Response of Nitrogen Metabolism in Masson Pine Needles to Elevated CO2
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Determination of the Content of Different Forms of Nitrogen
2.3. Free Amino Acid Detection
2.4. Nitrogen Metabolism Enzyme Activities
2.5. RNA Extraction, Complementary DNA (cDNA) Synthesis and Quantitative Real-Time PCR (qRT-PCR)
2.6. Statistical Analysis
3. Results
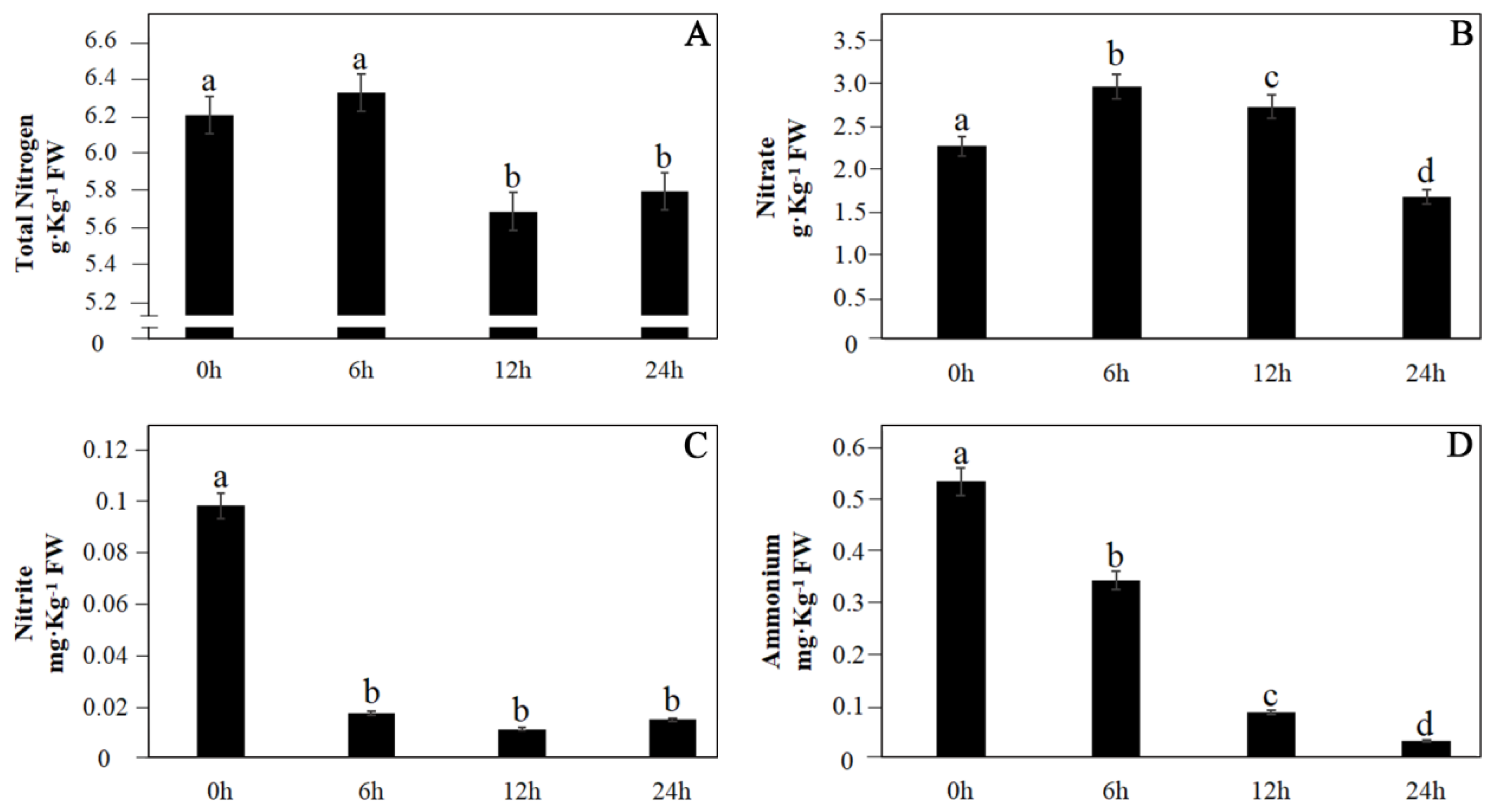
3.1. Changes in Different N Forms under CO2 Stress
3.2. Free Amino Acids
3.3. Enzyme Activities and Gene Expression
4. Discussion
4.1. Effects of Elevated CO2 on Different Nitrogen Forms
4.2. Enzyme Activities and Gene Expression Response to CO2 Stress
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gomez-Casanovas, N.; Blanc-Betes, E.; Gonzalez-Meler, M.; Azcon-Bieto, J. Changes in Respiratory Mitochondrial Machinery and Cytochrome and Alternative Pathway Activities in Response to Energy Demand Underlie the Acclimation of Respiration to Elevated CO2 in the Invasive Opuntia ficus-indica1 [OA]. Plant Physiol. 2007, 145, 49–61. [Google Scholar] [CrossRef] [PubMed]
- GCP. Carbon Budget. Available online: http://www.globalcarbonproject.org/carbonbudget (accessed on 13 March 2020).
- Ehleringer, J.R.; Schulze, E.D. Ecosystem Physiology Responses to Global Change; Cambridge University Press: Cambridge, UK; London, UK, 1999; pp. 14–16. [Google Scholar]
- Wang, W.M.; Wang, C.; Li, C.J.; Lin, W.H. Effects of elevated atmospheric CO2 concentrations on growth of plants. Acta Bot. Boreal. Occident. Sin. 2000, 20, 676–683. [Google Scholar]
- Thilakarathne, C.L.; Tausz-Posch, S.; Cane, K.; Norton, R.M.; Tausz, M.; Seneweera, S. Intraspecific variation in growth and yield response to elevated CO2 in wheat depends on the differences of leaf mass per unit area. Funct. Plant Boil. 2013, 40, 185–194. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, C.; Yu, Z.; Zhang, J.; Zhu, C.; Zhao, Z.; Xiong, J.; Chen, J. Effects of elevated CO2 and nitrogen addition on organic carbon and aggregates in soil planted with different rice cultivars. Plant Soil 2018, 432, 245–258. [Google Scholar] [CrossRef]
- Kleinhofs, A.; Warner, R.L. Advances in Nitrate Assimilation; Elsevier BV: Amsterdam, The Netherlands, 1990; pp. 89–120. [Google Scholar]
- Lea, P.J.; Robinson, S.A.; Stewart, G.R. The Enzymology and Metabolism of Glutamine, Glutamate and Asparagine Intermediary Nitrogen Metabolism the Biochemistry of Plants; Academic Press: New York, NY, USA, 2012; pp. 44–51. [Google Scholar]
- Men, Z.H.; Li, S.X. Effect of CO2 concentration on nitrogen metabolism of winter wheat. Zhongguo Nongye Kexue 2005, 38, 320–326. [Google Scholar]
- Xu, Y.B. Effect of CO2 Enrichment on Plant Growth and Nitrogen Use of Winter Wheat. Master’s Thesis, Northwest Agriculture and Forestry University, Xianyang, China, 2012. [Google Scholar]
- Bassirirad, H.; Thomas, R.B.; Reynolds, J.F. Differential responses of root uptake kinetics of NH4+ and NO3− to enriched atmospheric CO2 concentration in field-grown loblolly pine. Plant Cell Environ. 1996, 16, 957–962. [Google Scholar] [CrossRef]
- Su, W.L. Effects of Elevation CO2 on Nitrogen Uptake Characteristic and Growth of Larix Gmelinii and Pinus Sylvestris var. Mongolica. Master’s Thesis, Northeast Forestry University, Harbin, China, 2008. [Google Scholar]
- Johnson, D.; Cheng, W.; Ball, J. Effects of CO2 and N fertilization on decomposition and N immobilization in ponderosa pine litter. Plant Soil 2000, 224, 115–122. [Google Scholar] [CrossRef]
- Pang, J.; Zhu, J.G.; Liu, G. Effects of free-air CO2 enrichment (FACE) on concentrations of various N forms in rice tissues. J. Agro-Environ. Sci. 2005, 24, 833–837. [Google Scholar]
- Johansson, E.M.; Fransson, P.M.A.; Finlay, R.D.; Van Hees, P. Quantitative analysis of soluble exudates produced by ectomycorrhizal roots as a response to ambient and elevated CO2. Soil Boil. Biochem. 2009, 41, 1111–1116. [Google Scholar] [CrossRef]
- Chen, F.J.; Ge, F.; Liu, X.H. Responses of cotton to elevated CO2 and the effects on cotton aphid occurrences. Acta Ecol. Sin. 2004, 24, 991–996. [Google Scholar]
- Han, X. Effects of Free Air CO2 Enrichment on Wheat Growth and Yield: The Physiological Basis. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2012. [Google Scholar]
- Wang, X.J. Effect of CO2 Enrichment on Growth, Root Uptake Characteristics and Efficiency in Oilseed Rape; Hunan Agricultural University: Changsha, China, 2012. [Google Scholar]
- Li, Z.X. Effects of Elevated CO2 and Heat Stress on Growth and Development of Tea Plant. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2016. [Google Scholar]
- Yang, C.; Tan, T.; Zhang, L.; Yu, J.L.; Liao, Q.; Zhang, Z.H.; Liu, Q.; Rong, X.M.; Song, H.X.; Guan, C.Y. Effects of high CO2 concentration in atmosphere on nitrogen assimilation and plant growth of Oilseeds Rape (Brassica napus L.). Ecol. Environ. Sci. 2013, 22, 1688–1694. [Google Scholar]
- Hocking, P.; Meyer, C. Effects of CO2 Enrichment and Nitrogen Stress on Growth, and Partitioning of Dry Matter and Nitrogen in Wheat and Maize. Funct. Plant Boil. 1991, 18, 339–356. [Google Scholar] [CrossRef]
- Bauer, G.; Berntson, G.M. Ammonium and nitrate acquisition by plants in response to elevated CO2 concentration: The roles of root physiology and architecture. Tree Physiol. 2001, 21, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Constable, J.V.H.; BassiriRad, H.; Lussenhop, J.; Zerihun, A. Influence of elevated CO2 and mycorrhizae on nitrogen acquisition: Contrasting responses in Pinus taeda and Liquidambar styraciflua. Tree Physiol. 2001, 21, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Ye, Y.; Bai, T.; Xu, M.; Xu, L.-A. Complete Chloroplast Genome of Pinus massoniana (Pinaceae): Gene Rearrangements, Loss of ndh Genes, and Short Inverted Repeats Contraction, Expansion. Molecules 2017, 22, 1528. [Google Scholar] [CrossRef]
- Ni, Z.; Zhou, P.; Xu, M.; Xu, L.-A. Development and characterization of chloroplast microsatellite markers for Pinus massoniana and their application in Pinus (Pinaceae) species. J. Genet. 2018, 97, 53–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Yang, Q. Nitrogen (N) Deposition Impacts Seedling Growth of Pinus massoniana via N:P Ratio Effects and the Modulation of Adaptive Responses to Low P (Phosphorus). PLoS ONE 2013, 8, e79229. [Google Scholar] [CrossRef]
- Guan, L.L.; Wen, D. More nitrogen partition in structural proteins and decreased photosynthetic nitrogen-use efficiency of Pinus massoniana under in situ polluted stress. J. Plant Res. 2011, 124, 663–673. [Google Scholar] [CrossRef]
- Ni, C.; Wang, D.; Tao, Y. Variable weighted convolutional neural network for the nitrogen content quantization of Masson pine seedling leaves with near-infrared spectroscopy. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2019, 209, 32–39. [Google Scholar] [CrossRef]
- Treder, K.; Wanic, M.; Jastrzebska, M. The Influence of interaction between spring wheat and spring barley on accumulation of nitrogen, phosphorus and potassium in plants. Ann. UMCS, Agric. 2009, 64, 94–106. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Li, X.F. Experimental Guidance of Plant Physiology; Higher Education Press: Beijing, China, 2016; pp. 36–48. [Google Scholar]
- Zhu, P.; Ma, Y.; Zhu, L.; Chen, Y.; Li, R.; Ji, K.S.; Ji, K.S. Selection of Suitable Reference Genes in Pinus massoniana Lamb. Under Different Abiotic Stresses for qPCR Normalization. Forests 2019, 10, 632. [Google Scholar] [CrossRef]
- XCMS. Meta XCMS. Available online: http://metlin.scripps.edu/xcms (accessed on 5 January 2020).
- Li, M.; Li, Y.; Zhang, W.; Li, S.; Gao, Y.; Ai, X.; Zhang, D.; Liu, B.; Li, Q. Metabolomics analysis reveals that elevated atmospheric CO2 alleviates drought stress in cucumber seedling leaves. Anal. Biochem. 2018, 559, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.; Mayor, J.R.; Turner, B.L. Plasticity in nitrogen uptake among plant species with contrasting nutrient acquisition strategies in a tropical forest. Ecology 2017, 98, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Functions of mineral nurtients: Macronutrients. In The Mineral Nutrition of Higher Plants, 2nd ed.; Marschner, P., Ed.; Academic Press: New York, NY, USA, 1995; pp. 229–255. [Google Scholar]
- Zhao, X. Effect of Elevated Root-Zone CO2 Concentration on Melon Seeding Nitrogen Absorption, Metabolism and Transport in Root. Master’s Thesis, Shengyang Agricultural University, Shenyang, China, 2012. [Google Scholar]
- Thornton, B.; Robinson, D. Uptake and assimilation of nitrogen from solutions containing multiple N sources. Plant Cell Environ. 2005, 28, 813–821. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, J.; Sun, Y.; Yang, X.; Ma, J.; Li, T.; Wu, L. Elevated CO2 levels enhance the uptake and metabolism of organic nitrogen. Physiol. Plant. 2017, 162, 467–478. [Google Scholar] [CrossRef]
- Takatani, N.; Ito, T.; Kiba, T.; Mori, M.; Miyamoto, T.; Maeda, S.I.; Omata, T. Effects of high CO2 on growth and metabolism of Arabidopsis seedlings during growth with a constantly limited supply of nitrogen. Plant Cell Physiol. 2013, 55, 281–292. [Google Scholar] [CrossRef]
- Teng, N.; Wang, J.; Chen, T.; Wu, X.; Wang, Y.; Lin, J. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 2006, 172, 92–103. [Google Scholar] [CrossRef]
- Weigel, H.J.; Manderscheid, R. Crop growth responses to free air CO2 enrichment and nitrogen fertilization: Rotating barley, ryegrass, sugar beet and wheat. Eur. J. Agron. 2012, 43, 97–107. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; He, X.; Li, Q.; Liu, B.; Ai, X.; Zhang, D. Response of water balance and nitrogen assimilation in cucumber seedlings to CO2 enrichment and salt stress. Plant Physiol. Biochem. 2019, 139, 256–263. [Google Scholar] [CrossRef]
- Vega-Mas, I.; Pérez-Delgado, C.M.; Marino, D.; Fuertes-Mendizábal, T.; González-Murua, C.; Marquez, A.J.; Betti, M.; Estavillo, J.M.; González-Moro, M.B. Elevated CO2 Induces Root Defensive Mechanisms in Tomato Plants When Dealing with Ammonium Toxicity. Plant Cell Physiol. 2017, 58, 2112–2125. [Google Scholar] [CrossRef]
- Pan, R.C.; Wang, X.Q.; Li, N.H. Plant Physiology; Higher Education Press: Beijing, China, 2012; pp. 23–36. [Google Scholar]
- Torralbo, F.; Vicente, R.; Morcuende, R.; González-Murua, C.; Aranjuelo, I. C and N metabolism in barley leaves and peduncles modulates responsiveness to changing CO2. J. Exp. Bot. 2018, 70, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, U.; Robredo, A.; Miranda-Apodaca, J.; Lacuesta, M.; Muñoz-Rueda, A.; Petite, A.M. Carbon dioxide enrichment moderates salinity-induced effects on nitrogen acquisition and assimilation and their impact on growth in barley plants. Environ. Exp. Bot. 2013, 87, 148–158. [Google Scholar] [CrossRef]
- Zaghdoud, C.; Carvajal, M.; Ferchichi, A.; Ballesta, M.M. Water balance and N-metabolism in broccoli (Brassica oleracea L. var. Italica) plants depending on nitrogen source under salt stress and elevated CO2. Sci. Total. Environ. 2016, 571, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Anabel, R.; Usue, P.; Jon, M.; Maite, L.; Amaia, M.; Alberto, M. Elevated CO2 reduces the drought effect on nitrogen metabolism in barley plants during drought and subsequent recovery. Environ. Exp. Bot. 2011, 13, 399–408. [Google Scholar]
- Vicente, R.; Pérez, P.; Martinez-Carrasco, R.; Usadel, B.; Kostadinova, S.; Morcuende, R. Quantitative RT–PCR Platform to Measure Transcript Levels of C and N Metabolism-Related Genes in Durum Wheat: Transcript Profiles in Elevated [CO2] and High Temperature at Different Levels of N Supply. Plant Cell Physiol. 2015, 56, 1556–1573. [Google Scholar] [CrossRef] [PubMed]
- Reyes, T.H.; Pompeiano, A.; Pompeiano, A.; Ciurli, A.; Lu, Y.; Guglielminetti, L.; Yamaguchi, J. Nitrate Reductase Modulation in Response to Changes in C/N Balance and Nitrogen Source in Arabidopsis. Plant Cell Physiol. 2018, 59, 1248–1254. [Google Scholar] [CrossRef]
- Koch, M.; Naumann, M.; Pawelzik, E.; Gransee, A.; Thiel, H. The Importance of Nutrient Management for Potato Production Part I: Plant Nutrition and Yield. Potato Res. 2019, 63, 97–119. [Google Scholar] [CrossRef]
- Di Martino, C.; Fioretto, A.; Palmieri, D.; Torino, V.; Palumbo, G. Influence of Tomato Plant Mycorrhization on Nitrogen Metabolism, Growth and Fructification on P-Limited Soil. J. Plant Growth Regul. 2019, 38, 1183–1195. [Google Scholar] [CrossRef]
- Batnini, M.; Lopez-Gomez, M.; Palma, F.; Haddoudi, I.; Kallala, N.; Zribi, K.; Mrabet, M.; Mhadhbi, H. Sinorhizobium spp inoculation alleviates the effect of Fusarium oxysporum on Medicago truncatula plants by increasing antioxidant capacity and sucrose accumulation. Appl. Soil Ecol. 2020, 150, 103458. [Google Scholar] [CrossRef]
- Agtuca, B.J.; Stopka, S.; Tuleski, T.R.; Amaral, F.P.D.; Evans, S.; Liu, Y.; Xu, D.; Monteiro, R.A.; Koppenaal, D.W.; Tolic, L.P.; et al. In-Situ Metabolomic Analysis of Setaria viridis Roots Colonized by Beneficial Endophytic Bacteria. Mol. Plant-Microbe Interact. 2020, 33, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Moran-Duran, S.A.; Flynn, R.P.; Heerema, R.; VanLeeuwen, D. Leaf Net Photosynthesis, Leaf Greenness, and Shoot Lignin Content of Nonbearing Pecan Trees at Two Nitrogen and Nickel Application Rates. HortScience 2020, 55, 231–236. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology; Sinauer Associates Press: North Carolina, NY, USA, 2006; pp. 57–78. [Google Scholar]


| Primer | Sequence (5’→3’) | |
|---|---|---|
| qGS_F | Forward | GACTTCTGAACAGCAAAATGGTC |
| qGS_R | Reverse | GCAATCAGTTTAGATGGGCATAG |
| qNR_F | Forward | AACTGACAGCACTCTGAAACTCC |
| qNR_R | Reverse | AATATACATGTGGCCGTGAGAAG |
| qGDH_F | Forward | GGTCATTCTCCTGCAGTTGTTAC |
| qGDH_R | Reverse | ATGTTCAGCTAACAAGGCTTCTG |
| qNiR_F | Forward | GAAGACGGGAGACATAGAGGACT |
| qNiR_R | Reverse | TAGATATAATCCGGGTCCACCTT |
| qGOGAT_F | Forward | CAAATTCACTGTTGTGCAGAGAG |
| qGOGAT_R | Reverse | AACAGCAACAGCAGCTACTTCTC |
| 0 h | 6 h | 12 h | 24 h | |
|---|---|---|---|---|
| Aspartate | 0.030 ± 0.002a | 0.031 ± 0.052a | 0.010 ± 0.052a | 0.007 ± 0.001a |
| Serine | 0.357 ± 0.124a | 0.368 ± 0.156a | 0.199 ± 0.074b | 0.422 ± 0.084a |
| Threonine | 0.366 ± 0.075b | 0.490 ± 0.217b | 0.563 ± 0.200b | 1.111 ± 0.419a |
| Glutamate | 4.086 ± 0.657a | 3.387 ± 0.474ab | 3.396 ± 0.860ab | 2.555 ± 0.647b |
| Glycine | 0.027 ± 0.006a | 0.026 ± 0.005a | 0.031 ± 0.010a | 0.029 ± 0.005a |
| Alanine | 0.116 ± 0.022b | 0.267 ± 0.083a | 0.232 ± 0.073a | 0.275 ± 0.062a |
| Cysteine | 0.010 ± 0.006a | 0.008 ± 0.002a | 0.010 ± 0.007a | 0.011 ± 0.006a |
| Valine | 0.592 ± 0.284a | 0.34 ± 0.128b | 0.215 ± 0.129b | 0.243 ± 0.097b |
| Methionine | 0.048 ± 0.021a | 0.020 ± 0.007b | 0.013 ± 0.001b | 0.013 ± 0.004b |
| Isoleucine | 0.002 ± 0.001a | 0.001 ± 0.001b | 0.002 ± 0.001ab | 0.001 ± 0.001b |
| Phenylalanine | 0.299 ± 0.061a | 0.288 ± 0.055a | 0.285 ± 0.167a | 0.359 ± 0.162a |
| Lysine | 0.500 ± 0.920a | 0.556 ± 0.958a | 0.492 ± 0.209a | 0.517 ± 0.384a |
| Histidine | 0.300 ± 0.084b | 0.713 ± 0.528ab | 0.622 ± 0.726ab | 1.271 ± 0.994a |
| Arginine | 1.748 ± 1.687b | 4.616 ± 3.912ab | 3.892 ± 5.721ab | 7.702 ± 4.922a |
| Glutamine | 5.793 ± 3.521a | 3.734 ± 2.220ab | 3.125 ± 1.090ab | 1.895 ± 1.039b |
| Leucine | 0.873 ± 0.186b | 0.516 ± 0.144c | 0.72 ± 0.173bc | 1.327 ± 0.298a |
| Tyrosine | 0.276 ± 0.071a | 0.206 ± 0.096a | 0.236 ± 0.090a | 0.289 ± 0.113a |
| Tryptophan | 1.414 ± 0.815b | 1.794 ± 0.332ab | 2.362 ± 0.909a | 2.650 ± 0.537a |
| Proline | 0.648 ± 0.202a | 0.493 ± 0.151ab | 0.483 ± 0.208ab | 0.310 ± 0.071b |
| Asparagine | 0.114 ± 0.056b | 0.214 ± 0.091b | 0.426 ± 0.336b | 0.933 ± 0.733a |
| Total | 17.572 ± 1.490b | 18.068 ± 1.370b | 17.314 ± 1.239b | 21.230 ± 2.944a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Sun, X.; Hu, X.; Zou, B.; Lin, N.; Lin, J.; Ji, K. Response of Nitrogen Metabolism in Masson Pine Needles to Elevated CO2. Forests 2020, 11, 390. https://doi.org/10.3390/f11040390
Wu F, Sun X, Hu X, Zou B, Lin N, Lin J, Ji K. Response of Nitrogen Metabolism in Masson Pine Needles to Elevated CO2. Forests. 2020; 11(4):390. https://doi.org/10.3390/f11040390
Chicago/Turabian StyleWu, Fan, Xiaobo Sun, Xingfeng Hu, Bingzhang Zou, Nengqing Lin, Jingquan Lin, and Kongshu Ji. 2020. "Response of Nitrogen Metabolism in Masson Pine Needles to Elevated CO2" Forests 11, no. 4: 390. https://doi.org/10.3390/f11040390
APA StyleWu, F., Sun, X., Hu, X., Zou, B., Lin, N., Lin, J., & Ji, K. (2020). Response of Nitrogen Metabolism in Masson Pine Needles to Elevated CO2. Forests, 11(4), 390. https://doi.org/10.3390/f11040390





