Unveiling the Diversity of Tree Growth Patterns in Boreal Old-Growth Forests Reveals the Richness of Their Dynamics
Abstract
1. Introduction
2. Materials and Methods
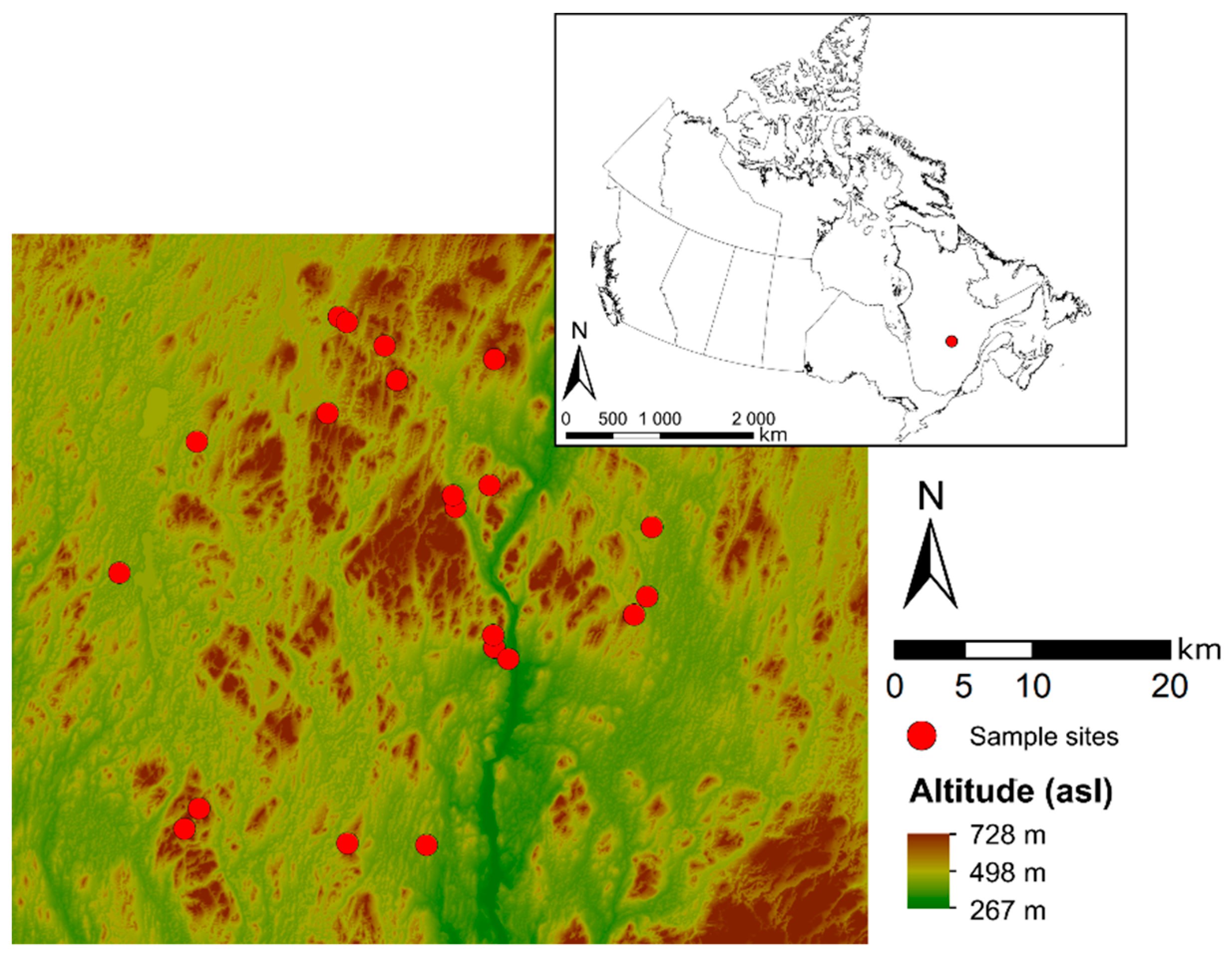
2.1. Sampling
2.2. Data Preparation
2.3. Statistical Analyses
3. Results
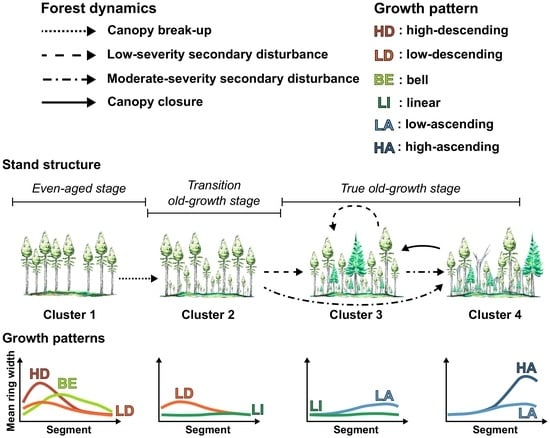
3.1. Radial Growth Patterns
3.2. Distribution of Radial Growth Patterns During Forest Succession
4. Discussion
4.1. Radial Growth Patterns as Accurate Indicators of Stand Dynamics
4.2. Changes in Dominant Radial Growth Patterns along an Old-Growth Succession
4.3. Implications for Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Food and Agriculture Organization of the United Nations. Global Forest Resources Assessment Progress towards Sustainable Forest Management; FAO: Rome, Italy, 2006. [Google Scholar]
- Watson, J.E.M.; Evans, T.; Venter, O.; Williams, B.; Tulloch, A.; Stewart, C.; Thompson, I.; Ray, J.C.; Murray, K.; Salazar, A.; et al. The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2018, 2, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Sarr, D.A.; Puettmann, K.J. Forest management, restoration, and designer ecosystems: Integrating strategies for a crowded planet. Ecoscience 2008, 15, 17–26. [Google Scholar] [CrossRef]
- Halme, P.; Allen, K.A.; Auniņš, A.; Bradshaw, R.H.W.; Brumelis, G.; Čada, V.; Clear, J.L.; Eriksson, A.M.; Hannon, G.; Hyvärinen, E.; et al. Challenges of ecological restoration: Lessons from forests in northern Europe. Biol. Conserv. 2013, 167, 248–256. [Google Scholar] [CrossRef]
- Kneeshaw, D.D.; Burton, P.J.; De Grandpré, L.; Gauthier, S.; Boulanger, Y. Is Management or Conservation of Old Growth Possible in North American Boreal Forests? In Ecology and Recovery of Eastern Old-Growth Forests; Barton, A.M., Keeton, W.S., Eds.; Island Press: Washington, DC, USA, 2018; pp. 139–157. [Google Scholar]
- Tikkanen, O.P.; Martikainen, P.; Hyvarinen, E.; Junninen, K.; Kouki, J. Red-listed boreal forest species of Finland: Associations with forest structure, tree species, and decaying wood. Ann. Zool. Fenn. 2006, 43, 373–383. [Google Scholar]
- Schmiegelow, F.K.A.; Monkkonen, M. Habitat Loss and Fragmentation in Dynamic Landscapes: Avian Perspectives From the Boreal Forest. Ecol. Appl. 2002, 12, 375–389. [Google Scholar]
- Jonsson, B.G.; Siitonen, J. Dead wood and sustainable forest management. In Biodiversity in Dead Wood; Stokland, J.N., Siitonen, J., Jonsson, B.G., Eds.; Cambridge University Press: New York, NY, USA, 2012; pp. 303–337. [Google Scholar]
- Maser, C.; Sedell, J.R. From the Forest to the Sea: The Ecology of Wood in Streams, Rivers, Estuaries and Oceans; St. Lucie Press: Delray Beach, FL, USA, 1994. [Google Scholar]
- Gauthier, S.; Bernier, P.; Kuuluvainen, T.; Shvidenko, A.Z.; Schepaschenko, D.G. Boreal forest health and global change. Science 2015, 349, 819–822. [Google Scholar] [CrossRef]
- Blumroeder, J.S.; Burova, N.; Winter, S.; Goroncy, A.; Hobson, P.R.; Shegolev, A.; Dobrynin, D.; Amosova, I.; Ilina, O.; Parinova, T.; et al. Ecological effects of clearcutting practices in a boreal forest (Arkhangelsk Region, Russian Federation) both with and without FSC certification. Ecol. Indic. 2019, 106, 105461. [Google Scholar] [CrossRef]
- Kuuluvainen, T. Natural variability of forests as a reference for restoring and managing biological diversity in boreal Fennoscandia. Silva Fenn. 2002, 36, 97–125. [Google Scholar] [CrossRef]
- Gauthier, S.; Vaillancourt, M.-A.; Leduc, A.; De Grandpré, L.; Kneeshaw, D.D.; Morin, H.; Drapeau, P.; Bergeron, Y. Ecosystem Management in the Boreal Forest; Presses de l’Université du Québec: Québec, QC, Canada, 2009. [Google Scholar]
- Vanha-Majamaa, I.; Lilja, S.; Ryömä, R.; Kotiaho, J.S.; Laaka-Lindberg, S.; Lindberg, H.; Puttonen, P.; Tamminen, P.; Toivanen, T.; Kuuluvainen, T. Rehabilitating boreal forest structure and species composition in Finland through logging, dead wood creation and fire: The EVO experiment. For. Ecol. Manag. 2007, 250, 77–88. [Google Scholar] [CrossRef]
- Kuuluvainen, T. Forest Management and Biodiversity Conservation Based on Natural Ecosystem Dynamics in Northern Europe: The Complexity Challenge. AMBIO 2009, 38, 309–315. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Aakala, T. Natural Forest Dynamics in Boreal Fennoscandia: A Review and Classification. Silva Fenn. 2011, 45, 823841. [Google Scholar] [CrossRef]
- Lorimer, C.G.; Halpin, C.R. Classification and dynamics of developmental stages in late-successional temperate forests. For. Ecol. Manag. 2014, 334, 344–357. [Google Scholar] [CrossRef]
- Reilly, M.J.; Spies, T.A.; Peters, D.P.C. Regional variation in stand structure and development in forests of Oregon, Washington, and inland Northern California. Ecosphere 2015, 6, 1–27. [Google Scholar] [CrossRef]
- Bergeron, Y.; Harper, K.A. Old-Growth Forests in the Canadian Boreal: The Exception Rather than the Rule? In Old-Growth Forests: Function, Fate and Value; Wirth, C., Gleixner, G., Heimann, M., Eds.; Springer: New York, NY, USA, 2009; pp. 285–300. [Google Scholar]
- Kneeshaw, D.D.; Bergeron, Y.; Kuuluvainen, T. Forest Ecosystem Structure and Disturbance Dynamics across the Circumboreal Forest. In Handbook of Biogeography; Millington, A., Ed.; SAGE: London, UK, 2011; pp. 261–278. [Google Scholar]
- Martin, M.; Fenton, N.J.; Morin, H. Structural diversity and dynamics of boreal old-growth forests case study in Eastern Canada. For. Ecol. Manag. 2018, 422, 125–136. [Google Scholar] [CrossRef]
- Portier, J.; Gauthier, S.; Cyr, G.; Bergeron, Y. Does time since fire drive live aboveground biomass and stand structure in low fire activity boreal forests? Impacts on their management. J. Environ. Manag. 2018, 225, 346–355. [Google Scholar] [CrossRef]
- Moussaoui, L.; Leduc, A.; Fenton, N.J.; Lafleur, B.; Bergeron, Y. Changes in forest structure along a chronosequence in the black spruce boreal forest: Identifying structures to be reproduced through silvicultural practices. Ecol. Indic. 2019, 97, 89–99. [Google Scholar] [CrossRef]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics, 4th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Bergeron, Y.; Leduc, A.; Harvey, B.D.; Gauthier, S. Natural fire regime: A guide for sustainable management of the Canadian boreal forest. Silva Fenn. 2002, 36, 81–95. [Google Scholar] [CrossRef]
- De Grandpré, L.; Gauthier, S.; Allain, C.; Cyr, D.; Périgon, S.; Pham, A.T.; Boucher, D.; Morissette, J.; Reyes, G.; Aakala, T.; et al. Towards an Ecosystem Approach to Managing the Boreal Forest in the North Shore Region: Disturbance Regime and Natural Forest Dynamics. In Ecosystem Management in the Boreal Forest; Presses de l’Université du Québec: Québec, QC, Canada, 2009; pp. 229–255. [Google Scholar]
- De Grandpré, L.; Waldron, K.; Bouchard, M.; Gauthier, S.; Beaudet, M.; Ruel, J.C.; Hébert, C.; Kneeshaw, D.D. Incorporating insect and wind disturbances in a natural disturbance-based management framework for the boreal forest. Forests 2018, 9, 20. [Google Scholar] [CrossRef]
- Martin, M.; Morin, H.; Fenton, N.J. Secondary disturbances of low and moderate severity drive the dynamics of eastern Canadian boreal old-growth forests. Ann. For. Sci. 2019, 76, 1–16. [Google Scholar] [CrossRef]
- Bouchard, M.; Pothier, D. Long-term influence of fire and harvesting on boreal forest age structure and forest composition in eastern Québec. For. Ecol. Manag. 2011, 261, 811–820. [Google Scholar] [CrossRef]
- Boucher, Y.; Perrault-Hébert, M.; Fournier, R.; Drapeau, P.; Auger, I. Cumulative patterns of logging and fire (1940–2009): Consequences on the structure of the eastern Canadian boreal forest. Landsc. Ecol. 2017, 32, 361–375. [Google Scholar] [CrossRef]
- Martin, M.; Boucher, Y.; Fenton, N.J.; Marchand, P.; Morin, H. Forest management has reduced the structural diversity of residual boreal old-growth forest landscapes in Eastern Canada. For. Ecol. Manag. 2020, 458, 1–10. [Google Scholar] [CrossRef]
- Bergeron, Y.; Gauthier, S.; Kafka, V.; Lefort, P.; Lesieur, D. Natural fire frequency for the eastern Canadian boreal forest: Consequences for sustainable forestry. Can. J. For. Res. 2001, 31, 384–391. [Google Scholar] [CrossRef]
- Garet, J.; Pothier, D.; Bouchard, M. Predicting the long-term yield trajectory of black spruce stands using time since fire. For. Ecol. Manag. 2009, 257, 2189–2197. [Google Scholar] [CrossRef]
- Cyr, D.; Gauthier, S.; Bergeron, Y.; Carcaillet, C. Forest management is driving the eastern North American boreal forest outside its natural range of variability. Front. Ecol. Environ. 2009, 7, 519–524. [Google Scholar] [CrossRef]
- [MFFP] Ministère des Forêts de la Faune et des Parcs. Intégration des Enjeux Écologiques Dans les Plans D’aménagement Forestier Intégré de 2018–2023, Cahier 2.1—Enjeux Liés à la Structure d’âge des Forêts; Forestiers, D. de L’aménagement et de L’environnement, Ed.; Gouvernement du Québec: Québec, QC, Canada, 2016. (In Franch)
- Bauhus, J.; Puettmann, K.; Messier, C. Silviculture for old-growth attributes. For. Ecol. Manag. 2009, 258, 525–537. [Google Scholar] [CrossRef]
- Fenton, N.J.; Imbeau, L.; Work, T.; Jacobs, J.; Bescond, H.; Drapeau, P.; Bergeron, Y. Lessons learned from 12 years of ecological research on partial cuts in black spruce forests of northwestern Québec. For. Chron. 2014, 89, 350–359. [Google Scholar] [CrossRef]
- Ruel, J.-C.; Fortin, D.; Pothier, D. Partial cutting in old-growth boreal stands: An integrated experiment. For. Chron. 2013, 89, 360–369. [Google Scholar] [CrossRef]
- Montoro Girona, M.; Morin, H.; Lussier, J.; Walsh, D. Radial growth response of black spruce stands ten years after experimental shelterwoods and seed-tree cuttings in boreal forest. Forests 2016, 7, 240. [Google Scholar] [CrossRef]
- Kneeshaw, D.D.; Burton, P.J.; De Grandpré, L.; Gauthier, S.; Boulanger, Y. Is management or conservation of old growth possible in North American boreal forests? Ecol. Recover. East. Old-Growth For. 2019, 8, 139–157. [Google Scholar]
- Bose, A.K.; Harvey, B.D.; Brais, S.; Beaudet, M.; Leduc, A. Constraints to partial cutting in the boreal forest of Canada in the context of natural disturbance-based management: A review. Forestry 2014, 87, 11–28. [Google Scholar] [CrossRef]
- Montoro Girona, M.; Morin, H.; Lussier, J.-M.; Ruel, J.-C. Post-cutting mortality following experimental silvicultural treatments in unmanaged boreal forest stands. Front. For. Glob. Chang. 2019, 2, 4. [Google Scholar] [CrossRef]
- Lorimer, C.G.; Frelich, L.E. A methodology for estimating canopy disturbance frequency and intensity in dense temperate forests. Can. J. For. Res. 1989, 19, 651–663. [Google Scholar] [CrossRef]
- Frelich, L.E. Forest Dynamics and Disturbance Regimes: Studies from Temperate Evergreen-Deciduous Forests; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Bleicher, N. Four levels of patterns in tree-rings: An archaeological approach to dendroecology. Veg. Hist. Archaeobot. 2014, 23, 615–627. [Google Scholar] [CrossRef]
- Nowacki, G.J.; Abrams, M.D. Radial-Growth Averaging Criteria for Reconstruction Disturbance Histories from Presettlement-Origin Oaks. Ecol. Monogr. 1997, 67, 225–249. [Google Scholar] [CrossRef]
- Fraver, S.; White, A.S. Identifying growth releases in dendrochronological studies of forest disturbance. Can. J. For. Res. 2005, 35, 1648–1656. [Google Scholar] [CrossRef]
- Druckenbrod, D.L.; Pederson, N.; Rentch, J.; Cook, E.R. A comparison of times series approaches for dendroecological reconstructions of past canopy disturbance events. For. Ecol. Manag. 2013, 302, 23–33. [Google Scholar] [CrossRef]
- Moreau, G.; Achim, A.; Pothier, D. A dendrochronological reconstruction of sugar maple growth and mortality dynamics in partially cut northern hardwood forests. For. Ecol. Manag. 2019, 437, 17–26. [Google Scholar] [CrossRef]
- Di Filippo, A.; Biondi, F.; Piovesan, G.; Ziaco, E. Tree ring-based metrics for assessing old-growth forest naturalness. J. Appl. Ecol. 2017, 54, 737–749. [Google Scholar] [CrossRef]
- Niukkanen, L.; Kuuluvainen, T. Radial growth patterns of dominant trees in two old-growth forests in eastern Fennoscandia. J. For. Res. 2011, 16, 228–236. [Google Scholar] [CrossRef]
- Lemay, A.; Krause, C.; Achim, A.; Bégin, J. Growth and wood quality of black spruce and balsam fir following careful logging around small merchantable stems (CLASS) in the boreal forest of Quebec, Canada. Forestry 2018, 91, 271–282. [Google Scholar] [CrossRef]
- Pamerleau-couture, É.; Rossi, S.; Pothier, D.; Krause, C.; Krause, C. Wood properties of black spruce (Picea mariana (Mill) BSP) in relation to ring width and tree height in even- and uneven-aged boreal stands. Ann. For. Sci. 2019, 76, 43. [Google Scholar] [CrossRef]
- Wirth, C.; Messier, C.; Bergeron, Y.; Frank, D.; Fankhänel, A. Old-Growth Forest Definitions: A Pragmatic View. In Old-Growth Forests: Function, Fate and Value; Wirth, C., Gleixner, G., Heimann, M., Eds.; Springer: Berlin, Germany, 2009; pp. 11–34. [Google Scholar]
- Pesklevits, A.; Duinker, P.N.; Bush, P.G. Old-growth forests: Anatomy of a wicked problem. Forests 2011, 2, 343–356. [Google Scholar] [CrossRef]
- Franklin, J.F.; Spies, T.A.; Van Pelt, R.; Carey, A.B.; Thornburgh, D.A.; Berg, D.R.; Lindenmayer, D.B.; Harmon, M.E.; Shaw, D.C.; Bible, K.; et al. Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. For. Ecol. Manag. 2002, 155, 399–423. [Google Scholar] [CrossRef]
- Halpin, C.R.; Lorimer, C.G. Trajectories and resilience of stand structure in response to variable disturbance severities in northern hardwoods. For. Ecol. Manag. 2016, 365, 69–82. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Wallenius, T.H.; Kauhanen, H.; Aakala, T.; Mikkola, K.; Demidova, N.; Ogibin, B. Episodic, patchy disturbances characterize an old-growth Picea abies dominated forest landscape in northeastern Europe. For. Ecol. Manag. 2014, 320, 96–103. [Google Scholar] [CrossRef]
- Khakimulina, T.; Fraver, S.; Drobyshev, I. Mixed-severity natural disturbance regime dominates in an old-growth Norway spruce forest of northwest Russia. J. Veg. Sci. 2016, 27, 400–413. [Google Scholar] [CrossRef]
- Trotsiuk, V.; Svoboda, M.; Janda, P.; Mikolas, M.; Bace, R.; Rejzek, J.; Samonil, P.; Chaskovskyy, O.; Korol, M.; Myklush, S. A mixed severity disturbance regime in the primary Picea abies (L.) Karst. forests of the Ukrainian Carpathians. For. Ecol. Manag. 2014, 334, 144–153. [Google Scholar] [CrossRef]
- Bače, R.; Schurman, J.S.; Brabec, M.; Čada, V.; Després, T.; Janda, P.; Lábusová, J.; Mikoláš, M.; Morrissey, R.C.; Mrhalová, H.; et al. Long-term responses of canopy–understorey interactions to disturbance severity in primary Picea abies forests. J. Veg. Sci. 2017, 28, 1128–1139. [Google Scholar] [CrossRef]
- Meigs, G.W.; Morrissey, R.C.; Bače, R.; Chaskovskyy, O.; Čada, V.; Després, T.; Donato, D.C.; Janda, P.; Lábusová, J.; Seedre, M.; et al. More ways than one: Mixed-severity disturbance regimes foster structural complexity via multiple developmental pathways. For. Ecol. Manag. 2017, 406, 410–426. [Google Scholar] [CrossRef]
- Burrascano, S.; Keeton, W.S.; Sabatini, F.M.; Blasi, C. Commonality and variability in the structural attributes of moist temperate old-growth forests: A global review. For. Ecol. Manag. 2013, 291, 458–479. [Google Scholar] [CrossRef]
- Oliver, C.D. Forest development in North America following major disturbances. For. Ecol. Manag. 1981, 3, 153–168. [Google Scholar] [CrossRef]
- Shorohova, E.; Kneeshaw, D.D.; Kuuluvainen, T.; Gauthier, S. Variability and dynamics of old-growth forests in the circumboreal zone: Implications for conservation, restoration and management. Silva Fenn. 2011, 45, 785–806. [Google Scholar] [CrossRef]
- Robitaille, A.; Saucier, J.-P. Paysages Régionaux du Québec Méridional; Les Publications du Québec: Montréal, QC, Canada, 1998. [Google Scholar]
- Couillard, P.-L.; Frégeau, M.; Payette, S.; Grondin, P.; Lavoie, M.; Laflamme, J. Dynamique et Variabilité Naturelle de la Pessière à Mousses au Nord de la Région du Lac-Saint-Jean; Presses de l’Université du Québec: Québec, QC, Canada, 2016. (In Franch) [Google Scholar]
- Martin, M.; Fenton, N.J.; Morin, H. Boreal old-growth forest structural diversity challenges aerial photographic survey accuracy. Can. J. For. Res. 2020, 50, 155–169. [Google Scholar] [CrossRef]
- Farr, T.G.; Rosen, P.A.; Caro, E.; Crippen, R.; Duren, R.; Hensley, S.; Kobrick, M.; Paller, M.; Rodriguez, E.; Roth, L.; et al. The Shuttle Radar Topography Mission. Rev. Geophys. 2007, 45, 33. [Google Scholar] [CrossRef]
- Uhlig, P.A.; Harris, G.; Craig, C.; Bowling, B.; Chambers, B.; Naylor, B.; Beemer, G. Old-Growth Forest Definitions for Ontario; Ontario Ministry of Natural Resources, Ed.; Queen’s Printer for Ontario: Toronto, ON, Canada, 2001. [Google Scholar]
- Gauthier, S.; Boucher, D.; Morissette, J.; De Grandpré, L. Fifty-seven years of composition change in the eastern boreal forest of Canada. J. Veg. Sci. 2010, 21, 772–785. [Google Scholar] [CrossRef]
- Holmes, R.l. Computer-assisted quality control in tree-ring dating measurement. Tree-Ring Bull. 1983, 69–78. [Google Scholar]
- Kneeshaw, D.D.; Gauthier, S. Old growth in the boreal forest: A dynamic perspective at the stand and landscape level. Environ. Rev. 2003, 11, S99–S114. [Google Scholar] [CrossRef]
- Hartigan, J.A.; Wong, M.A. Algorithm AS 136: A k-means clustering algorithm. J. R. Stat. Soc. 1979, 28, 100–108. [Google Scholar] [CrossRef]
- Dolnicar, S. Analyzing destination images: A perceptual charting approach. J. Travel Tour. Mark. 1999, 8, 43–57. [Google Scholar] [CrossRef]
- Williams, L.J.; Abdi, H. Fisher’s Least Significant Difference Test. In Encyclopedia of Research Design; Salkind, N., Ed.; SAGE: Thousand Oaks, CA, USA, 2010; pp. 1–6. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Adv. Microbiol. 2019, 9, 8. [Google Scholar]
- Oksanen, J.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; MCGlinn, D.; Minchin, P.R.B.; O’Hara, B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community ecology package. J. Geosci. Environ. Protect. 2018, 5, 11. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.2-2017. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 22 January 2020).
- Rossi, S.; Tremblay, M.J.; Morin, H.; Savard, G. Growth and productivity of black spruce in even- and uneven-aged stands at the limit of the closed boreal forest. For. Ecol. Manag. 2009, 258, 2153–2161. [Google Scholar] [CrossRef]
- Tremblay, M.-J.; Rossi, S.; Morin, H. Growth dynamics of black spruce in stands located between the 51st and 52nd parallels in the boreal forest of Quebec, Canada. Can. J. For. Res. 2011, 41, 1769–1778. [Google Scholar] [CrossRef]
- Morin, H. Dynamics of balsam fir in relation to spruce budworm outbreaks in the Boreal Zone of Quebec. Can. J. For. Res. 1994, 24, 730–741. [Google Scholar] [CrossRef]
- Harvey, B.D.; Leduc, A.; Gauthier, S.; Bergeron, Y. Stand-landscape integration in natural disturbance-based management of the southern boreal forest. For. Ecol. Manag. 2002, 155, 369–385. [Google Scholar] [CrossRef]
- Firm, D.; Nagel, T.A.; Diaci, J. Disturbance history and dynamics of an old-growth mixed species mountain forest in the Slovenian Alps. For. Ecol. Manag. 2009, 257, 1893–1901. [Google Scholar] [CrossRef]
- Dang, H.; Zhang, K.; Zhang, Y.; Jiang, M.; Zhang, Q. Disturbance dynamics and history of an old-growth subalpine fir (Abies fargesii) forest in central China. Plant Ecol. 2014, 215, 1111–1121. [Google Scholar] [CrossRef]
- Trotsiuk, V.; Svoboda, M.; Weber, P.; Pederson, N.; Klesse, S.; Janda, P.; Martin-Benito, D.; Mikolas, M.; Seedre, M.; Bace, R.; et al. The legacy of disturbance on individual tree and stand-level aboveground biomass accumulation and stocks in primary mountain Picea abies forests. For. Ecol. Manag. 2016, 373, 108–115. [Google Scholar] [CrossRef]
- Robichaud, E.; Methven, I.R. The effect of site quality on the timing of stand breakup, tree longevity, and the maximum attainable height of black spruce. Can. J. For. Res. 1993, 1514–1519. [Google Scholar] [CrossRef]
- Larson, D.W. The paradox of great longevity in a short-lived tree species. Exp. Gerontol. 2001, 36, 651–673. [Google Scholar] [CrossRef]
- Pham, A.T.; De Grandpré, L.; Gauthier, S.; Bergeron, Y. Gap dynamics and replacement patterns in gaps of the northeastern boreal forest of Quebec. Can. J. For. Res. 2004, 34, 353–364. [Google Scholar] [CrossRef]
- Le Goff, H.; De Grandpré, L.; Kneeshaw, D.D.; Bernier, P. L’aménagement durable des vieilles forêts boréales: Mythes, pistes de solutions et défis. For. Chron. 2010, 86, 63–69. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Gauthier, S. Young and old forest in the boreal: Critical stages of ecosystem dynamics and management under global change. For. Ecosyst. 2018, 5, 26. [Google Scholar] [CrossRef]
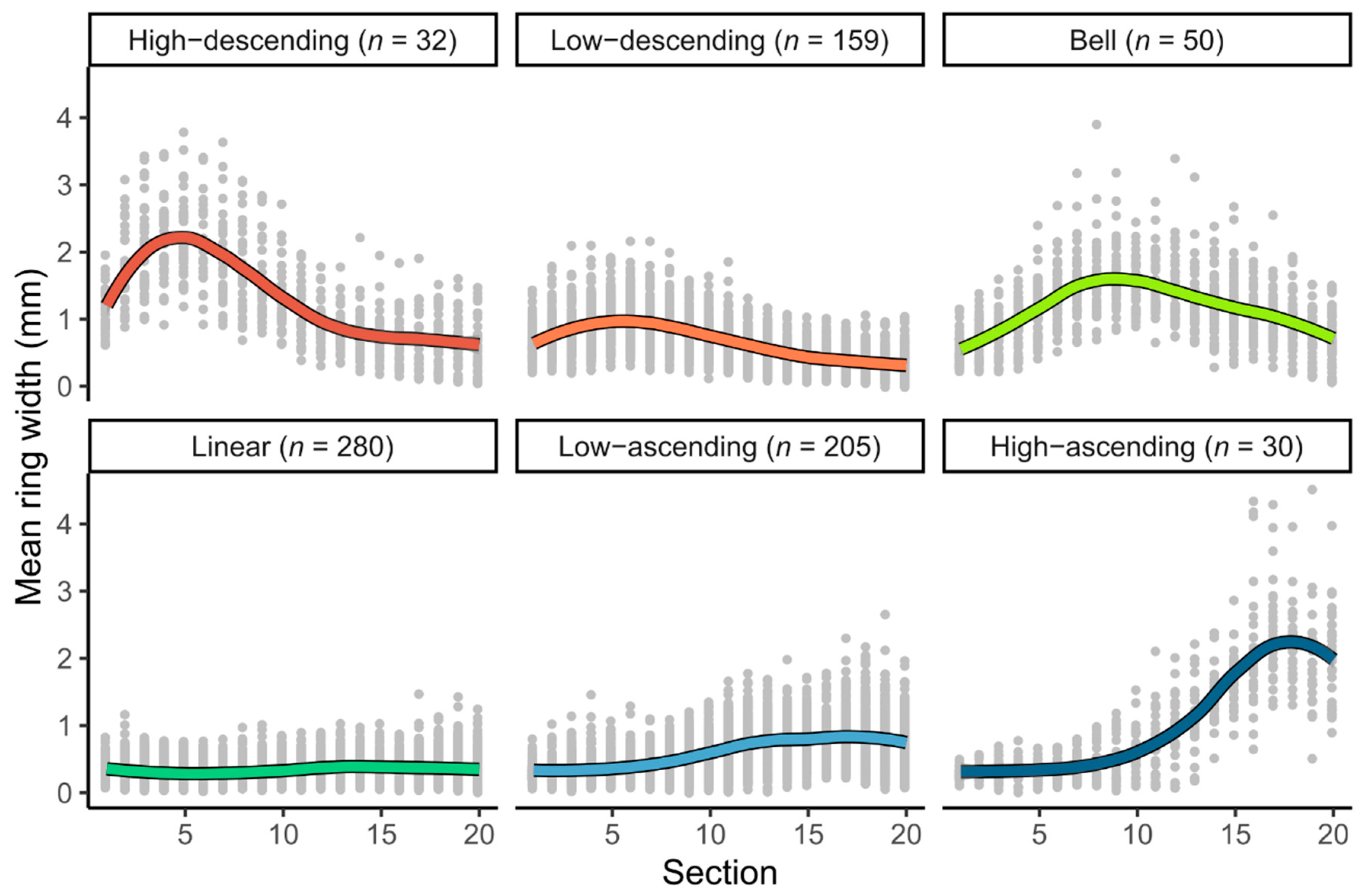
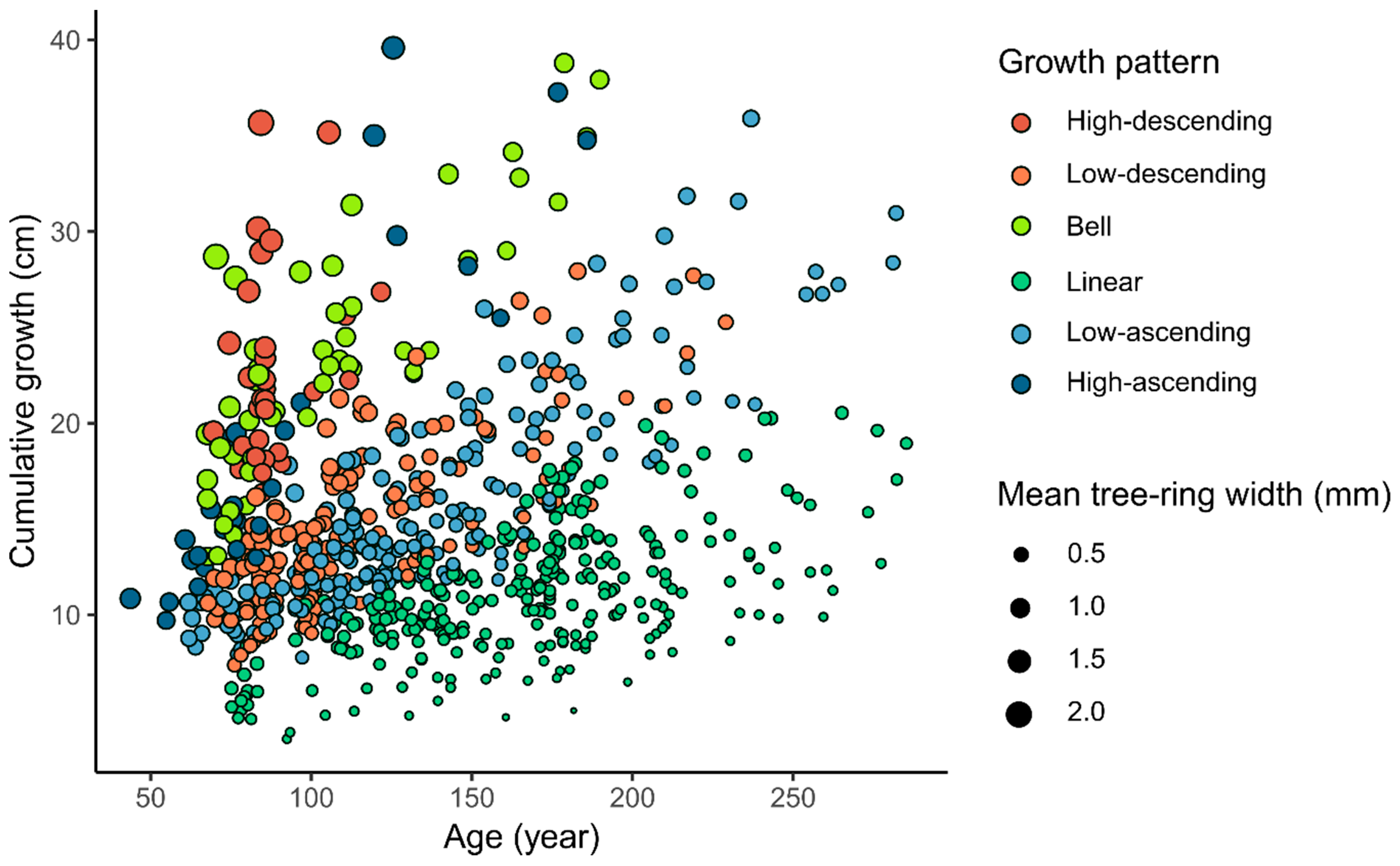
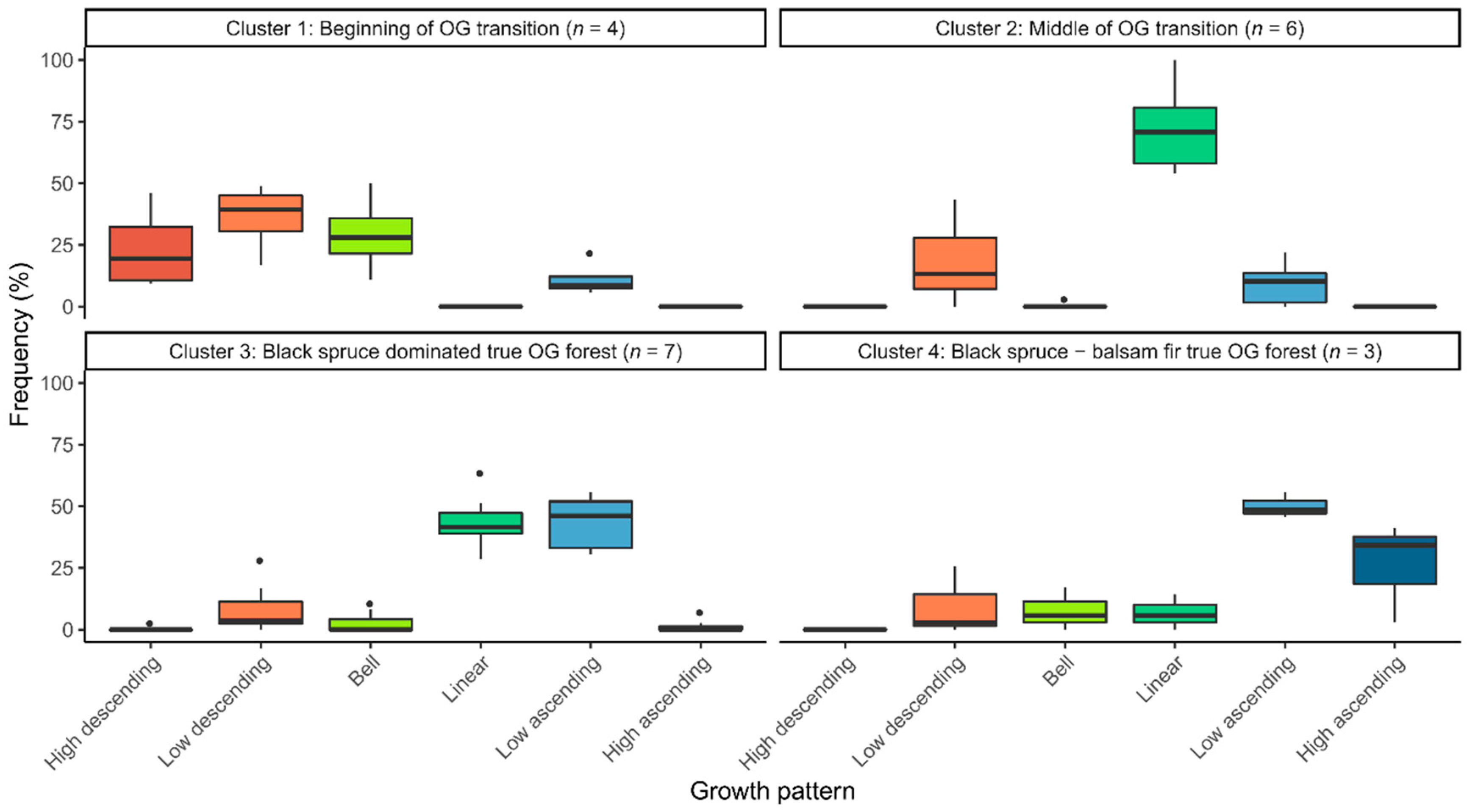
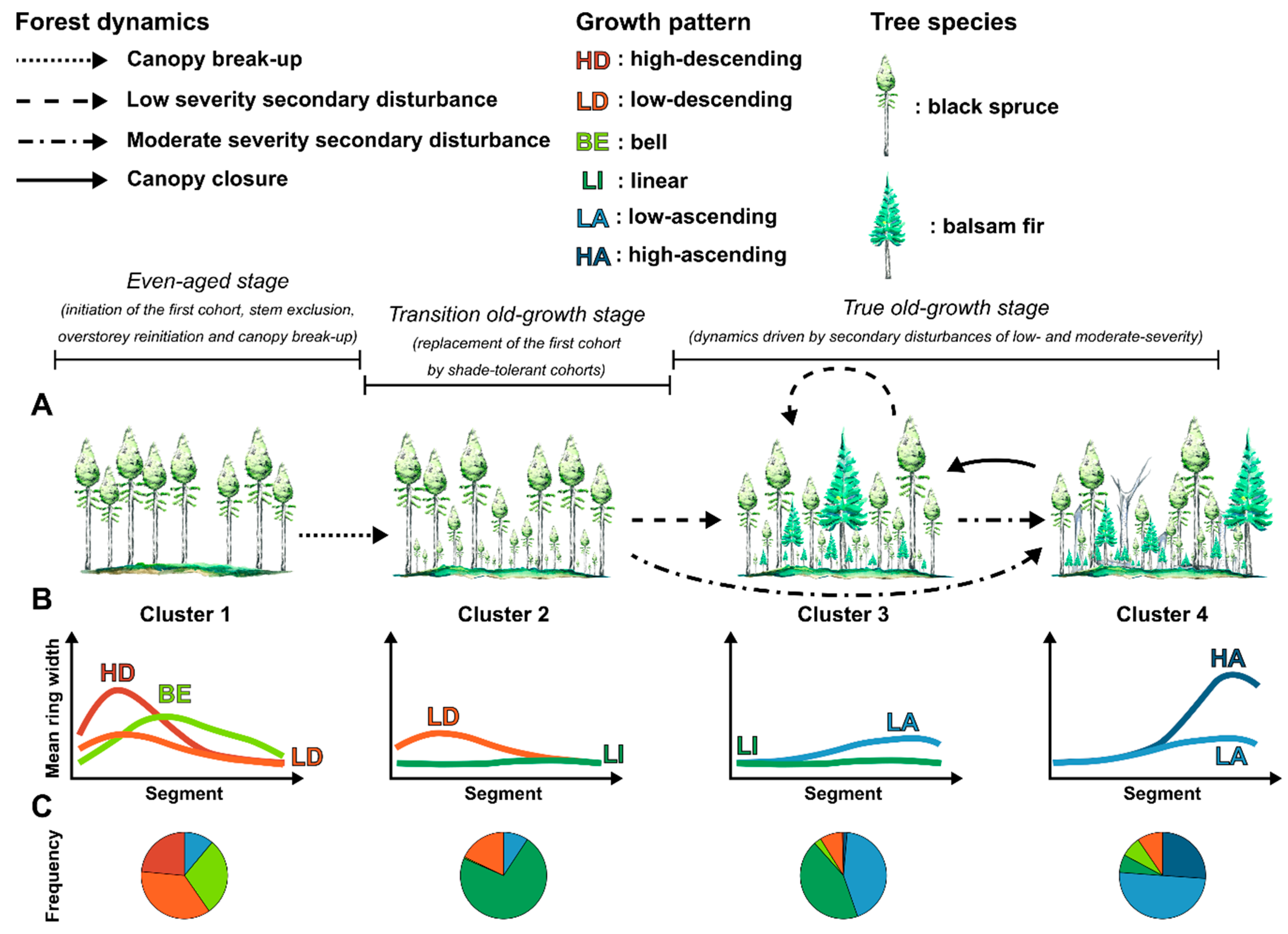
| Growth Pattern | Species | Total | |
|---|---|---|---|
| Black Spruce | Balsam Fir | ||
| High-descending | 26 | 2 | 28 |
| 4.2% | 1.8% | 3.8% | |
| Low-descending | 128 | 16 | 144 |
| 20.5% | 14% | 19.5% | |
| Bell | 47 | 3 | 50 |
| 7.5% | 2.6% | 6.8% | |
| Linear | 242 | 38 | 280 |
| 38.8% | 33.3% | 38% | |
| Low-ascending | 151 | 54 | 205 |
| 24.2% | 47.4% | 27.8% | |
| High-ascending | 29 | 1 | 30 |
| 4.7% | 0.9% | 4.1% | |
| Total | 623 | 114 | 737 |
| 100% | 100% | 100% | |
| Growth Attribute | High-Descending (n = 32) | Low-Descending (n = 159) | Bell (n = 50) | Linear (n = 280) | Low-Ascending (n = 205) | High-Ascending (n = 30) |
|---|---|---|---|---|---|---|
| Tree mean ring width (mm) | 1.29 ± 0.28 a | 0.64 ± 0.13 b | 1.15 ± 0.22 a | 0.33 ± 0.08 d | 0.59 ± 0.11 c | 1.04 ± 0.17 a |
| Ring width standard deviation (mm·year−1) | 0.72 ± 0.2 a | 0.33 ± 0.11 c | 0.49 ± 0.15 b | 0.16 ± 0.05 d | 0.33 ± 0.11 c | 0.86 ± 0.23 a |
| Ring width 5th percentile (mm·year−1) | 0.45 ± 0.25 a | 0.22 ± 0.12 b | 0.43 ± 0.19 a | 0.13 ± 0.05 d | 0.19 ± 0.09 c | 0.16 ± 0.07 c |
| Ring width 95th percentile (mm·year−1) | 2.59 ± 0.57 a | 1.24 ± 0.3 c | 1.97 ± 0.44 b | 0.64 ± 0.17 d | 1.21 ± 0.3 c | 2.64 ± 0.64 a |
| Age (years) | 89.1 ± 10.9 d | 116 ± 34.9 c | 106 ± 35.2 d | 167 ± 44.5 a | 134 ± 47.6 b | 91.2 ± 36.8 d |
| Cumulative radial growth (cm) | 22.8 ± 5.12 a | 14.7 ± 4.2 c | 23.6 ± 6.37 a | 11.1 ± 3.36 d | 15.6 ± 5.53 c | 19 ± 8.63 b |
| Type | Attribute | Cluster 1 (n = 4) | Cluster 2 (n = 6) | Cluster 3 (n = 7) | Cluster 4 (n = 3) |
|---|---|---|---|---|---|
| Structural | Merchantable tree density (n·ha−1) | 1194 ± 314 a | 688 ± 378 a | 904 ± 331 a | 833 ± 151 a |
| Basal area (m2 ha−1) | 27.3 ± 9.71 a | 11.2 ± 7.1 b | 18.1 ± 8.31 a,b | 16.6 ± 1.7 a,b | |
| Sapling density (n·ha−1) | 1238 ± 1359 a | 2071 ± 1384 a | 1961 ± 842 a | 4183 ± 3790 a | |
| Maximum height (m) | 15.8 ± 2.78 b | 15.7 ± 4.34 a,b | 19.5 ± 2.82 a,b | 20.6 ± 2.57 a | |
| Balsam fir proportion (%) | 13.1 ± 18.7 b,c | 0.26 ± 0.64 c | 16.4 ± 28.5 a,b | 32.3 ± 16.7 a | |
| Coarse woody debris volume (m3·ha−1) | 16 ± 14.2 c | 24.1 ± 20.5 c | 54.8 ± 26.5 b | 146 ± 46.8 a | |
| Successional | Min. time since the last fire (year) | 106 ± 29.3 c | 191 ± 54.7 b | 259 ± 28.2 a | 179 ± 43.2 b,c |
| Cohort basal area proportion | 0.13 ± 0.12 b | 0.47 ± 0.4 a,b | 0.8 ± 0.35 a | 0.82 ± 0.3 a | |
| Environmental | Slope (%) | 14 ± 14.7 a | 4 ± 4.05 a | 14.7 ± 6.66 a | 14.4 ± 11.7 a |
| Depth of the organic horizon (cm) | 20 ± 13.4 b | 42.2 ± 16.4 a | 29.4 ± 10.4 a,b | 27.3 ± 15.4 a,b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, M.; Krause, C.; Fenton, N.J.; Morin, H. Unveiling the Diversity of Tree Growth Patterns in Boreal Old-Growth Forests Reveals the Richness of Their Dynamics. Forests 2020, 11, 252. https://doi.org/10.3390/f11030252
Martin M, Krause C, Fenton NJ, Morin H. Unveiling the Diversity of Tree Growth Patterns in Boreal Old-Growth Forests Reveals the Richness of Their Dynamics. Forests. 2020; 11(3):252. https://doi.org/10.3390/f11030252
Chicago/Turabian StyleMartin, Maxence, Cornélia Krause, Nicole J. Fenton, and Hubert Morin. 2020. "Unveiling the Diversity of Tree Growth Patterns in Boreal Old-Growth Forests Reveals the Richness of Their Dynamics" Forests 11, no. 3: 252. https://doi.org/10.3390/f11030252
APA StyleMartin, M., Krause, C., Fenton, N. J., & Morin, H. (2020). Unveiling the Diversity of Tree Growth Patterns in Boreal Old-Growth Forests Reveals the Richness of Their Dynamics. Forests, 11(3), 252. https://doi.org/10.3390/f11030252