siberian spruce forests. That means that we observed a gradual increase in the proportion of green mosses. During the period of maximal participation of birch in the stand (50–80 years after a fire), the cover of the layer decreased due to abundant leaf litter, inhibiting the development of the layer. The stabilization of the total cover of the moss–lichen layer in the studied forests was observed at ca 120 years after a fire.
Both the inhibition of the layer and the stabilization of its cover in Siberian spruce–Scots pine and Siberian spruce forests happened simultaneously. The stabilization of the total cover of the layer in Scots pine forests of the green moss–lichen type takes a shorter period of 60–100 years after a fire due to the earlier stabilization of species structure and basal area of the stand. Pleurozium schreberi absolutely dominated the layer from 80 years after a fire in the studied forests as well as in Scots pine forests of the moss–lichen type, whereas in Siberian spruce forests, it co-dominates with Hylocomium splendens [72].
A maximal number of species and structure of species coverings with a higher evenness in Siberian spruce–Scots pine forests were registered in two periods (15–20 years and ca 300 years after a fire). This was associated with a high diversity of microhabitats, with a disturbed cover that could be populated by mosses and lichens in open post-fire areas and in the late succession community after a transition to gap dynamics.
5. Conclusions
This study has identified some features of the structure and recovery dynamics of Siberian spruce–Scots pine forests, a rare type of community typical of the north-western sector of the European taiga. The parameters of the size, vitality structure of the stands and renewal characteristics of the two forest-forming species Pinus sylvestris and Picea obovata have significant differences, mainly in the first half of succession up to ~200 years after a fire. At the late stage of post-fire recovery (~400 years after fire), the stands’ structures and the regeneration activity of Scots pine and Siberian spruce show a high degree of similarity, which indicates the parity state of the two species and the sustainable development of Siberian spruce–Scots pine forest in the study region. The presence of Scots pine significantly changed the characteristics of the Siberian spruce population at all stages of post-fire succession in the mixed Siberian spruce–Scots pine forest compared to the pure Siberian spruce one.
There were substantial changes in the value of the total cover both in the dwarf shrub–herb and moss–lichen layers within 150 years after a fire. Those changes were closely related to the dynamics of the structure of the tree layer and in particular to the birch proportion. Changes in the species structure were observed during the whole investigated period of 380 years after a fire.
Author Contributions
Conceptualization, N.I.S. and V.V.G.; methodology, N.I.S. and V.V.G.; field data collection, N.I.S., V.V.G., P.N.K. and I.J.B.; data analysis, N.I.S., V.V.G., P.N.K. and I.J.B.; writing—original draft, N.I.S., V.V.G., P.N.K. and I.J.B.; writing—review and editing, N.I.S. and V.V.G. All authors have read and agreed to the published version of the manuscript.
Funding
The work was carried out within the framework of the state task of the Komarov Botanical Institute of the Russian Academy of Sciences (the planned theme №AAAA-A19-119030690058-2).
Acknowledgments
The authors are grateful to anonymous reviewers for their valuable comments and suggestions that helped to improve the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Esseen, P.-A.; Ehnström, B.; Ericson, L.; Sjöberg, K. Boreal forests. Ecol. Bull. 1997, 46, 16–47. [Google Scholar]
- Gauthier, S.; Bernier, P.; Kuuluvainen, T.; Shvidenko, A.Z.; Schepaschenko, D.G. Boreal forest health and global change. Science 2015, 349, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Kuuluvainen, T.; Wallenius, T.H.; Kauhanen, H.; Aakala, T.; Mikkola, K.; Demidova, N.; Ogibin, B. Episodic, patchy disturbances characterize an old-growth Picea abies dominated forest landscape in northeastern Europe. For. Ecol. Manag. 2014, 320, 96–103. [Google Scholar] [CrossRef]
- Kuuluvainen, T. Forest management and biodiversity conservation based on natural ecosystem dynamics in northern Europe: The complexity challenge. AMBIO 2009, 38, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Kuuluvainen, T.; Gauthier, S. Young and old forest in the boreal: Critical stages of ecosystem dynamics and management under global change. For. Ecosyst. 2018, 5, 26. [Google Scholar] [CrossRef]
- Zackrisson, O. The influence of forest fires in the North Swedish boreal forest. Oikos 1977, 29, 22–32. [Google Scholar] [CrossRef]
- Angelstam, P.; Andersson, L. Estimates of the needs for forest reserves in Sweden. Scand. J. For. Res. Suppl. 2001, 3, 38–51. [Google Scholar] [CrossRef]
- Gromtsev, A. Natural disturbance dynamics in the boreal forests of European Russia: A review. Silva Fenn. 2002, 36, 41–55. [Google Scholar] [CrossRef]
- Wallenius, T.H.; Kauhanen, H.H.; Herva, H.; Pennanen, J. Long fire cycle in northern boreal Pinus forests in Finnish Lapland. Can. J. For. Res. 2010, 40, 2027–2035. [Google Scholar] [CrossRef]
- Goldammer, J.G.; Furyaev, V.V. (Eds.) Fire in Ecosystems of Boreal Eurasia; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; 524p. [Google Scholar] [CrossRef]
- Korovin, G.N. Analysis of the distribution of forest fires in Russia. In Fire in Ecosystems of Boreal Eurasia; Goldammer, J.G., Furyaev, V.V., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 112–128. [Google Scholar]
- Linder, P.; Elfving, B.; Zackrisson, O. Stand structure and successional trends in virgin boreal forest reserves in Sweden. For. Ecol. Manag. 1997, 98, 17–33. [Google Scholar] [CrossRef]
- Boulanger, Y.; Gauthier, S.; Burton, P.J. A refinement of models projecting future Canadian fire regimes using homogeneous fire regime zones. Can. J. For. Res. 2014, 44, 365–376. [Google Scholar] [CrossRef]
- Harper, K.A.; Bergeron, Y.; Gauthier, S.; Drapeau, P. Post-fire development of canopy structure and composition in black spruce forests of Abitibi, Québec: A landscape scale study. Silva Fenn. 2002, 36, 249–263. [Google Scholar] [CrossRef]
- Auvinen, A.-P.; Hildén, M.; Toivonen, H.; Primmer, E.; Niemelä, J.; Aapala, K.; Bäck, S.; Härmä, P.; Ikävalko, J.; Järvenpää, E.; et al. Evaluation of the Finnish National Biodiversity Action Plan 1997–2005. Monographs of Boreal Environment Research; Finnish Environment Institute: Helsinki, Finland, 2007; pp. 1–55. [Google Scholar]
- Bergeron, Y.; Fent, N.J. Boreal forests of eastern Canada revisited: Old growth, nonfire disturbances, forest succession, and biodiversity. Botany 2012, 90, 509–523. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Siitonen, J. Fennoscandian boreal forests as complex adaptive systems. Properties, management challenges and opportunities. In Managing Forests as Complex Adaptive Systems. Building Resilience to the Challenge of Global Change; Messier, C., Puettmaan, K.J., Coates, K.D., Eds.; Earthscan: New York, NY, USA, 2013; pp. 244–268. [Google Scholar]
- Stavrova, N.I.; Gorshkov, V.V.; Katyutin, P.N. Structure formation of forest tree species coenopopulations during post-fire recovery of northern taiga forest. Trans. Karelian Res. Cent. Russ. Acad. Sci. Ser. Biogeogr. 2016, 3, 10–28, (In Russian with English Summary). [Google Scholar]
- Korchagin, A.A. The impact of fires on vegetation and vegetation recovery after fires in the European North. Proc. Bot. Inst. USSR Acad. Sci. Ser. III 1954, 9, 75–149. (In Russian) [Google Scholar]
- Sirén, G. The development of spruce forest on raw humus sites in Northern Finland and its ecology. Acta For. Fenn. 1955, 62. [Google Scholar] [CrossRef]
- Dyrenkov, S.A. Structure and Dynamics of the Boreal Spruce Forest; Nauka: Leningrad, USSR, 1984; 174p. (In Russian) [Google Scholar]
- Steijlen, I.; Zackrisson, O. Long-term regeneration dynamics and successional trends in northern Swedish coniferous forest stand. Can. J. Bot. 1987, 65, 839–848. [Google Scholar] [CrossRef]
- Ågren, J.; Zackrisson, O. Age and size structure of Pinus sylvestris populations on mires in Central and Northern Sweden. J. Ecol. 1990, 78, 1049–1062. [Google Scholar] [CrossRef]
- Engelmark, O. Early post-fire tree regeneration in a Picea-Vaccinium forest in northern Sweden. J. Veg. Sci. 1993, 4, 791–794. [Google Scholar] [CrossRef]
- Lähde, E.; Laiho, O.; Norokorpi, Y.; Saksa, T. Structure and yield of all-sized and even-sized conifer-dominated stands on fertile sites. Ann. For. Sci. 1994, 51, 97–109. [Google Scholar] [CrossRef]
- Hörnberg, G.; Ohlson, M.; Zackrisson, O. Stand dynamics, regeneration patterns and long-term continuity in boreal old-growth Picea abies swamp-forests. J. Veg. Sci. 1995, 6, 291–298. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Syrjänen, K.; Kalliola, R. Structure of a pristine Picea abies forest in northeastern Europe. J. Veg. Sci. 1998, 9, 563–574. [Google Scholar] [CrossRef]
- Volkov, A.D. Comparative assessment of the ecological role and biological specificity of indigenous and derived forests in the North-West of the taiga zone of Russia. In Proceedings of the Primary Forests of the Taiga Zone of Europe: Current State and Conservation Problems: Mater, International Scientific-Practical Conference, Petrozavodsk, Russia, 6–8 July 1999; pp. 9–16. (In Russian). [Google Scholar]
- Kuuluvainen, T.; Mäki, J.; Karjalainen, L.; Lehtonen, H. Tree age distributions in old-growth forest sites in Vienansalo wilderness, eastern Fennoscandia. Silva Fenn. 2002, 36, 169–184. [Google Scholar] [CrossRef]
- Regel, K. Die Pflanzendecke der Halbinsel Kola. Memories de la Faculte des Sciences de L’Universite de Lithuanie. Tail II. Lapponia Ponoensis und Lapponia Imandrae; L′Universite de Lithuanie: Kaunas, Lithuania, 1927; pp. 164–293. [Google Scholar]
- Nekrasova, T.P. The relationship of pine and spruce in the forests of the Kola Peninsula. In Forests of the Kola Peninsula and Their Renewal; USSR Academy of Sciences: Moscow, USSR, 1961; pp. 63–70. (In Russian) [Google Scholar]
- Nicklasson, M.; Hörnberg, G.; Zackrisson, O. Age structure and disturbance history in boreal Pinus-Picea forest, N. Sweden; Department of Forest Vegetation Ecolology, Swedish University of Agricultural Sciences: Umeaa, Sweden, 1998; 23p. [Google Scholar]
- Stavrova, N.I.; Gorshkov, V.V.; Mishko, A.E. Ontogenesis of Siberian spruce (Picea obovata Ledeb.) in North taiga shrub-green-moss pine-spruce forests. Bot. J. 2017, 102, 163–185, (In Russian with English Summary). [Google Scholar]
- Bebiya, S.M. Differentiation of trees in the forest, their classification and determination of the life state of stands. Lesovedenie 2000, 4, 35–43, (In Russian with English Summary). [Google Scholar]
- Torlopova, N.V.; Ilchukov, S.V. Vital state of native pine forests at the Pechora-Ilych biosphere reserve. Lesovedenie 2003, 3, 34–40, (In Russian with English Summary). [Google Scholar]
- Stavrova, N.I.; Gorshkov, V.V.; Katyutin, P.N. Dynamics of vitality structure of Picea obovata and Betula pubescens coenopopulations during post fire succession in northern taiga spruce forest. Botanicheskii Zhurnal 2003, 95, 1550–1565, (In Russian with English Summary). [Google Scholar]
- World Reference Base for Soil Resources 2014, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Report No. 106; Food and Agriculture Organization: Rome, Italy, 2015.
- Pereverzev, V.N. Forest Soils of the Kola Peninsula; Nauka: Moscow, Russia, 2004; 232p, (In Russian with English Summary). [Google Scholar]
- Storozhenko, V.G. Dating of decomposition of fir windfall. Ekologiya 1990, 6, 66–69, (In Russian with English Summary). [Google Scholar]
- Alekseev, V.A. Some problems of diagnosis and classification of forest ecosystems damaged by air pollution. In Forest Ecosystems and Atmospheric Pollution; Alekseev, V.A., Ed.; Nauka: Leningrad, USSR, 1990; pp. 38–54. [Google Scholar]
- Yarmishko, V.T.; Gorshkov, V.V.; Stavrova, N.I. Vitality structure of Pinus sylvestris L. in forest communities with varying degrees and types of anthropogenic disturbance. Plant Resour. 2003, 39, 1–19, (In Russian with English Summary). [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing Company: Oxford, UK, 2004; 256p. [Google Scholar]
- Bailey, R.L.; Dell, T.R. Quantifying diameter distributions with Weibull function. For. Sci. 1973, 19, 97–104. [Google Scholar]
- Maltamo, M.; Kangasb, A.; Uutterac, J.; Torniainenc, T.; Saramäki, J. Comparison of percentile based prediction methods and the Weibull distribution in describing the diameter distribution of heterogeneous Scots pine stands. For. Ecol. Manag. 2000, 133, 263–274. [Google Scholar] [CrossRef]
- Mulverhill, C.; Coops, N.C.; White, J.C.; Tompalski, P.; Marshall, P.L.; Bailey, T. Enhancing the estimation of stem-size distributions for unimodal and bimodal stands in a boreal mixedwood forest with airborne laser scanning data. Forests 2018, 9, 95. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, L.; Mulverhill, C.; Liu, H.; Pang, Y.; Li, Z. Prediction of Diameter Distributions with Multimodal Models Using LiDAR Data in Subtropical Planted Forests. Forests 2019, 10, 125. [Google Scholar] [CrossRef]
- Melekhov, I.S. The Impact of Fires on Forest; State Forestry Publishing House: Moscow: Leningrad, Russia, 1948; 126p. (In Russian) [Google Scholar]
- Sannikov, S.N.; Sannikova, N.S.; Petrova, I.V. Outlines of Theory of Forest Populational Biology; Ural Branch of the Russian Academy of Sciences: Ekaterinburg, Russia, 2012; 272p, (In Russian with English Summary). [Google Scholar]
- Steijlen, I.; Nilsson, M.-C.; Zackrisson, O. Seed regeneration of Scots pine in boreal forests stands dominated by lichen and feather moss. Can. J. For. Res. 1995, 25, 713–723. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Juntunen, P. Propeties and importance of tree regeneration microhabitats in a small windthrow gap in a boreal Pinus sylvestris dominated forest. J. Veg. Sci. 1998, 9, 551–562. [Google Scholar] [CrossRef]
- Nguyen-Xuan, T.; Bergeron, Y.; Simard, D.; Fyles, J.W.; Paré, D. The importance of forest floor disturbance in the early regeneration patterns of the boreal forest of western and Central Quebec: A wildfire versus logging comparison. Can. J. For. Res. 2000, 30, 1353–1364. [Google Scholar] [CrossRef]
- Grenfell, R.; Aakala, T.; Kuuluvainen, T. Microsite occupancy and the spatial structure of understorey regeneration in three late-successional Norway spruce forests in northern Europe. Silva Fenn. 2011, 45, 1093–1110. [Google Scholar] [CrossRef]
- Leemans, R. Canopy gaps and establishment patterns of spruce (Picea abies L. Karst.) in two old-growth coniferous forests in central Sweden. Vegetatio 1991, 93, 157–165. [Google Scholar] [CrossRef]
- Korotkov, V.N.; Morozov, A.S.; Yaroshenko, A.Y. Mosaic organization and spontaneous dynamics of quasiclimax boreal forests. In Eastern European Forests: History in the Holocene and Modernity; Smirnova, O.V., Ed.; Nauka: Moscow, Russia, 2004; Volume 2, pp. 330–346. (In Russian) [Google Scholar]
- Smirnova, O.V.; Toropova, N.A. Succession and climax as the ecosystem process. Biol. Bull. Rev. 2008, 128, 129–144, (In Russian with English Summary). [Google Scholar]
- Kuuluvainen, T. Gap disturbance, ground microtopography, and the regeneration dynamics of boreal forests in Finland: A review. Ann. Zool. Fenn. 1994, 31, 35–51. [Google Scholar]
- Stavrova, N.I.; Kalimova, I.B.; Gorshkov, V.V.; Drozdova, I.V.; Alekseeva-Popova, N.V.; Bakkal, I.Y. Long-term postfire changes of soil characteristics in dark coniferous forests of the European North. Eurasian Soil Sci. 2019, 52, 218–227. [Google Scholar] [CrossRef]
- Sannikov, S.N.; Sannikova, N.S. Ecology of Natural Regeneration of Pine under the Forest Canopy; Gorchakovsky, P.L., Ed.; Nauka: Moscow, USSR, 1985; 149p, (In Russian with English Summary). [Google Scholar]
- Hofgaard, A. 50 years of change in a Swedish boreal old-growth Picea abies forests. J. Veg. Sci. 1993, 4, 773–782. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Kalmari, R. Regeneration microsites of Picea abies seedlings in a windthrow area of a boreal old-growth forests in Finland. Ann. Bot. Fenn. 2003, 40, 401–413. [Google Scholar]
- Juntunen, V.; Neuvonen, S. Natural regeneration of Scots pine and Norway spruce close to the timberline in northern Finland. Silva Fenn. 2006, 40, 443–458. [Google Scholar] [CrossRef]
- Zielonka, T. When does dead wood turn into a substrate for spruce replacement? J. Veg. Sci. 2006, 17, 739–746. [Google Scholar] [CrossRef]
- Sannikov, S.N.; Sannikova, N.S.; Petrova, I.V. Natural Forest Regeneration in Western Siberia (Ecological-Geograpfical Essay); Ural Branch of the Russian Academy of Sciences: Ekaterinburg, Russia, 2004; 198p, (In Russian with English Summary). [Google Scholar]
- Ulanova, N.G. The effect of windthrow on forests at different spatial scales: A review. For. Ecol. Manag. 2000, 135, 155–167. [Google Scholar] [CrossRef]
- Ahlström, M.A.; Lundqvist, L. Stand development during 16–57 years in partially harvested sub-alpine uneven-aged Norway spruce stands reconstructed from increment cores. For. Ecol. Manag. 2015, 350, 81–86. [Google Scholar] [CrossRef]
- Hett, J.M.; Loucks, O.L. Age structure of balsam fir and eastern hemlock. J. Ecol. 1976, 64, 1029–1044. [Google Scholar] [CrossRef]
- McCarthy, J.W.; Weetman, G. Age and size structure of gap dynamic, old-growth boreal forest stand in Newfoundland. Silva Fenn. 2006, 40, 209–230. [Google Scholar] [CrossRef]
- Lundqvist, L. Tamm Review: Selection system reduces long-term volume growth in Fennoscandic uneven-aged Norway spruce forests. For. Ecol. Manag. 2017, 391, 362–375. [Google Scholar] [CrossRef]
- Ushakova, G.I. Biogeocenotic features of spruce and birch trees in rare-coniferous spruce forests of the Far North and their role in migration of elements. In Fertility of Soil and Productivity of Phytocenoses; KSC of the USSR Academy of Sciences: Apatity, USSR, 1991; pp. 55–72. (In Russian) [Google Scholar]
- Orlova, M.A.; Lukina, N.V.; Smirnov, V.E.; Isaeva, L.G. Fertility of soils of birch forests at the northern margin of their distribution. Pochvovedenie 2014, 3, 327–339. [Google Scholar]
- Pereverzev, V.N. Soil Formation on Loose and Crystalline Rocks in Northern Fennoscandia; Kola Scientific Center RAS: Apatity, Russia, 2013; 158p. (In Russian) [Google Scholar]
- Gorshkov, V.V.; Bakkal, I.J. Recovery of the ground layer in spruce (Picea obovata Ledeb.) forests. In Dynamics of Forest Communities in the North-West of Russia; Yarmishko, V.T., Ed.; VVM: St.-Petersburg, Russia, 2009; pp. 197–204. (In Russian) [Google Scholar]
- Gorshkov, V.V.; Bakkal, I.J. Features of post-fire recovery dynamics of communities with domination of lichens. News Samara Sci. Cent. RAS 2012, 14, 1223–1227. (In Russian) [Google Scholar]
- Pushkina, N.M. Regeneration of vegetation on forest fire areas. Works Lapland State Reserve 1960, IV, 5–124, (In Russian with English Summary). [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).