Impacts of Global Warming on the Radial Growth and Long-Term Intrinsic Water-Use Efficiency (iWUE) of Schrenk Spruce (Picea schrenkiana Fisch. et Mey) in the Sayram Lake Basin, Northwest China
Abstract
1. Introduction
2. Data and Methods
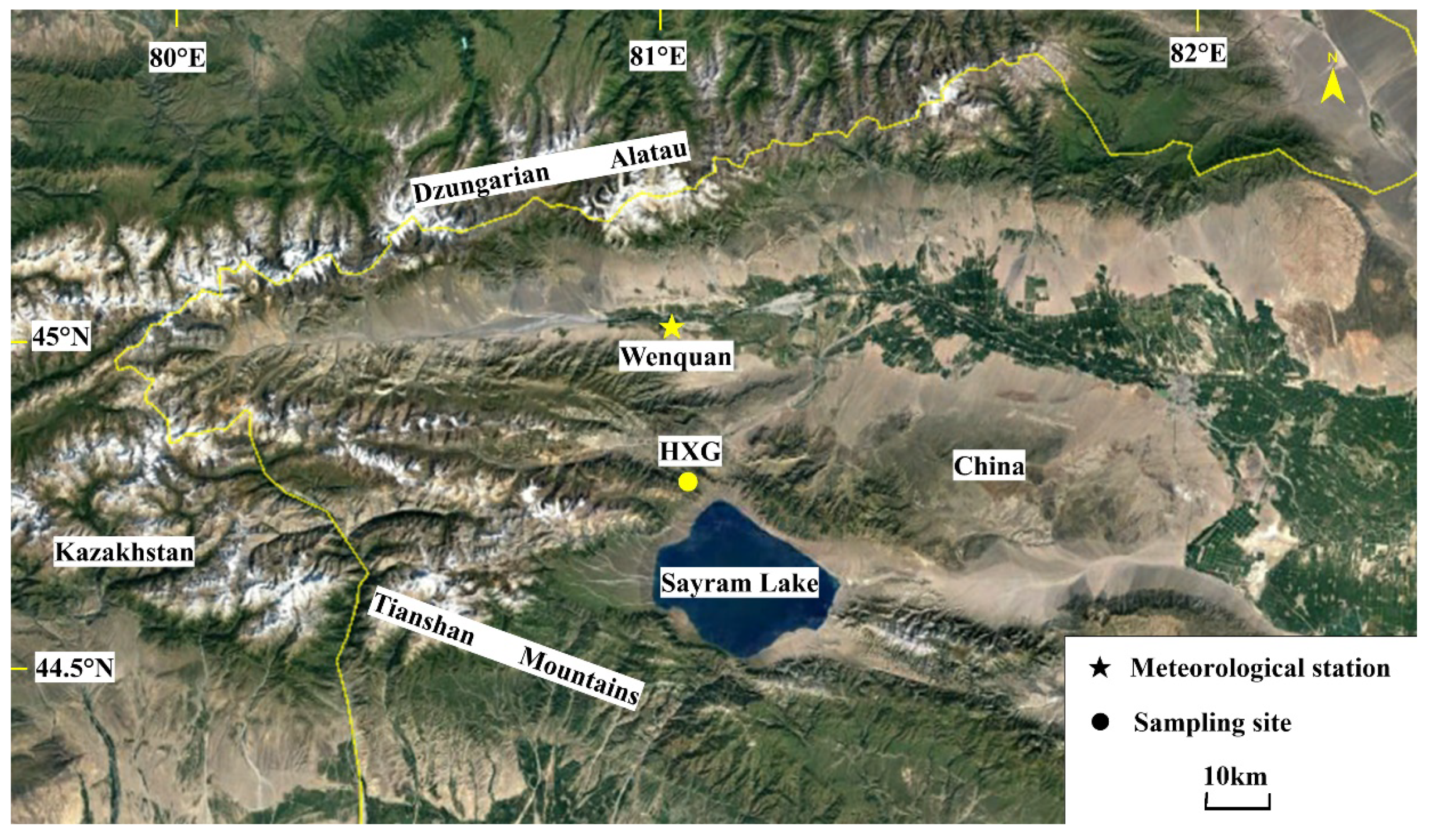
2.1. Study Area and Tree-Ring Sampling Site
2.2. Establishing the Tree-Ring-Width Chronology
2.3. Stable Carbon Isotopic Extraction
2.4. Long-Term Intrinsic Water-Use Efficiency (iWUE) Calculations
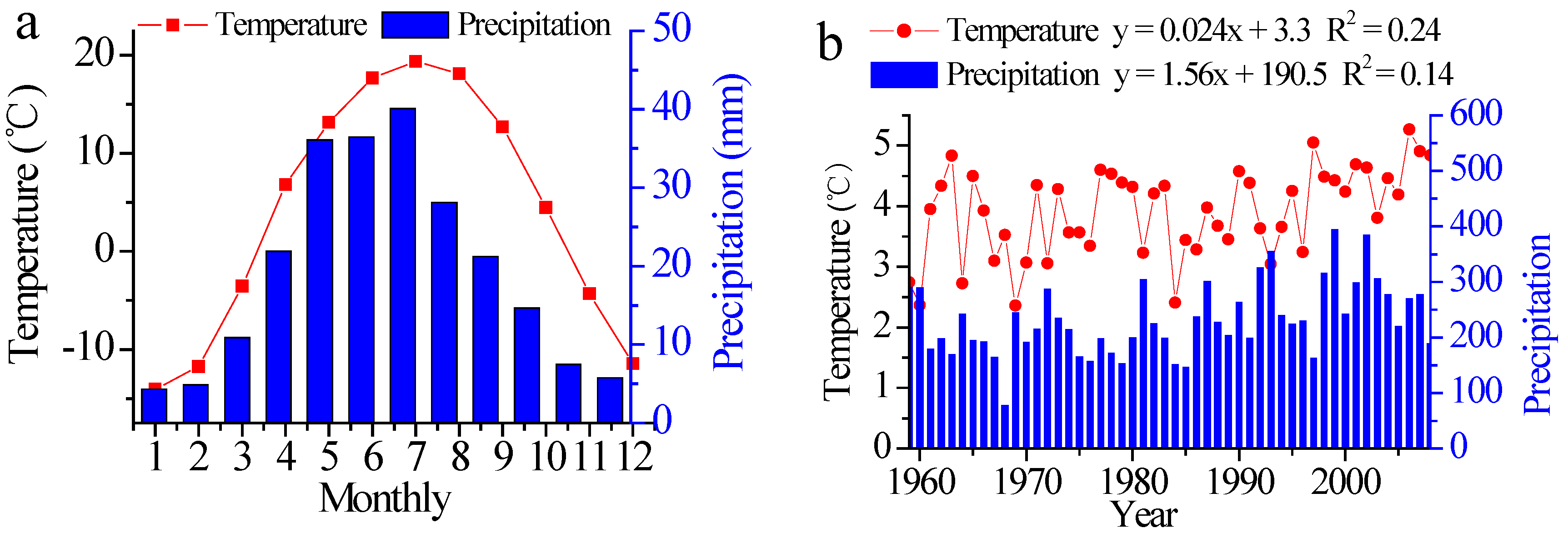
2.5. Climate Data
2.6. Statistical Methodology
3. Results and Discussion
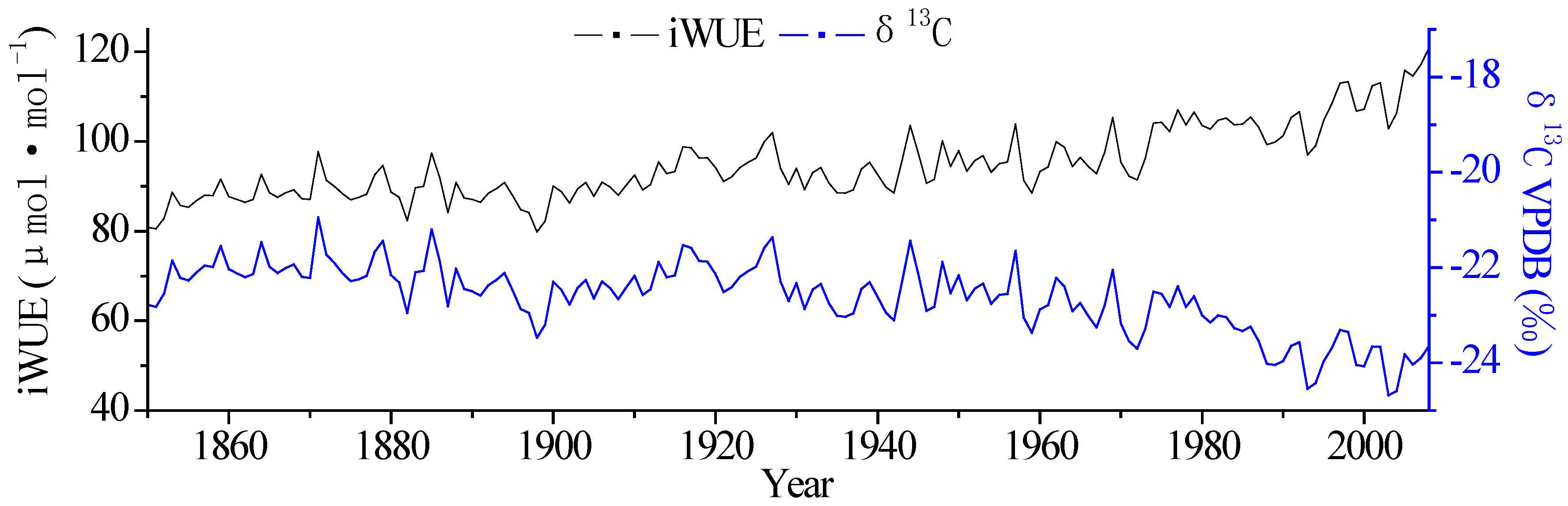
3.1. Changes in Tree-Ring δ13C and iWUE
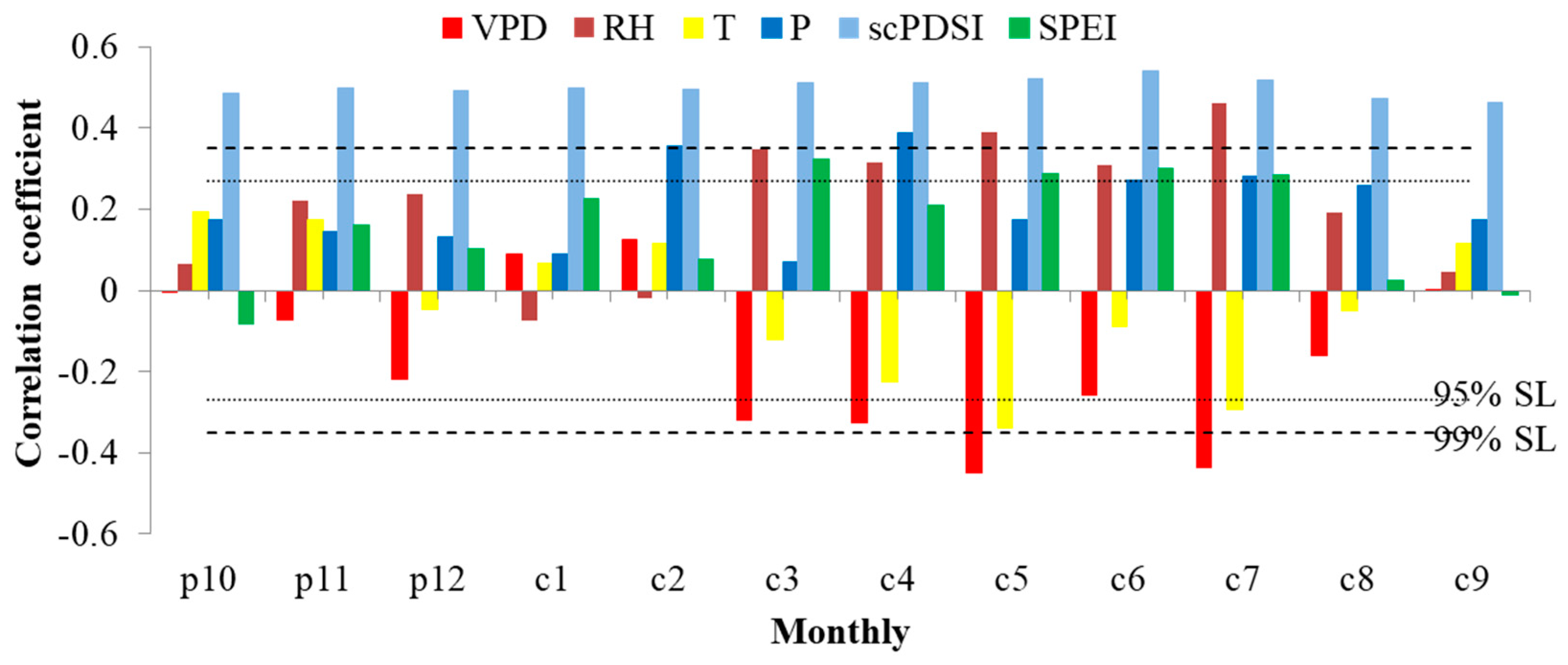
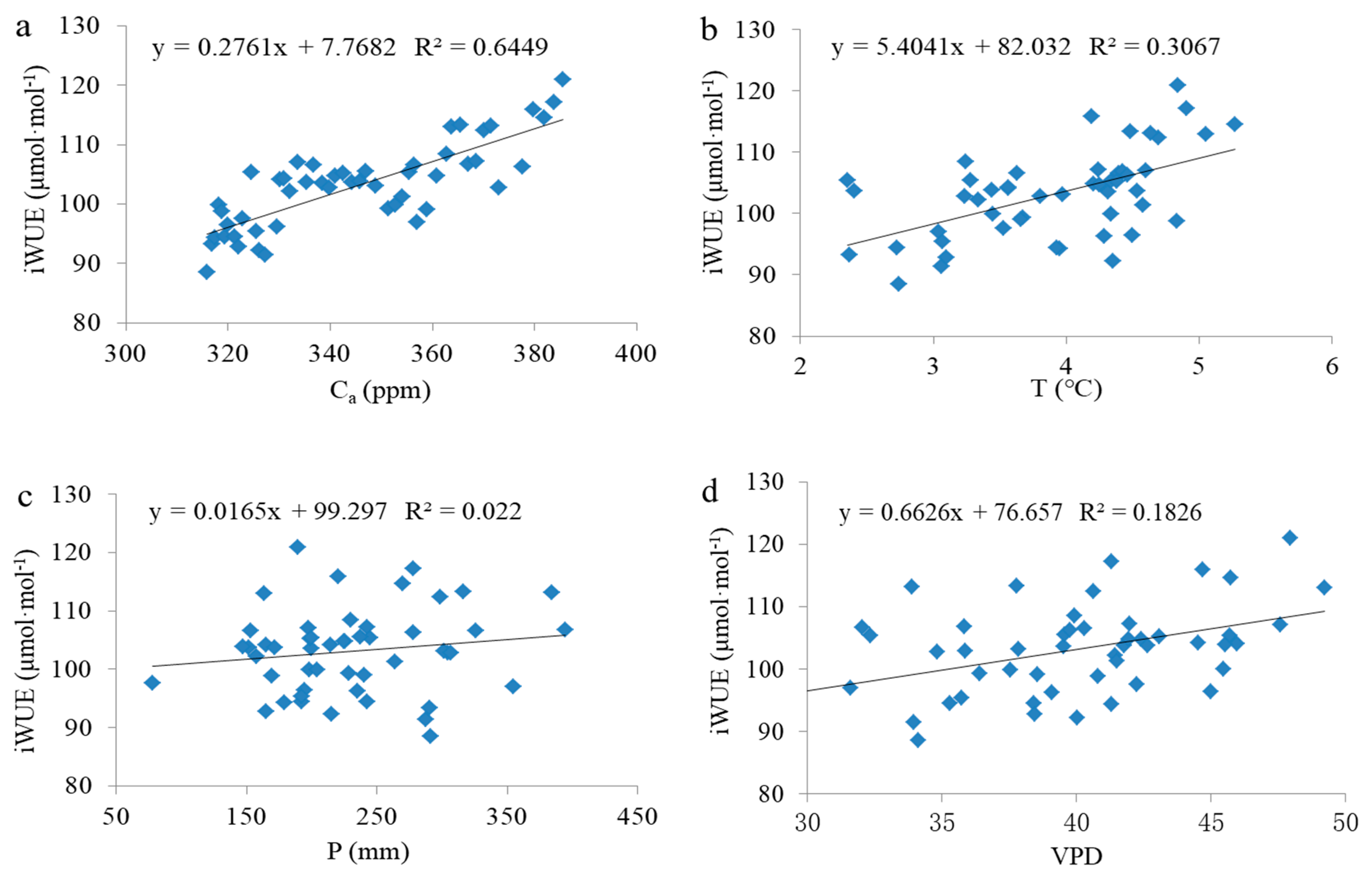
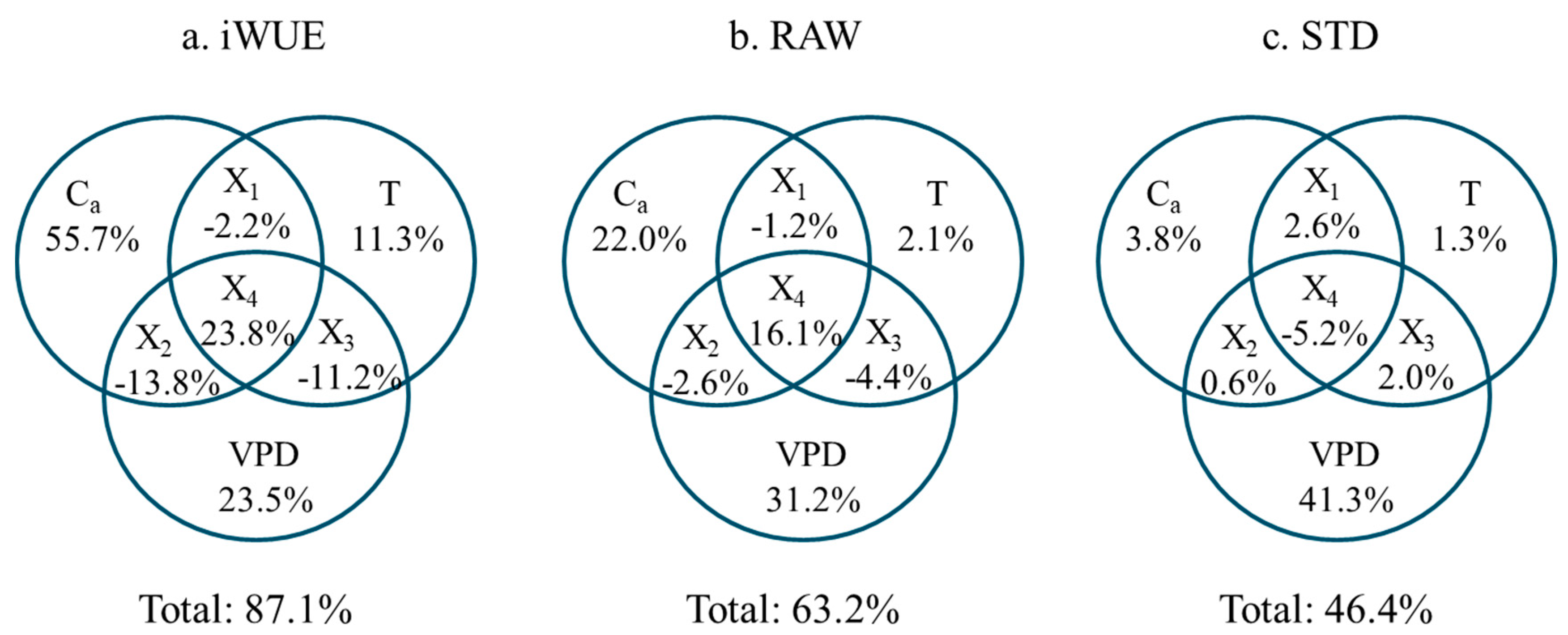
3.2. Response of Tree Radial Growth and iWUE to Environmental Factors
3.3. The Relationship between Radial Growth and iWUE
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis [M/OL]; Cambridge University Press: Cambridge, UK, 2013; Available online: http://www.ipcc.ch/report/ar5/wg1/#.Uq_tD7KBRR1.2013 (accessed on 5 January 2019).
- McCarroll, D.; Loader, N.J. Stable isotopes in tree rings. Quat. Sci. Rev. 2004, 23, 771–801. [Google Scholar] [CrossRef]
- Dlugokenky, E.; Tans, P. Trends in Atmospheric Carbon Dioxide; National Oceanic & Atmospheric Administration: Boulder, CO, USA, 2019. Available online: http://www.esrl.noaa.gov/gmd/ccgg/trends/global.html (accessed on 1 December 2019).
- Hughes, L. Biological consequences of global warming: Is the signal already apparent? Trends Ecol. Evol. 2000, 15, 56–61. [Google Scholar] [CrossRef]
- Barber, V.A.; Juday, G.P.; Finney, B.P. Reduced growth of Alaskan white spruce in the twentieth century from temperature-induced drought stress. Nature 2000, 405, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Hayles, L.; Planells, O.; Gutierrez, E.; Muntan, E.; Helle, G.; Anchukaitis, K.J.; Schleser, G.H. Long tree-ring chronologies reveal 20th century increases in water-use efficiency but no enhancement of tree growth at five Iberian pine forests. Glob. Chang. Biol. 2011, 17, 2095–2112. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Fang, O.; Shao, X. Long-term changes in the tree radial growth and intrinsic water-use efficiency of Chuanxi spruce (Picea likiangensis var. balfouriana) in southwestern China. J. Geogr. Sci. 2018, 28, 833–844. [Google Scholar] [CrossRef]
- Morgan, J.; Pataki, D.; Körner, C.; Clark, H.; Del Grosso, S.; Grűnzweig, J.; Knapp, A.; Mosier, A.; Newton, P.; Niklaus, P. Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia 2004, 140, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Babst, F.; Alexander, M.R.; Szejner, P.; Bouriaud, O.; Klesse, S.; Roden, J.; Ciais, P.; Poulter, B.; Frank, D.; Moore, D.J.; et al. A tree-ring perspective on the terrestrial carbon cycle. Oecologia 2014, 176, 307–322. [Google Scholar] [CrossRef]
- Saurer, M.; Siegwolf, R.T.W.; Schweingruber, F.H. Carbon isotope discrimination indicates improving wateruse efficiency of trees in northern Eurasia over the last 100 years. Glob. Chang. Biol. 2004, 10, 2109–2120. [Google Scholar] [CrossRef]
- Waterhouse, J.S.; Switsur, V.R.; Barker, A.C.; Carter, A.H.C.; Hemming, D.L.; Loader, N.J.; Robertson, I. Northern European trees show a progressively diminishing response to increasing atmospheric carbon dioxide concentrations. Quat. Sci. Rev. 2004, 23, 803–810. [Google Scholar] [CrossRef]
- Liu, X.; Shao, X.; Liang, E.; Zhao, L.; Chen, T.; Qin, D.; Ren, J. Speciesdependent responses of juniper and spruce to increasing CO2 concentration and to climate in semi-arid and arid areas of northwestern China. Plant Ecol. 2007, 193, 195–209. [Google Scholar] [CrossRef]
- Liu, X.; Shao, X.; Wang, L.; Liang, E.; Qin, D.; Ren, J. Response and dendroclimatic implications of δ13C in tree rings to increasing drought on the northeastern Tibetan Plateau. J. Geophys. Res. Atmos. 2008, 113, G03015. [Google Scholar] [CrossRef]
- Huang, J.; Bergeron, Y.; Denneler, B.; Berninger, F.; Tardif, J. Response of forest trees to increased atmospheric CO2. Crit. Rev. Plant Sci. 2007, 26, 265–283. [Google Scholar] [CrossRef]
- Lévesque, M.; Siegwolf, R.; Saurer, M.; Eilmann, B.; Rigling, A. Increased water-use efficiency does not lead to enhanced tree growth under xeric and mesic conditions. New Phytol. 2014, 203, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.C.R.; Anand, M. Probing for the influence of atmospheric CO2 and climate change on forest ecosystems across biomes. Glob. Ecol. Biogeogr. 2013, 22, 83–92. [Google Scholar] [CrossRef]
- Peñuelas, J.; Canadell, J.G.; Ogaya, R. Increased water use efficiency during the 20th century did not translate into enhanced tree growth. Glob. Ecol. Biogeogr. 2011, 20, 597–608. [Google Scholar] [CrossRef]
- Wu, C.; Hember, R.A.; Chen, J.; Kurz, W.A.; Price, D.T.; Boisvenue, C.; Gonsamo, A.; Ju, W. Accelerating forest growth enhancement due to climate and atmospheric changes in British Colombia, Canada over 1956–2001. Sci. Rep. 2014, 4, 4461. [Google Scholar] [CrossRef]
- Feng, X. Trends in intrinsic water-use efficiency of natural trees for the past 100–200 years: A response to atmospheric CO2 concentration. Geochim. Cosmochim. Acta 1999, 63, 1891–1903. [Google Scholar] [CrossRef]
- Li, D.; Fang, K.; Li, Y.; Chen, D.; Liu, X.; Dong, Z.; Zhou, F.; Guo, G.; Shi, F.; Xu, C.; et al. Climate, intrinsic water-use efficiency and tree growth over the past 150 years in humid subtropical China. Glob. Planet. Chang. 2017, 148, 217–226. [Google Scholar] [CrossRef]
- Huang, R.; Zhu, H.; Liu, X.; Liang, E.; Grießinger, J.; Wu, G.; Li, X.; Brauning, A. Does increasing intrinsic water use efficiency (iWUE) stimulate tree growth at natural alpine timberline on the southeastern Tibetan Plateau? Glob. Planet. Chang. 2017, 148, 217–226. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Xu, G.; Zeng, X.; Wu, G.; Zhang, X.; Qin, D. Tree growth and intrinsic water-use efficiency of inland riparian forests in northwestern China: Evaluation via δ13C and δ18O analysis of tree rings. Tree Physiol. 2014, 34, 966–980. [Google Scholar] [CrossRef]
- Xu, G.; Liu, X.; Qin, D.; Chen, T.; Wang, W.; Wu, G.; Sun, W.; An, W.; Zeng, X. Tree-ring δ18O evidence for the drought history of eastern Tianshan Mountains, northwest China since 1700 AD. Int. J. Climatol. 2013, 34, 3336–3347. [Google Scholar] [CrossRef]
- Xu, G.; Liu, X.; Qin, D.; Chen, T.; Sun, W.; An, W.; Wang, W.; Wu, G.; Zeng, X.; Ren, J. Drought history inferred from tree ring δ13C and δ18O in the central Tianshan Mountains of China and linkage with the North Atlantic Oscillation. Theor. Appl. Climatol. 2014, 113, 385–401. [Google Scholar] [CrossRef]
- Yu, S.; Yuan, Y.; Wei, W.; Chen, F.; Zhang, T.; Shang, H.; Zhang, R.; Qin, L. A 352-year record of summer temperature reconstruction in the western Tianshan Mountains, China, as deduced from tree-ring density. Quat. Res. 2013, 80, 158–166. [Google Scholar] [CrossRef]
- Yuan, Y.; Jin, L.; Shao, X.; He, Q.; Li, Z.; Li, J. Variations of the spring precipitation day numbers reconstructed from tree rings in the Urumqi River drainage, Tianshan Mts. over the last 370 years. Chin. Sci. Bull. 2003, 48, 1507–1510. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, J.; Zhang, J. 348-year precipitation reconstruction from tree-rings for the North Slope of the middle Tianshan Mountains. Acta Meteorol. Sin. 2001, 15, 95–104. [Google Scholar]
- Zhang, R.; Shang, H.; Yu, S.; He, Q.; Yuan, Y.; Bolatov, K.; Mambetov, B.T. Tree-ring-based precipitation reconstruction in southern Kazakhstan, reveals drought variability since A.D. 1770. Int. J. Climatol. 2017, 37, 741–750. [Google Scholar] [CrossRef]
- Zhang, R.; Yuan, Y.; Gou, X.; He, Q.; Shang, H.; Zhang, T.; Chen, F.; Ermenbaev, B.; Yu, S.; Qin, L.; et al. Tree-ring-based moisture in western Tianshan Mountains since A.D. 1882 and its possible driving mechanism. Agric. For. Meteorol. 2016, 218–219, 267–276. [Google Scholar] [CrossRef]
- Solomina, O.N.; Dolgova, E.A.; Maximova, O.E. Tree-ring based hydrometeorological reconstructions in Crimea, Caucasus and Tien-Shan. Nestor Hist. Mosc. St.-Petersbg. 2012, 232. (In Russian, with extended English summary). [Google Scholar]
- Solomina, O.N.; Maximova, O.E.; Cook, E.R. Picea Schrenkiana ring width and density at the upper and lower tree limits in the Tien Shan mts Kyrgyz republic as a source of paleoclimatic information. Geogr. Environ. Sustain. 2014, 1, 66–79. [Google Scholar] [CrossRef]
- Li, J.; Cook, E.; Chen, F.; Gou, X.; D’Arrigo, R.D.; Yuan, Y. An extreme drought event in the central Tien Shan area in the year 1945. J. Arid Environ. 2010, 74, 1225–1231. [Google Scholar] [CrossRef]
- Li, J.; Gou, X.; Cook, E.R.; Chen, F. Tree-ring based drought reconstruction for the central Tien Shan area in northwest China. Geophys. Res. Lett. 2006, 33, L07715. [Google Scholar] [CrossRef]
- Zhang, R.; Ermenbaev, B.; Zhang, T.; Ali, M.; Qin, L.; Satylkanov, R. The radial growth of schrenk spruce (Picea schrenkiana Fisch. et Mey.) records the hydroclimatic changes in the Chu River Basin over the past 175 Years. Forests 2019, 10, 223. [Google Scholar] [CrossRef]
- Zhang, R.; Yuan, Y.; Yu, S.; Chen, F.; Zhang, T. Past changes of spring drought in the inner Tianshan Mountains, China, as recorded by tree rings. Boreas 2017, 46, 688–696. [Google Scholar] [CrossRef]
- Zhang, R.; Qin, L.; Shang, H.; Yu, S.; Gou, X.; Mambetov, B.T.; Bolatov, K.; Zheng, W.; Ainur, U.; Bolatova, A. Climatic change in southern Kazakhstan since 1850 C.E. inferred from tree rings. Int. J. Biometeorol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wei, W.; Shang, H.; Yu, S.; Gou, X.; Qin, L.; Bolatov, K.; Mambetov, B.T. A tree ring-based record of annual mass balance changes for the TS.Tuyuksuyskiy Glacier and its linkages to climate change in the Tianshan Mountains. Quat. Sci. Rev. 2019, 205, 10–21. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, T.; Kelgenbayev, N.; He, Q.; Mambetov, B.T.; Dosmanbetov, D.; Shang, H.; Yu, S.; Yuan, Y. A 189-year tree-ring record of drought for the Dzungarian Alatau, arid Central Asia. J. Asian Earth Sci. 2017, 148, 305–314. [Google Scholar] [CrossRef]
- Wu, G.; Liu, X.; Chen, T.; Xu, G.; Wang, W.; Zeng, X.; Zhang, X. Elevation-dependent variations of tree growth and intrinsic water-use efficiency in Schrenk spruce (Picea schrenkiana) in the western Tianshan Mountains, China. Front. Plant Sci. 2015, 6, 309. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liu, X.; Chen, T.; Xu, G.; Wang, W.; Zeng, X.; Wang, B.; Zhang, X. Long-term variation of tree growth and intrinsic water-use efficiency in Schrenk spruce with increasing CO2 concentration and climate warming in the western Tianshan Mountains, China. Acta Physiol. Plant. 2015, 37, 150. [Google Scholar] [CrossRef]
- Speer, J.H. Fundamentals of Tree-ring Research; The University of Arizona Press: Tucson, AZ, USA, 2010; p. 333. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Bull. 1983, 43, 69–75. [Google Scholar]
- Cook, E.R. A Time Series Analysis Approach to Tree Ring Standardization (Dendrochronology, Forestry, Dendroclimatology, Autoregressive Process); The University of Arizona: Tucson, AZ, USA, 1985; p. 185. [Google Scholar]
- Cook, E.R.; Holmes, R.L. Users manual for program ARSTAN. In: Detecting dryness and wetness signals from tree-rings in Shenyang, Northeast China. Palaeogeogr. Palaeoecol. 1986, 302, 301–310. [Google Scholar]
- Brendel, O.; Iannetta, P.P.M.; Stewart, D. A rapid and simple method to isolate pure alpha-cellulose. Phytochem. Anal. 2000, 11, 7–10. [Google Scholar] [CrossRef]
- Evans, M.N.; Schrag, D.P. A stable isotope-based approach to tropical dendroclimatology. Geochim. Cosmochim. Acta 2004, 68, 3295–3305. [Google Scholar] [CrossRef]
- Coplen, T.B. Discontinuance of SMOW and PDB. Nature 1995, 375, 285. [Google Scholar] [CrossRef]
- IAEA. Reference and intercomparison materials for stable isotopes of light elements. In Proceedings of the Consultants Meeting, TECDOC-825, International Atomic Energy Agency, Vienna, Austria, 1–3 December 1995. [Google Scholar]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 36. [Google Scholar] [CrossRef]
- Ehleringer, J.R. Carbon and water relations in desert plants: An isotopic perspective. In Stable Isotopes and Plant Carbon- Water Relations; Saugier, B., Ehleringer, J.R., Hall, A., Eds.; Academic Press: San Diego, CA, USA, 1993; pp. 155–172. [Google Scholar]
- Linares, J.C.; Camarero, J.J. From pattern to process: Linking intrinsic water-use efficiency to drought-induced forest decline. Glob. Chang. Biol. 2012, 18, 1000–1015. [Google Scholar] [CrossRef]
- Shi, Y.; Shen, Y.; Kang, E.; Li, D.; Ding, Y.; Zhang, G.; Hu, R. Recent and future climate change in Northwest China. Clim. Chang. 2007, 80, 379–393. [Google Scholar] [CrossRef]
- Campbell, G.S.; Norman, J.M. An Introduction to Environmental Biophysics, 2nd ed.; Springer: New York, NY, USA, 1998. [Google Scholar]
- Zhang, R.; Yuan, Y.; Gou, X.; Zhang, T.; Zou, C.; Ji, C.; Fan, Z.; Qin, L.; Shang, H.; Li, X. Intra-annual radial growth of Schrenk spruce (Picea schrenkiana Fisch. et Mey) and its response to climate on the northern slopes of the Tianshan Mountains. Dendrochronologia 2016, 40, 36–42. [Google Scholar] [CrossRef]
- McVicar, T.R.; Roderick, M.L.; Donohue, R.J.; Li, L.; Van Niel, T.G.; Thomas, A.; Grieser, J.; Jhajharia, D.; Himri, Y.; Mahowald, N.M.; et al. Global review and synthesis of trends in observed terrestrial near-surface wind speeds: Implications for evaporation. J. Hydrol. 2012, 416–417, 182–205. [Google Scholar] [CrossRef]
- Eamus, D.; Boulain, N.; Cleverly, J.; Breshears, D.D. Global change type drought-induced tree mortality: Vapor pressure deficit is more important than temperature per se in causing decline in tree health. Ecol. Evol. 2013, 3, 2711–2729. [Google Scholar] [CrossRef]
- Feng, X. Long-term Ci/Ca response of trees in western North America to atmospheric CO2 concentration derived from carbon isotope chronologies. Oecologia 1998, 117, 19–25. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Cerling, T.E. Atmospheric CO2 and the ratio of intercellular to ambient CO2 concentrations in plants. Tree Physiol. 1995, 15, 105–111. [Google Scholar] [CrossRef]
- Hietz, P.; Wanek, W.; Dunisch, O. Long-term trends in cellulose 13C and water-use efficiency of tropical Cedrela and Swietenia from Brazil. Tree Physiol. 2005, 25, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Giguere-Croteau, C.; Boucher, E.; Bergeron, Y.; Girardin, M.P.; Drobyshev, I.; Silv, L.C.R.; Helie, J.F.; Garneau, M. North America’s oldest boreal trees are more efficient water users due to increased [CO2], but do not grow faster. Proc. Natl. Acad. Sci. USA 2019, 116, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, X.; He, X.; Davi, N.K.; Li, L.; Bai, X. Response of radial growth to warming and CO2 enrichment in southern Northeast China: A case of Pinus tabulaeformis. Clim. Chang. 2015, 130, 559–571. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Zhang, Q.; Zeng, X.; Xu, G.; Wu, G.; Wang, W. Species-specific tree growth and intrinsic water-use efficiency of Dahurian larch (Larix gmelinii) and Mongolian pine (Pinus sylvestris var. mongolica) growing in a boreal permafrost region of the Greater Hinggan Mountains, Northeastern China. Agric. For. Meteorol. 2018, 248, 145–155. [Google Scholar] [CrossRef]
- Tezara, W.; Mitchell, V.J.; Driscoll, S.D.; Lawlor, D.W. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 1999, 401, 914–917. [Google Scholar] [CrossRef]



| Maximum | Minimum | Mean | Standard Deviation | Variance | Skewness | Kurtosis | |
|---|---|---|---|---|---|---|---|
| δ13C (‰) | −20.95 | −24.68‰ | −22.62‰ | 0.717 | 0.514 | −0.616 | 0.256 |
| iWUE (μmol/mol) | 120.93 | 79.78 | 94.73 | 7.98 | 63.736 | 0.849 | 0.457 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, L.; Yuan, Y.; Shang, H.; Yu, S.; Liu, W.; Zhang, R. Impacts of Global Warming on the Radial Growth and Long-Term Intrinsic Water-Use Efficiency (iWUE) of Schrenk Spruce (Picea schrenkiana Fisch. et Mey) in the Sayram Lake Basin, Northwest China. Forests 2020, 11, 380. https://doi.org/10.3390/f11040380
Qin L, Yuan Y, Shang H, Yu S, Liu W, Zhang R. Impacts of Global Warming on the Radial Growth and Long-Term Intrinsic Water-Use Efficiency (iWUE) of Schrenk Spruce (Picea schrenkiana Fisch. et Mey) in the Sayram Lake Basin, Northwest China. Forests. 2020; 11(4):380. https://doi.org/10.3390/f11040380
Chicago/Turabian StyleQin, Li, Yujiang Yuan, Huaming Shang, Shulong Yu, Weiping Liu, and Ruibo Zhang. 2020. "Impacts of Global Warming on the Radial Growth and Long-Term Intrinsic Water-Use Efficiency (iWUE) of Schrenk Spruce (Picea schrenkiana Fisch. et Mey) in the Sayram Lake Basin, Northwest China" Forests 11, no. 4: 380. https://doi.org/10.3390/f11040380
APA StyleQin, L., Yuan, Y., Shang, H., Yu, S., Liu, W., & Zhang, R. (2020). Impacts of Global Warming on the Radial Growth and Long-Term Intrinsic Water-Use Efficiency (iWUE) of Schrenk Spruce (Picea schrenkiana Fisch. et Mey) in the Sayram Lake Basin, Northwest China. Forests, 11(4), 380. https://doi.org/10.3390/f11040380






