Temporal Changes in Community Structure over a 5-Year Successional Stage in a Subtropical Forest
Abstract
1. Introduction
2. Materials and Methods
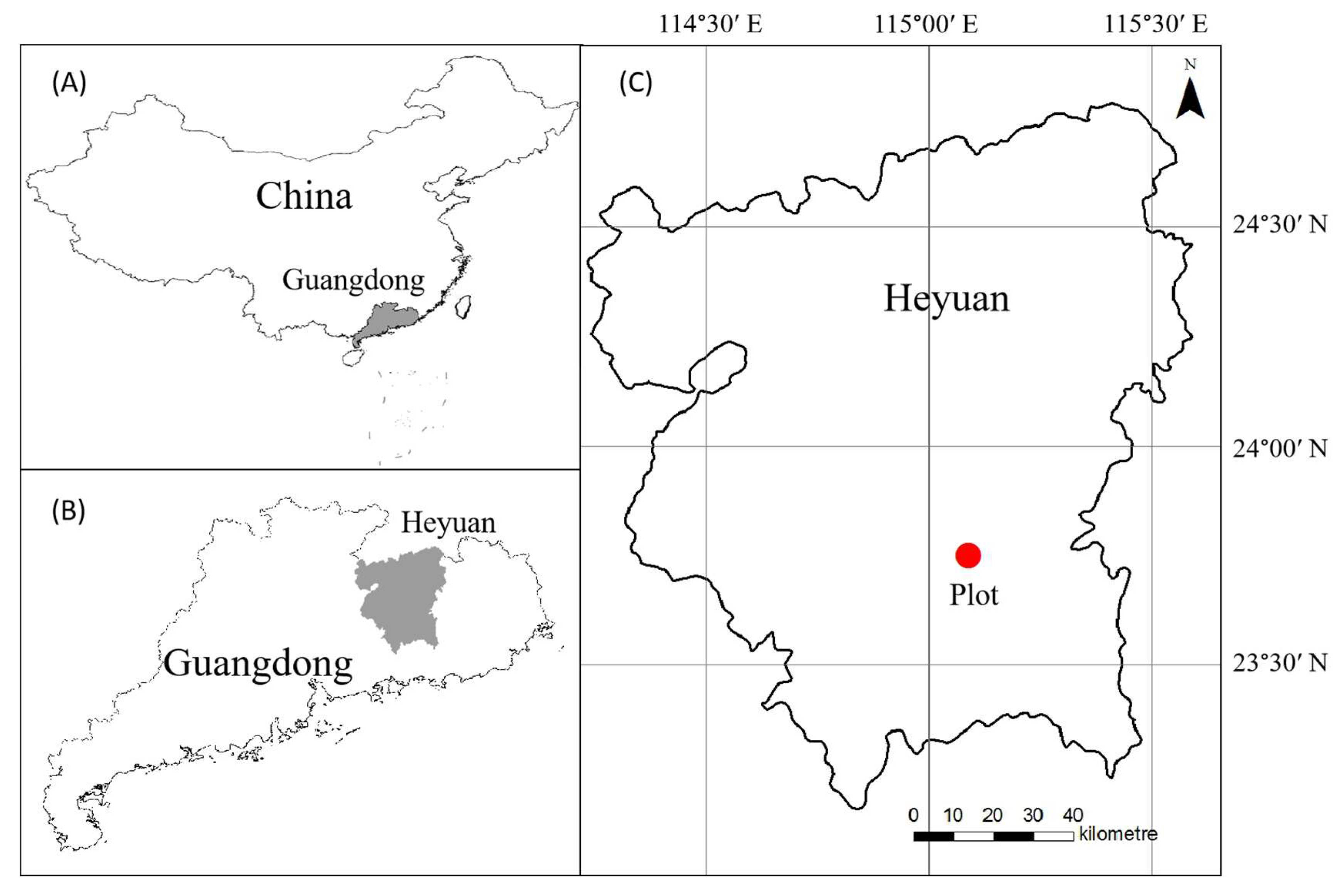
2.1. Study Area
2.2. Sampling Design
2.3. Data Collection and Measurements
2.4. Data Analysis
3. Results
3.1. Characteristics of DBH Classes during Forest Succession
3.2. Plot-Based Differences in Community Characteristics during Forest Succession
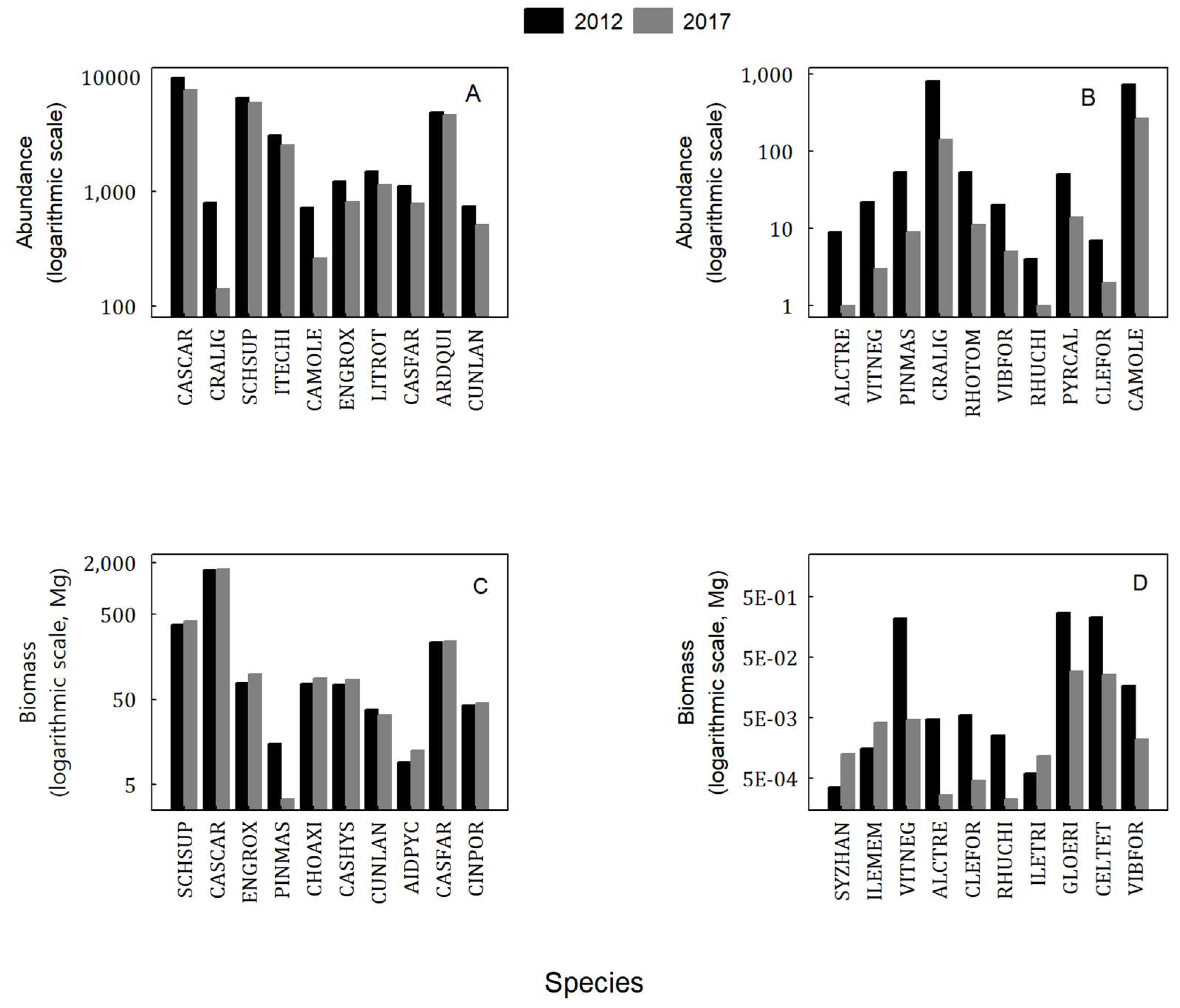
3.3. Changes in Indicator Species in Different DBH Classes during Forest Succession
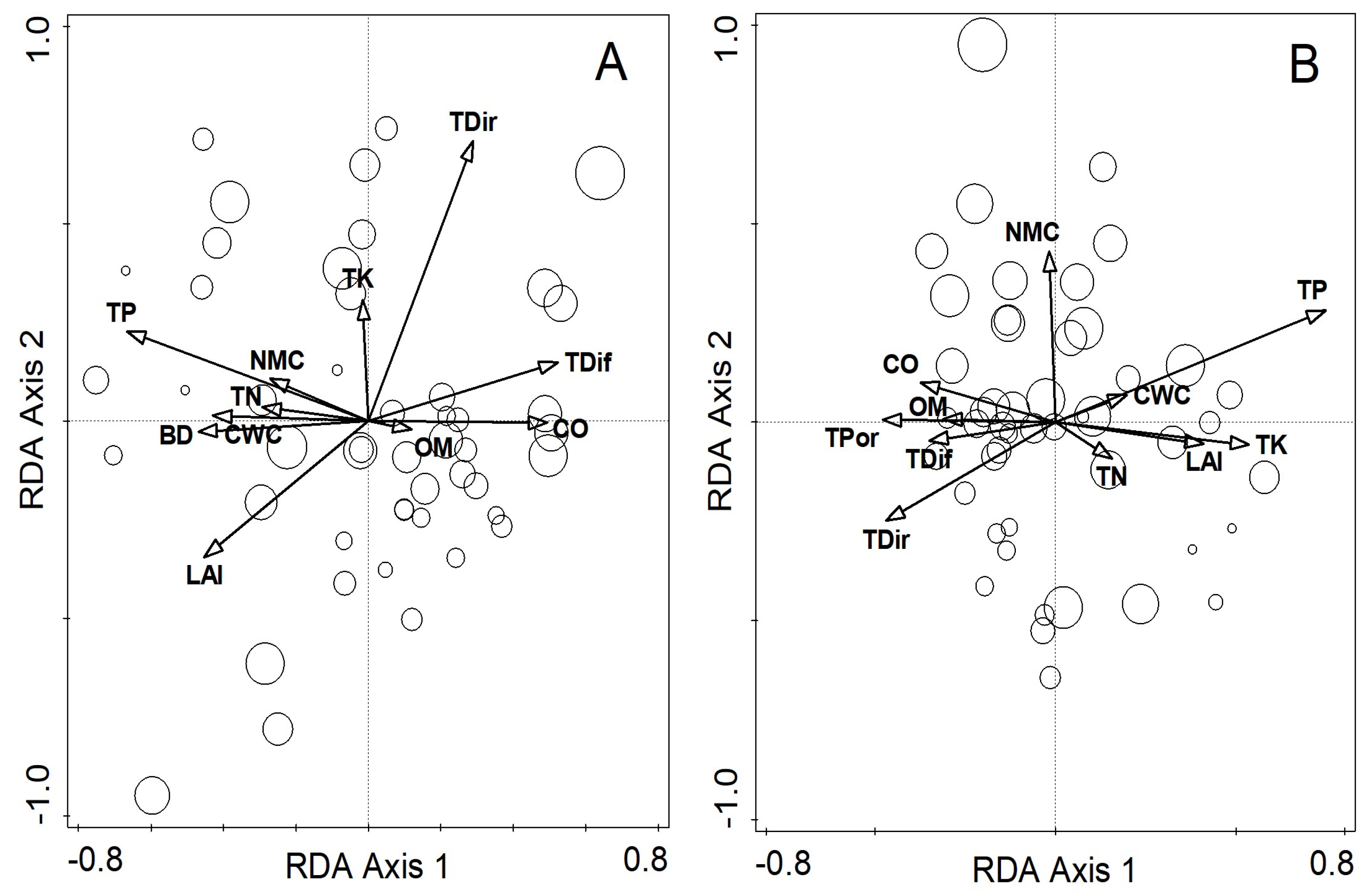
3.4. Major Drivers for Community Succession
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, G.Y.; Peng, C.H.; Li, Y.L.; Liu, S.Z.; Zhang, Q.M.; Tang, X.L.; Liu, J.X.; Yan, J.H.; Zhang, D.Q.; Chu, G.W. A climate change-induced threat to the ecological resilience of a subtropical monsoon evergreen broad-leaved forest in Southern China. Glob. Chang. Biol. 2013, 19, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Labrecque-Foy, J.-P.; Morin, H.; Girona, M.M. Dynamics of territorial occupation by North American beavers in Canadian boreal forests: A novel dendroecological approach. Forests 2020, 11, 221. [Google Scholar] [CrossRef]
- Lavoie, J.; Girona, M.M.; Morin, H. Vulnerability of conifer regeneration to spruce budworm outbreaks in the Eastern Canadian boreal forest. Forests 2019, 10, 14. [Google Scholar] [CrossRef]
- Girona, M.M.; Morin, H.; Lussier, J.M.; Ruel, J.C. Post-cutting mortality following experimental silvicultural treatments in unmanaged boreal forest stsands. Front. For. Glob. Chang 2019, 2. [Google Scholar] [CrossRef]
- Navarro, L.; Morin, H.; Bergeron, Y.; Girona, M.M. Changes in spatiotemporal patterns of 20th century spruce budworm outbreaks in eastern Canadian boreal forests. Front. Plant Sci. 2018, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Montoro Girona, M.; Lussier, J.M.; Morin, H.; Thiffault, N. Conifer regeneration after experimental shelterwood and seed-tree treatments in boreal forests: Finding silvicultural alternatives. Front. Plant Sci. 2018, 9, 1145. [Google Scholar] [CrossRef] [PubMed]
- Bretfeld, M.; Ewers, B.E.; Hall, J.S. Plant water use responses along secondary forest succession during the 2015–2016 El Niño drought in Panama. New Phytol. 2018, 219, 885–899. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef]
- Song, L.; Liu, W.Y.; Nadkarni, N.M. Response of non-vascular epiphytes to simulated climate change in a montane moist evergreen broad-leaved forest in southwest China. Biol. Conserv. 2012, 152, 127–135. [Google Scholar] [CrossRef]
- Nakao, K.; Matsui, T.; Horikawa, M.; Tsuyama, I.; Tanaka, N. Assessing the impact of land use and climate change on the evergreen broad-leaved species of Quercus acuta in Japan. Plant Ecol. 2011, 212, 229–243. [Google Scholar] [CrossRef]
- Yang, X.D.; Yan, E.R.; Chang, S.X.; Da, L.J.; Wang, X.H. Tree architecture varies with forest succession in evergreen broad-leaved forests in Eastern China. Trees 2015, 29, 43–57. [Google Scholar] [CrossRef]
- Michalski, S.G.; Bruelheide, H.; Durka, W. Phylogenetic turnover during subtropical forest succession across environmental and phylogenetic scales. Ecol. Evol. 2017, 7, 11079–11091. [Google Scholar] [PubMed]
- Kreutz, A.; Aakala, T.; Grenfell, R.; Kuuluvainen, T. Spatial tree community structure in three stands across a forest succession gradient in northern boreal Fennoscandia. Silva. Fenn. 2015, 49, 1–17. [Google Scholar] [CrossRef]
- Moroni, M.T.; Musk, R.; Wardlaw, T.J. Forest succession where trees become smaller and wood carbon stocks reduce. For. Ecol. Manag. 2017, 393, 74–80. [Google Scholar] [CrossRef]
- Laflower, D.M.; Hurteau, M.D.; Koch, G.W.; North, M.P.; Hungate, B.A. Climate-driven changes in forest succession and the influence of management on forest carbon dynamics in the Puget Lowlands of Washington State, USA. For. Ecol. Manag. 2016, 362, 194–204. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Guo, C.Z.; Ni, J. Dynamics of major forest vegetations in Tiantong National Forest Park during the last 30 years. Chin. J. Appl. Ecol. 2014, 25, 1547–1554. [Google Scholar]
- Ali, A.; Ma, W.J.; Yang, X.D.; Sun, B.W.; Xu, M.S. Biomass and carbon stocks in Schima superba dominated subtropical forests of eastern China. J. For. Sci. 2014, 60, 198–207. [Google Scholar] [CrossRef]
- Yang, T.H.; Song, K.; Da, L.J.; Li, X.P.; Wu, J.P. The biomass and aboveground net primary productivity of Schima superba-Castanopsis carlesii forests in east China. Sci. China Life Sci. 2010, 53, 811–821. [Google Scholar] [CrossRef]
- Taylor, B.N.; Patterson, A.E.; Ajayi, M.; Arkebauer, R.; Bao, K.; Bray, N.; Elliott, R.M.; Gauthier, P.P.G.; Gersony, J.; Gibson, R. Growth and physiology of a dominant understory shrub, Hamamelis virginiana, following canopy disturbance in a temperate hardwood forest. Can J. For. Res. 2016, 47, 193–202. [Google Scholar] [CrossRef]
- Rojas, A.M.Q.; Schwarzkopf, T.; García, C.; Rico, M.J. Light environment in the understory of an Andean cloud forest: Canopy structure and climatic seasonality. Rev. Biol. Trop. 2016, 64, 1699–1707. [Google Scholar]
- Gendreau-Berthiaume, B.; Macdonald, S.E.; Stadt, J.J.; Hnatiuk, R.J. How dynamic are understory communities and the processes structuring them in mature conifer forests? Ecosphere 2015, 6, 1–49. [Google Scholar] [CrossRef]
- McKenzie, D.; Halpern, C.B.; Nelson, C.R. Overstory influences on herb and shrub communities in mature forests of western Washington, U.S.A. Can J. For. Res. 2000, 30, 1655–1666. [Google Scholar] [CrossRef]
- Su, X.P.; Wang, M.H.; Huang, Z.Q.; Fu, S.L.; Chen, H.Y.H. Forest Understorey Vegetation: Colonization and the Availability and Heterogeneity of Resources. Forests 2019, 10, 944. [Google Scholar] [CrossRef]
- Pallardy, S.G. Physiology of Woody Plants; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Cano, F.J.; Sánchez-Gómez, D.; Gascó, A.; Rodriguez-Calcerrada, J.; Gil, L.; Warren, C.R.; Aranda, I. Light acclimation at the end of the growing season in two broadleaved oak species. Photosynthetica 2011, 49, 581–592. [Google Scholar] [CrossRef]
- Lhotka, J.M.; Loewenstein, E.F. Influence of canopy structure on the survival and growth of underplanted seedlings. New Forests 2008, 35, 89–104. [Google Scholar] [CrossRef]
- Wang, D.P.; Ji, S.Y.; Chen, F.P.; Xing, F.W.; Peng, S.L. Diversity and relationship with succession of naturally regenerated southern subtropical forests in Shenzhen, China and its comparison with the zonal climax of Hong Kong. For. Ecol. Manag. 2006, 222, 384–390. [Google Scholar] [CrossRef]
- Sarker, S.K.; Rashid, S.; Sharmin, M.; Haque, M.M.; Sonet, S.S.; Nur-Un-Nabi, M. Environmental correlates of vegetation distribution in tropical Juri forest, Bangladesh. Trop. Ecol. 2014, 55, 177–193. [Google Scholar]
- Thuiller, W. On the importance of edaphic variables to predict plant species distributions–limits and prospects. J. Veg. Sci. 2013, 24, 591–592. [Google Scholar] [CrossRef]
- Rubio, A.; Escudero, A. Small-scale spatial soil-plant relationship in semi-arid gypsum environments. Plant Soil 2000, 220, 139–150. [Google Scholar] [CrossRef]
- Lasky, J.R.; Uriarte, M.; Boukili, V.K.; Erickson, D.L.; John Kress, W.; Chazdon, R.L.; Vila, M. The relationship between tree biodiversity and biomass dynamics changes with tropical forest succession. Ecol. Lett. 2014, 17, 1158–1167. [Google Scholar] [CrossRef]
- Wang, Q.G.; Xing, Y.J.; Zhou, X.F.; Han, S.J. Relationship between diversity of forest plant and community dynamics in eastern mountain area of Heilongjiang Province, China. J. For. Res. 2006, 17, 289–292. [Google Scholar] [CrossRef]
- Girona, M.M.; Rossi, S.; Lussier, J.M.; Walsh, D.; Morin, H. Understanding tree growth responses after partial cuttings: A new approach. PLoS ONE 2017, 12, e0172653. [Google Scholar]
- Rozendaal, D.M.; Chazdon, R.L. Demographic drivers of tree biomass change during secondary succession in northeastern Costa Rica. Ecol. Appl. 2015, 25, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.L.; Hou, Y.P.; Chen, B.M. Establishment of Markov successional model and its application for forest restoration reference in Southern China. Ecol. Model. 2010, 221, 1317–1324. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Su, Z.Y.; Li, W.B.; Li, J.P.; Ke, X.D. Influence of tree species composition and community structure on carbon density in a subtropical forest. PLoS ONE 2015, 10, e0136984. [Google Scholar] [CrossRef] [PubMed]
- HU, Y.Q.; LI, W.B.; CUI, J.Y.; SU, Z.Y. Spatial point patterns of dominant species by individual trees and biomass in a subtropical evergreen broad-leaved forest. Acta Ecol. Sin. 2016, 36, 1066–1072. [Google Scholar]
- Condit, R. Tropical Forest Census Plots: Methods and Results from Barro Colorado Island, Panama and a Comparison with Other Plots; Springer-Verlag: New York, NY, USA, 1998. [Google Scholar]
- Li, J.P.; Xu, M.F.; Su, Z.Y.; Sun, Y.D.; Hu, Y.Q. Soil fertility quality assessment under different vegetation restoration patterns. Acta Ecol. Sin. 2014, 34, 2297–2307. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; Agriculture Press: Beijing, China, 2005. [Google Scholar]
- Beaudet, M.; Messier, C. Variation in canopy openness and light transmission following selection cutting in northern hardwood stands: An assessment based on hemispherical photographs. Agric. For. Meteorol. 2002, 110, 217–228. [Google Scholar] [CrossRef]
- Frazer, G.W.; Canham, C.D.; Lertzman, K.P. Gap Light Analyzer (GLA), Version 2.0: Imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, users manual and program documentation. In Simon Fraser University, Burnaby, British Columbia, and the Institute of Ecosystem Studies; Millbrook: New York, NY, USA, 1999; Volume 36. [Google Scholar]
- Guangdong Provincial Forestry Bureau. Guangdong Academy of Forest Inventory and Planning. Common Forest Tree Volume Tables for Forest Inventory in Guangdong Province; Guangdong Provincial Forestry Bureau: Guangzhou, China, 2009.
- Li, J.L.; Shi, Z.G. Methodologies for Forestry Carbon Sequestration Projects; China Forestry Publishing House: Beijing, China, 2016. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- He, S.Y.; Zhong, Y.L.; Sun, Y.D.; Su, Z.Y.; Jia, X.R.; Hu, Y.Q.; Zhou, Q. Topography-associated thermal gradient predicts warming effects on woody plant structural diversity in a subtropical forest. Sci. Rep. 2017, 7, 40387. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and User’s Guide: Software for Ordination, 5th ed.; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Zhang, R.; Yang, H.; Zhou, Z.; Shen, B.; Xiao, J.; Wang, B. A high-density genetic map of Schima superba based on its chromosomal characteristics. BMC Plant Biol. 2019, 19, 41. [Google Scholar] [CrossRef]
- Webb, C.O.; Gilbert, G.S.; Donoghue, M.J. Phylodiversity-dependent seedling mortality, size structure, and disease in a Bornean rain forest. Ecology 2006, 87, S123–S131. [Google Scholar] [CrossRef]
- Zhu, Y.; Mi, X.C.; Ren, H.B.; Ma, K.P. Density dependence is prevalent in a heterogeneous subtropical forest. Oikos 2010, 119, 109–119. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Chazdon, R.L. Light gradient partitioning by tropical tree seedlings in the absence of canopy gaps. Oecologia 2002, 131, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kruger, L.M.; Midgley, J.J. The influence of resprouting forest canopy species on richness in southern Cape forests, South Africa. Glob. Ecol. Biogeogr. 2001, 10, 567–572. [Google Scholar] [CrossRef]
- Overman, A.R.; Scholtz, R.V. Accumulation of biomass and mineral elements with calendar time by cotton: Application of the expanded growth model. PLoS ONE 2013, 8, e72810. [Google Scholar] [CrossRef]
- Zheng, D.X.; Cai, Y.X.; Yang, Y.F.; Zheng, Z.Q.; Miao, S.H.; Wu, W.B. Stoichiometric characteristics of C, N and P in the dominant species sapling organs of Castanopsis fissa natural forest in northern Fujian. For. Res. 2017, 30, 154–159. [Google Scholar]
- Vandermeer, J.; de la Cerda, I.G. Height dynamics of the thinning canopy of a tropical rain forest: 14 years of succession in a post-hurricane forest in Nicaragua. For. Ecol. Manag. 2004, 199, 125–135. [Google Scholar] [CrossRef]
- Grime, J.P. Plant strategy theories: A comment on Craine (2005). J. Ecol. 2007, 95, 227–230. [Google Scholar] [CrossRef]
- Grime, J.P. Vegetation classification by reference to strategies. Nature 1974, 250, 26–31. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, J.T. Individual and modular biomass of Lespedeza bicolor Turcz. populations in western mountain areas of Beijing. Chin. Bull. Bot. 2007, 24, 581–589. [Google Scholar]
- Zhang, J.T. Quantitative Ecology, 3rd ed.; Science Press: Beijing, China, 2018. [Google Scholar]
- Pulsford, S.A.; Lindenmayer, D.B.; Driscoll, D.A. A succession of theories: Purging redundancy from disturbance theory. Biol. Rev. 2016, 91, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Egler, F.E. Vegetation science concepts I. Initial floristic composition, a factor in old-field vegetation development. Vegetatio 1954, 4, 412–417. [Google Scholar] [CrossRef]
- Benjamin, O.K.; Stephen, G.P. Forty-eight years of forest succession: Tree species change across four forest types in Mid-Missouri. Forests 2018, 9, 633. [Google Scholar]
- Satdichanh, M.; Ma, H.X.; Yan, K.; Dossa, G.G.O.; Winowiecki, L.; Vågen, T.G.; Gassner, A.; Xu, J.C.; Harrison, R.D.; Swenson, N. Phylogenetic diversity correlated with above-ground biomass production during forest succession: Evidence from tropical forests in Southeast Asia. J. Ecol. 2018, 107, 1419–1432. [Google Scholar] [CrossRef]
- Montoro Girona, M.; Morin, H.; Lussier, J.M.; Walsh, D. Radial growth response of black spruce stands ten years after experimental shelterwoods and seed-tree cuttings in boreal forest. Forests 2016, 7, 240. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Wang, B.S.; Li, M.G.; Zan, Q.J. The community dynamics of the forest secondary succession in Heishiding Natural Reserve of Guangdong Province. Acta Bot. Sin. 1999, 41, 877–886. [Google Scholar]



| Species Group | D (g/cm2) | BEF (t) | R |
|---|---|---|---|
| Cunninghamia lanceolata (Lamb.) Hook. | 0.307 | 1.634 | 0.246 |
| Pinus massoniana Lamb. | 0.380 | 1.472 | 0.187 |
| Mixed broad-leaved trees | 0.482 | 1.514 | 0.262 |
| Index | DBH Classes (2012) | DBH Classes (2017) | ||||||
|---|---|---|---|---|---|---|---|---|
| All | Small | Medium | Large | All | Small | Medium | Large | |
| Plot-Level | ||||||||
| Abundance | 200.28 | 152.40 | 36.79 | 11.09 | 160.46 | 113.66 | 34.10 | 12.70 |
| Species Richness | 25.14 | 22.62 | 8.05 | 3.10 | 22.83 | 19.64 | 7.98 | 3.34 |
| Shannon–Wiener index | 2.37 | 2.35 | 1.55 | 0.75 | 2.30 | 2.25 | 1.55 | 0.81 |
| Biomass (Mg) | 11.70 | 0.43 | 4.29 | 6.98 | 12.05 | 0.37 | 3.80 | 7.89 |
| Community-Level | ||||||||
| Abundance | 50,070 | 38,100 | 9198 | 2772 | 40,115 | 28,415 | 8524 | 3176 |
| Species Richness | 153 | 138 | 99 | 55 | 147 | 127 | 95 | 47 |
| Biomass (Mg) | 2925.4 | 107.0 | 1072.3 | 1746.1 | 3012.9 | 91.6 | 949.5 | 1971.8 |
| Groups Compared | T | A | P |
|---|---|---|---|
| All (2012) vs. All (2017) | −4.98 | 0.003 | 0.002 |
| Small (2012) vs. Small (2017) | −7.22 | 0.005 | 0.000 |
| Medium (2012) vs. Medium (2017) | −1.00 | 0.001 | 0.131 |
| Large (2012) vs. Large (2017) | −0.37 | 0.001 | 0.231 |
| Groups Compared | 2012 | 2017 | ||||
|---|---|---|---|---|---|---|
| T | A | P | T | A | P | |
| Overall Comparison | −253.01 | 0.171 | < 10−7 | −254.56 | 0.168 | < 10−7 |
| Pairwise Comparison | ||||||
| All vs. Small | −28.79 | 0.019 | <10−7 | −42.74 | 0.029 | <10−7 |
| All vs. Medium | −184.74 | 0.140 | <10−7 | −176.13 | 0.134 | <10−7 |
| All vs. Large | −242.39 | 0.221 | <10−7 | −244.07 | 0.217 | <10−7 |
| Small vs. Medium | −147.58 | 0.099 | <10−7 | −136.61 | 0.093 | <10−7 |
| Small vs. Large | −216.30 | 0.172 | <10−7 | −211.61 | 0.167 | <10−7 |
| Medium vs. Large | −99.01 | 0.096 | < 10−7 | −88.23 | 0.080 | < 10−7 |
| Species Code | 2012 | 2017 | Rate of Abundance Change (%) | Rate of IV Change (%) | ||||
|---|---|---|---|---|---|---|---|---|
| DBH Class | IV | Abundance (Small) | DBH Class | IV | Abundance (Small) | |||
| ITECHI | 1 | 86.4 * | 3052 | 1 | 81.8 * | 2454 | −19.6 | −5.3 |
| ARDQUI | 1 | 73.2 * | 4918 | 1 | 73.1 * | 4636 | −5.7 | −0.1 |
| ELACHI | 1 | 65.7 * | 792 | 1 | 61.6 * | 664 | −16.2 | −6.2 |
| EURMAC | 1 | 65.7 * | 628 | 1 | 64.6 * | 567 | −9.7 | −1.7 |
| ILEPUB | 1 | 65.7 * | 775 | 1 | 63.1 * | 609 | −21.4 | −4 |
| DIOMOR | 1 | 64 * | 589 | 1 | 59.4 * | 496 | −15.8 | −7.2 |
| MACVEL | 1 | 61.9 * | 774 | 1 | 57.8 * | 596 | −23 | −6.6 |
| SCHSUP | 1 | 59.8 * | 4283 | 1 | 53.3 * | 3489 | −18.5 | −10.9 |
| BEITSA | 1 | 55.9 * | 895 | 1 | 51.4 * | 804 | −10.2 | −8.1 |
| PHOPRU | 1 | 52.8 * | 700 | 1 | 45.3 * | 529 | −24.4 | −14.2 |
| MACCHI | 1 | 52 * | 519 | 1 | 41 * | 319 | −38.5 | −21.2 |
| STYODO | 1 | 49.4 * | 457 | 1 | 43.2 * | 342 | −25.2 | −12.6 |
| FICVAR | 1 | 46.4 * | 280 | 1 | 35.6 * | 192 | −31.4 | −23.3 |
| AIDCAN | 1 | 45.7 * | 1421 | 1 | 47.7 * | 1368 | −3.7 | 4.4 |
| EURBRE | 1 | 44.0 * | 327 | 1 | 40.5 * | 270 | −17.4 | −8 |
| ADIMIL | 1 | 42.7 * | 565 | 1 | 37.2 * | 335 | −40.7 | −12.9 |
| SCHOCT | 1 | 42.6 * | 376 | 1 | 37.1 * | 325 | −13.6 | −12.9 |
| CASCAR | 1 | 42.2 * | 4826 | 2 | 31 | 2813 | −41.7 | − |
| TARMOL | 1 | 39.5 * | 298 | 1 | 34 * | 237 | −20.5 | −13.9 |
| ENGROX | 1 | 34.2 * | 783 | 1 | 16.9 | 369 | −52.9 | −50.6 |
| CASVIL | 1 | 33.8 * | 251 | 1 | 28.3 * | 195 | −22.3 | −16.3 |
| CRYCHI | 1 | 31.8 * | 252 | 1 | 29.5 * | 231 | −8.3 | −7.2 |
| AIDPYC | 1 | 31.7 * | 1699 | 1 | 31.5 * | 1598 | −5.9 | −0.6 |
| CAMOLE | 1 | 30.8 * | 730 | 1 | 22.4 * | 264 | −63.8 | −27.3 |
| CINPOR | 1 | 28.2 * | 329 | 2 | 25.7 * | 181 | −45 | − |
| STYSUB | 1 | 23.2 * | 340 | 1 | 17.4 | 244 | −28.2 | −25 |
| DAPOLD | 1 | 22.2 * | 153 | 1 | 12.2 | 80 | −47.7 | −45 |
| Statistic | Axis 1 | Axis 2 | Axis 3 | Axis 4 |
|---|---|---|---|---|
| 2012 | ||||
| Eigenvalues | 0.1457 | 0.0739 | 0.0377 | 0.0314 |
| Cumulative explained variation (%) | 14.57 | 21.97 | 25.74 | 28.88 |
| Pseudo-canonical correlation | 0.8074 | 0.7611 | 0.7432 | 0.8244 |
| Cumulative explained fitted variation (%) | 39.86 | 60.08 | 70.38 | 78.97 |
| p value | 0.001 | 0.028 | 0.675 | 0.797 |
| 2017 | ||||
| Eigenvalues | 0.1703 | 0.0477 | 0.0354 | 0.0295 |
| Cumulative explained variation (%) | 17.03 | 21.8 | 25.34 | 28.29 |
| Pseudo-canonical correlation | 0.8563 | 0.6435 | 0.7476 | 0.7901 |
| Cumulative explained fitted variation (%) | 47.63 | 60.97 | 70.89 | 79.14 |
| p value | 0.001 | 0.559 | 0.838 | 0.919 |
| Environmental Variables | Explained Variation | Explained Fitted Variation (%) | p (adj) |
|---|---|---|---|
| 2012 | |||
| Total phosphorus (g/kg) | 7.7 | 21.1 | 0.012 |
| Transmitted direct solar radiation (mols·m−2·d−1) | 5.6 | 15.3 | 0.012 |
| Organic matter (g/kg) | 4.7 | 12.9 | 0.024 |
| Capillary water capacity (g/kg) | 3.8 | 10.4 | 0.024 |
| 2017 | |||
| Total phosphorus (g/kg) | 10.4 | 29.0 | 0.012 |
| Total potassium (g/kg) | 5.1 | 14.2 | 0.024 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Liu, T.; Xie, P.; Chen, H.; Su, Z. Temporal Changes in Community Structure over a 5-Year Successional Stage in a Subtropical Forest. Forests 2020, 11, 438. https://doi.org/10.3390/f11040438
Xu M, Liu T, Xie P, Chen H, Su Z. Temporal Changes in Community Structure over a 5-Year Successional Stage in a Subtropical Forest. Forests. 2020; 11(4):438. https://doi.org/10.3390/f11040438
Chicago/Turabian StyleXu, Mingfeng, Ting Liu, Peiyun Xie, Hongyu Chen, and Zhiyao Su. 2020. "Temporal Changes in Community Structure over a 5-Year Successional Stage in a Subtropical Forest" Forests 11, no. 4: 438. https://doi.org/10.3390/f11040438
APA StyleXu, M., Liu, T., Xie, P., Chen, H., & Su, Z. (2020). Temporal Changes in Community Structure over a 5-Year Successional Stage in a Subtropical Forest. Forests, 11(4), 438. https://doi.org/10.3390/f11040438





