Forest Landscape Heterogeneity Increases Shrub Diversity at the Expense of Tree Seedling Diversity in Temperate Mixedwood Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Landscape Selection and Characterisation of Heterogeneity
2.3. Field Sampling
2.4. Gap Proportion Assessment
2.5. Statistical Analyses
2.5.1. Landscape Heterogeneity Characterization
2.5.2. Alpha- and Beta-Diversity Characterization
3. Results
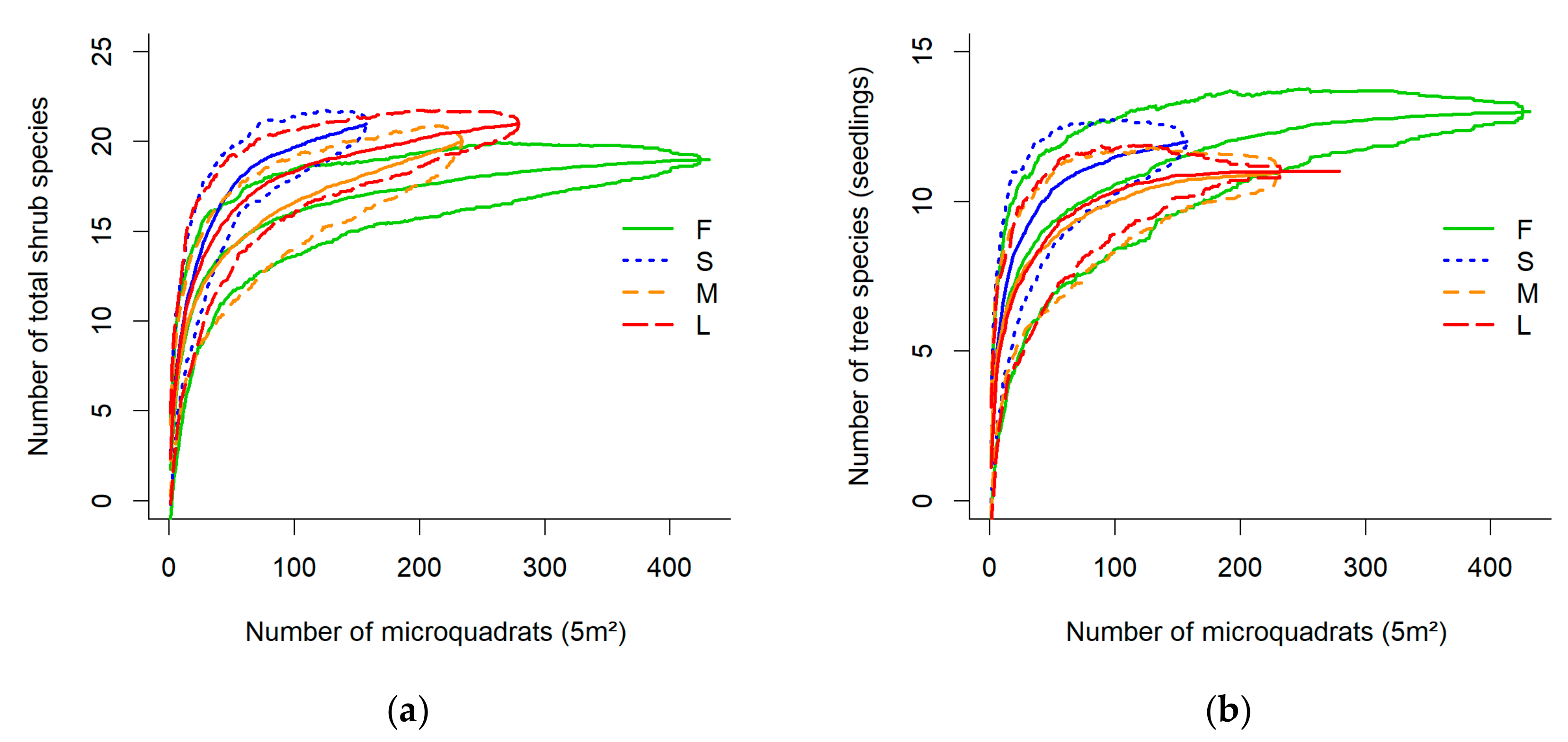
3.1. Effect of Landscape Heterogeneity
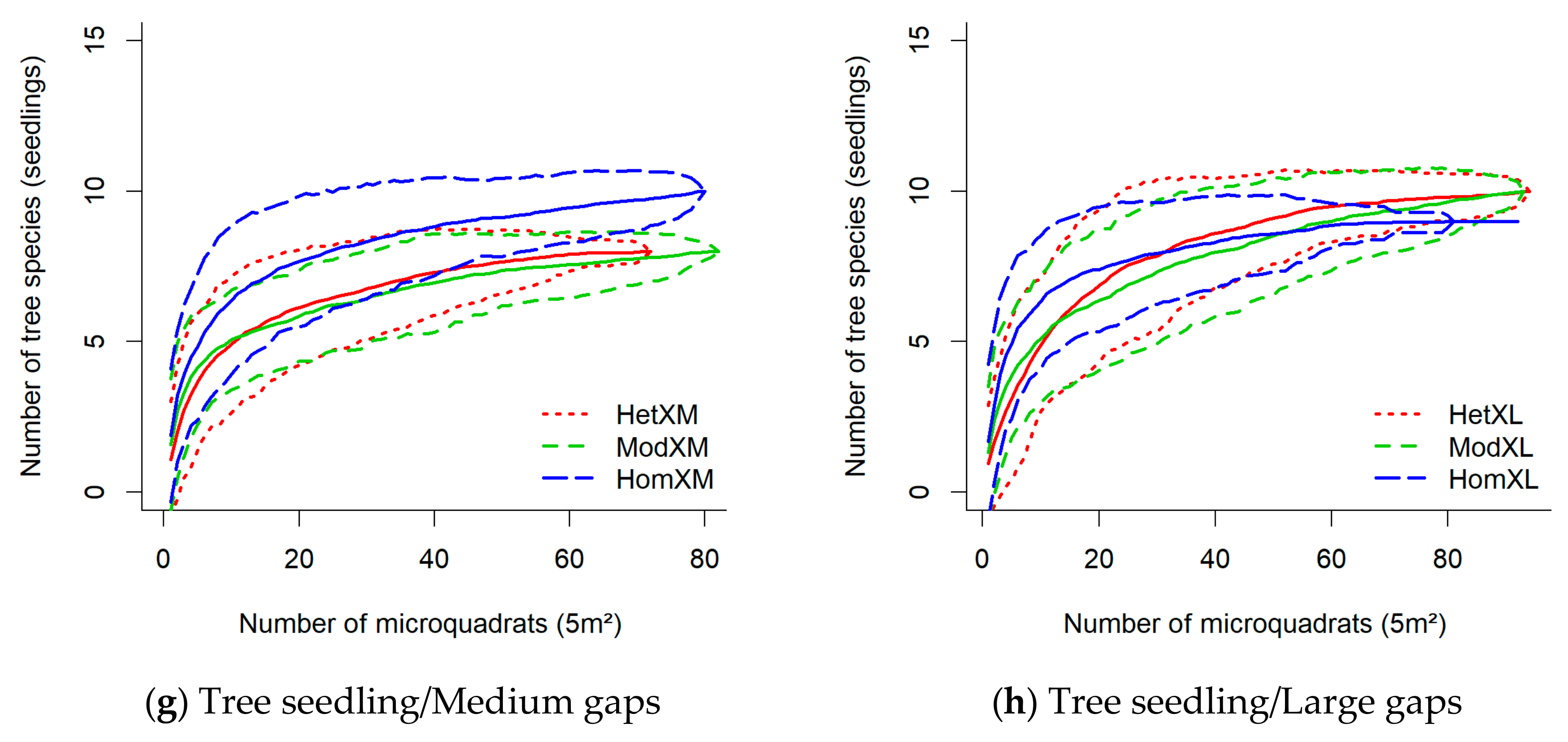
3.2. Diversity in Response to Gap/Forest Environment
4. Discussion
4.1. Heterogeneity–Diversity Relationship or Biotic Interaction?
4.2. Diversity–Resource Relationship and Species Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Levin, S.A. The problem of pattern and scale in ecology: The Robert H. MacArthur award lecture. Ecology 1992, 73, 1943–1967. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Willis, K.J.; Field, R. Scale and species richness: towards a general, hierarchical theory of species diversity. J. Biogeogr. 2001, 28, 453–470. [Google Scholar] [CrossRef]
- Sarr, D.A.; Hibbs, D.E.; Huston, M.A. A hierarchical perspective of plant diversity. Q. Rev. Biol. 2005, 80, 187–212. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.G. Landscape ecology: the effect of pattern on process. Annu. Rev. Ecol. S. 1989, 20, 171–197. [Google Scholar] [CrossRef]
- Wiens, J.A. Ecological heterogeneity: an ontogeny of concepts and approaches. In The Ecological Consequences of Environmental Heterogeneity; Hutchings, M.J., John, E.A., Stewart, A.J.A., Eds.; Blackwell Science: Oxford, UK, 2000; pp. 9–31. [Google Scholar]
- Fahrig, L.; Baudry, J.; Brotons, L.; Burel, F.G.; Crist, T.O.; Fuller, R.J.; Sirami, C.; Siriwardena, G.M.; Martin, J.L. Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 2010, 14, 101–112. [Google Scholar] [CrossRef]
- Tscharntke, T.; Tylianakis, J.M.; Rand, T.A.; Didham, R.K.; Fahrig, L.; Batary, P.; Bengtsson, J.; Clough, Y.; Crist, T.O.; Dormann, C.F.; et al. Landscape moderation of biodiversity patterns and processes-eight hypotheses. Biol. Rev. 2012, 87, 661–685. [Google Scholar] [CrossRef]
- Gámez-Virués, S.; Perović, D.J.; Gossner, M.M.; Börschig, C.; Blüthgen, N.; De Jong, H.; Simons, N.K.; Klein, A.M.; Krauss, J.; Maier, G.; et al. Landscape simplification filters species traits and drives biotic homogenization. Nat. Commun. 2015, 6, 8568. [Google Scholar]
- Sutcliffe, L.M.; Batáry, P.; Becker, T.; Orci, K.M.; Leuschner, C. Both local and landscape factors determine plant and Orthoptera diversity in the semi-natural grasslands of Transylvania, Romania. Biodivers. Conserv. 2015, 24, 229–245. [Google Scholar] [CrossRef]
- Kumar, S.; Stohlgren, T.J.; Chong, G.W. Spatial heterogeneity influences native and nonnative plant species richness. Ecology 2006, 87, 3186–3199. [Google Scholar] [CrossRef]
- Dufour, A.; Gadallah, F.; Wagner, H.H.; Guisan, A.; Buttler, A. Plant species richness and environmental heterogeneity in a mountain landscape: effects of variability and spatial configuration. Ecography 2006, 29, 573–584. [Google Scholar] [CrossRef]
- Poggio, S.L.; Chaneton, E.J.; Ghersa, C.M. Landscape complexity differentially affects alpha, beta, and gamma diversities of plants occurring in fencerows and crop fields. Biol. Conserv. 2010, 143, 2477–2486. [Google Scholar] [CrossRef]
- Zelený, D.; Li, C.F.; Chytrý, M. Pattern of local plant species richness along a gradient of landscape topographical heterogeneity: result of spatial mass effect or environmental shift? Ecography 2010, 33, 578–589. [Google Scholar] [CrossRef]
- Gabriel, D.; Thies, C.; Tscharntke, T. Local diversity of arable weeds increases with landscape complexity. Perspect. Plant Ecol. 2005, 7, 85–93. [Google Scholar] [CrossRef]
- Gaba, S.; Chauvel, B.; Dessaint, F.; Bretagnolle, V.; Petit, S. Weed species richness in winter wheat increases with landscape heterogeneity. Agr. Ecosyst. Environ. 2010, 138, 318–323. [Google Scholar] [CrossRef]
- Young, T.P.; Peffer, E. “Recalcitrant understory layers” revisited: arrested succession and the long life-spans of clonal mid-successional species. Can. J. For. Res. 2010, 40, 1184–1188. [Google Scholar] [CrossRef]
- Valladares, F.; Guzmán, B. Canopy structure and spatial heterogeneity of understory light in an abandoned Holm oak woodland. Ann. For. Sci. 2006, 63, 749–761. [Google Scholar] [CrossRef]
- Leibold, M.A.; McPeek, M.A. Coexistence of the niche and neutral perspectives in community ecology. Ecology 2006, 87, 1399–1410. [Google Scholar] [CrossRef]
- Adler, P.B.; HilleRisLambers, J.; Levine, J.M. A niche for neutrality. Ecol. Lett. 2007, 10, 95–104. [Google Scholar] [CrossRef]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef]
- Grace, J.B. The factors controlling species density in herbaceous plant communities: an assessment. Perspect. Plant Ecol. 1999, 2, 1–28. [Google Scholar] [CrossRef]
- Woods, K.D. Intermediate disturbance in a late-successional hemlock-northern hardwood forest. J. Ecol. 2004, 92, 464–476. [Google Scholar] [CrossRef]
- Kneeshaw, D.D.; Prévost, M. Natural canopy gap disturbances and their role in maintaining mixed-species forests of central Québec, Canada. Can. J. For. Res. 2007, 37, 1534–1544. [Google Scholar] [CrossRef]
- Royo, A.A.; Carson, W.P. On the formation of dense understory layers in forests worldwide: consequences and implications for forest dynamics, biodiversity, and succession. Can. J. For. Res. 2006, 36, 1345–1362. [Google Scholar] [CrossRef]
- De Blois, S.; Domon, G.; Bouchard, A. Landscape issues in plant ecology. Ecography 2002, 25, 244–256. [Google Scholar] [CrossRef]
- Kern, C.C.; Montgomery, R.A.; Reich, P.B.; Strong, T.F. Harvest-created canopy gaps increase species and functional trait diversity of the forest ground-layer community. Forest Science 2014, 60, 335–344. [Google Scholar] [CrossRef]
- Busing, R.T.; Brokaw, N. Tree species diversity in temperate and tropical forest gaps: the role of lottery recruitment. Folia Geobot. 2002, 37, 33–43. [Google Scholar] [CrossRef]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- Raymond, P.; Royo, A.A.; Prévost, M.; Dumais, D. Assessing the single-tree and small group selection cutting system as intermediate disturbance to promote regeneration and diversity in temperate mixedwood stands. Forest Ecol. Manag. 2018, 430, 21–32. [Google Scholar] [CrossRef]
- Balvanera, P.; Lott, E.; Segura, G.; Siebe, C.; Islas, A. Patterns of beta-diversity in a Mexican tropical dry forest. J. Veg. Sci. 2002, 13, 145–158. [Google Scholar] [CrossRef]
- Alahuhta, J.; Kosten, S.; Akasaka, M.; Auderset, D.; Azzella, M.M.; Bolpagni, R.; Bove, C.P.; Chambers, P.A.; Chappuis, E.; Clayton, J.; et al. Global variation in the beta diversity of lake macrophytes is driven by environmental heterogeneity rather than latitude. J. Biogeogr. 2017, 44, 1758–1769. [Google Scholar] [CrossRef]
- Robitaille, A.; Saucier, J.-P. Paysages régionaux du Québec méridional; Les Publications du Québec: Québec, QC, Canada, 1998. [Google Scholar]
- Prévost, M.; Roy, V.; Raymond, P. Sylviculture et régénération des forêts mixtes du Québec (Canada): une approche qui respecte la dynamique naturelle des peuplements; Direction de la recherche forestière, Ministère des ressources naturelles: Québec, QC, Canada, 2003. [Google Scholar]
- Grenier, D.; Bergeron, Y.; Kneeshaw, D.; Gauthier, S. Fire frequency for the transitional mixedwood forest of Timiskaming, Québec, Canada. Can. J. For. Res. 2005, 35, 656–666. [Google Scholar] [CrossRef]
- Bouchard, M.; Kneeshaw, D.; Bergeron, Y. Forest dynamics after successive spruce budworm outbreaks in mixedwood forests. Ecology 2006, 87, 2319–2329. [Google Scholar] [CrossRef]
- Cumming, S.G. Effective fire suppression in boreal forests. Can. J. For. Res. 2005, 35, 772–786. [Google Scholar] [CrossRef]
- Boucher, Y. Registre des états de référence; Direction de la recherche forestiére, Gouvernement du Québec: Québec, QC, Canada, 2011. [Google Scholar]
- Robitaille, L.; Majcen, Z. Traitements sylvicoles visant à favoriser la régénération et la croissance du bouleau jaune. L’Aubelle 1991, 82, 10–12. [Google Scholar]
- Majcen, Z. Historique des coupes de jardinage dans les forêts inéquiennes au Québec. Rev. For. Fr. 1994, 4, 375–384. [Google Scholar] [CrossRef]
- Raymond, P.; Dumais, D.; Prévost, M. Écologie et sylviculture la forêt mixte: Qu’avons-nous appris au cours de la dernière décennie? Carrefour Forêt Innovations: Québec, QC, Canada, 2012. [Google Scholar]
- Gastaldello, P.; Ruel, J.C.; Lussier, J.M. Remise en production des bétulaies jaunes résineuses dégradées: étude du succès d’installation de la régénération. For. Chron. 2007, 83, 742–753. [Google Scholar] [CrossRef]
- Roy, V.; Prévost, M. Caractérisation des bétulaies jaunes résineuses dégradées de la sapinière à bouleau jaune; Programme de mise en valeur des ressources du milieu forestier, Volet 1: Québec, QC, Canada, 2001. [Google Scholar]
- Bresee, M.K.; Le Moine, J.; Mather, S.; Brosofske, K.D.; Chen, J.; Crow, T.R.; Rademacher, J. Disturbance and landscape dynamics in the Chequamegon National Forest Wisconsin, USA, from 1972 to 2001. Landsc. Ecol. 2004, 19, 291–309. [Google Scholar] [CrossRef]
- Peck, J.E.; Zenner, E.K.; Brang, P.; Zingg, A. Tree size distribution and abundance explain structural complexity differentially within stands of even-aged and uneven-aged structure types. Eur. J. Forest Res. 2014, 133, 335–346. [Google Scholar] [CrossRef]
- McElhinny, C.; Gibbons, P.; Brack, C.; Bauhus, J. Forest and woodland stand structural complexity: its definition and measurement. Forest Ecol. Manag. 2005, 218, 1–24. [Google Scholar] [CrossRef]
- Senécal, J.F.; Doyon, F.; Messier, C. Management implications of varying gap detection height thresholds and other canopy dynamics processes in temperate deciduous forests. Forest Ecol. Manag. 2018, 410, 84–94. [Google Scholar] [CrossRef]
- MFFP. Guide d’utilisation des produits dérivés du LiDAR; Direction des inventaires forestiers, Ministère des Forêts, de la Faune et des Parcs, Secteur des forêts: Québec, QC, Canada, 2016. [Google Scholar]
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S.M. A mélange of curves – further dialogue about species – area relationships. Glo. Ecol. Biogeogr. 2004, 13, 479–484. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Colwell, R.K. Estimating species richness. Biological diversity: frontiers in measurement and assessment 2011, 12, 39–54. [Google Scholar]
- Cumming, G. Inference by eye: Pictures of confidence intervals and thinking about levels of confidence. Teaching Statistics 2007, 29, 89–93. [Google Scholar] [CrossRef]
- R Development Core Team. R: A language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Deutschewitz, K.; Lausch, A.; Kühn, I.; Klotz, S. Native and alien plant species richness in relation to spatial heterogeneity on a regional scale in Germany. Glob. Ecol. Biogeogr. 2003, 12, 299–311. [Google Scholar] [CrossRef]
- Hofer, G.; Wagner, H.H.; Herzog, F.; Edwards, P.J. Effects of topographic variability on the scaling of plant species richness in gradient dominated landscapes. Ecography 2008, 31, 131–139. [Google Scholar] [CrossRef]
- Loos, J.; Turtureanu, P.D.; von Wehrden, H.; Hanspach, J.; Dorresteijn, I.; Frink, J.P.; Fischer, J. Plant diversity in a changing agricultural landscape mosaic in Southern Transylvania (Romania). Agr. Ecosyst. Environ. 2015, 199, 350–357. [Google Scholar] [CrossRef]
- McGarigal, K.; Romme, W.H.; Crist, M.; Roworth, E. Cumulative effects of roads and logging on landscape structure in the San Juan Mountains, Colorado (USA). Landsc. Ecol. 2001, 16, 327–349. [Google Scholar] [CrossRef]
- Markgraf, R.; Doyon, F.; Roy, M.È.; Kneeshaw, D. Landscape heterogeneity influences tree and shrub species abundance while local gap environments influence growth in temperate mixedwood forests. Under review.
- Lundholm, J.T. Plant species diversity and environmental heterogeneity: spatial scale and competing hypotheses. J. Veg. Sci. 2009, 20, 377–391. [Google Scholar] [CrossRef]
- Tamme, R.; Hiiesalu, I.; Laanisto, L.; Szava-Kovats, R.; Pärtel, M. Environmental heterogeneity, species diversity and co-existence at different spatial scales. J. Veg. Sci. 2010, 21, 796–801. [Google Scholar] [CrossRef]
- Costanza, J.K.; Moody, A.; Peet, R.K. Multi-scale environmental heterogeneity as a predictor of plant species richness. Lands. Ecol. 2011, 26, 851–864. [Google Scholar] [CrossRef]
- Gazol, A.; Tamme, R.; Takkis, K.; Kasari, L.; Saar, L.; Helm, A.; Pärtel, M. Landscape- and small-scale determinants of grassland species diversity: direct and indirect influences. Ecography 2012, 35, 944–951. [Google Scholar] [CrossRef]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialist species: toward a global functional homogenization? Front. Ecol. Environ. 2011, 9, 222–228. [Google Scholar] [CrossRef]
- Allouche, O.; Kalyuzhny, M.; Moreno-Rueda, G.; Pizarro, M.; Kadmon, R. Area–heterogeneity tradeoff and the diversity of ecological communities. Proc. Natl. Acad. Sci. 2012, 109, 17495–17500. [Google Scholar] [CrossRef] [PubMed]
- Redon, M.; Berges, L.; Cordonnier, T.; Luque, S. Effects of increasing landscape heterogeneity on local plant species richness: how much is enough? Landsc. Ecol. 2014, 29, 773–787. [Google Scholar] [CrossRef]
- Bartels, S.F.; Chen, H.Y. Is understory plant species diversity driven by resource quantity or resource heterogeneity? Ecology 2010, 91, 1931–1938. [Google Scholar] [CrossRef]
- Reich, P.B.; Frelich, L.E.; Voldseth, R.A.; Bakken, P.; Adair, E.C. Understorey diversity in southern boreal forests is regulated by productivity and its indirect impacts on resource availability and heterogeneity. J. Ecol. 2012, 100, 539–545. [Google Scholar] [CrossRef]
- Mingke, L.I.; Maclean, D.A.; Hennigar, C.R.; Ogilvie, J. Previous year outbreak conditions and spring climate predict spruce budworm population changes in the following year. Forest Ecol. Manag. 2019, 117737. [Google Scholar]
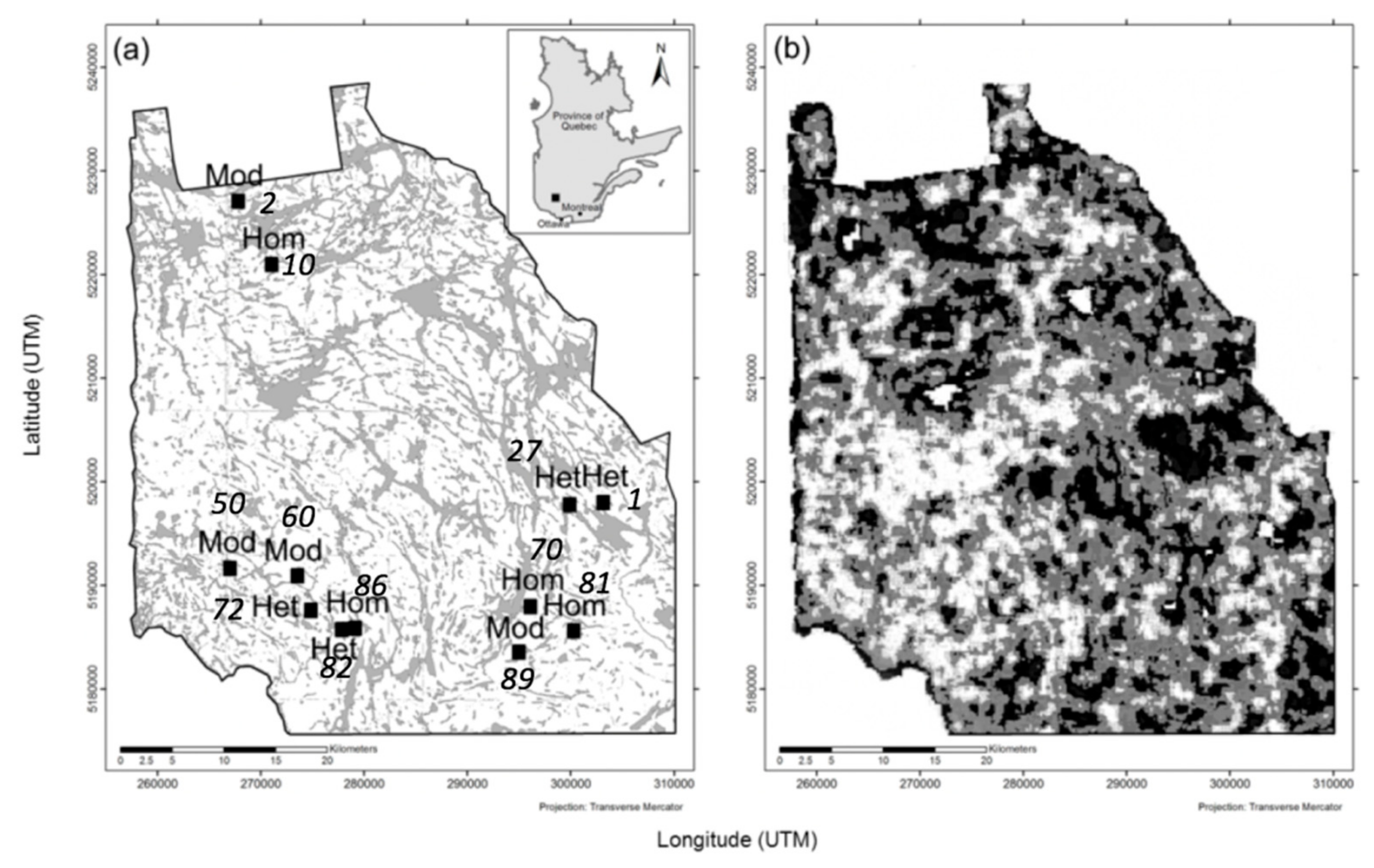

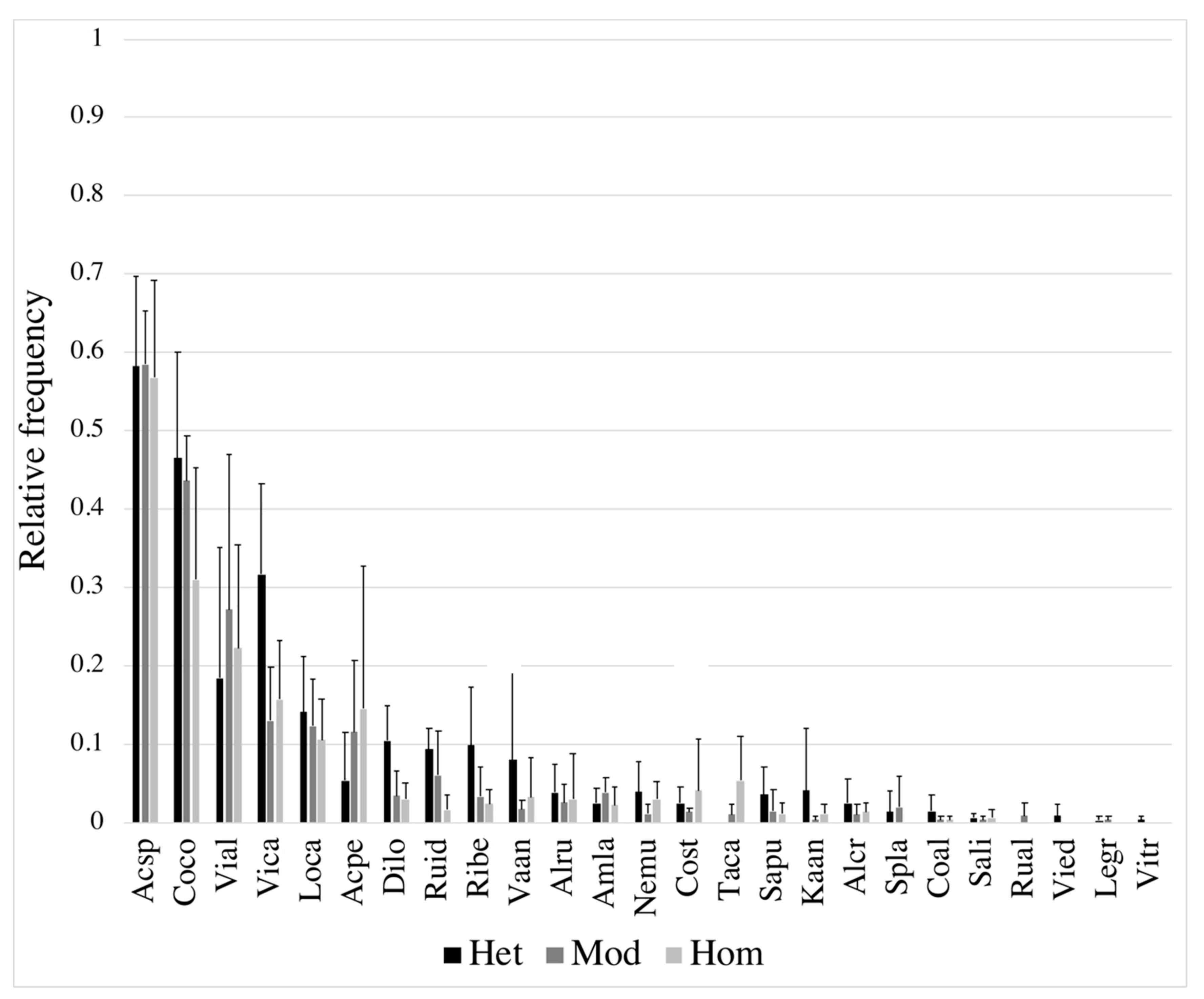
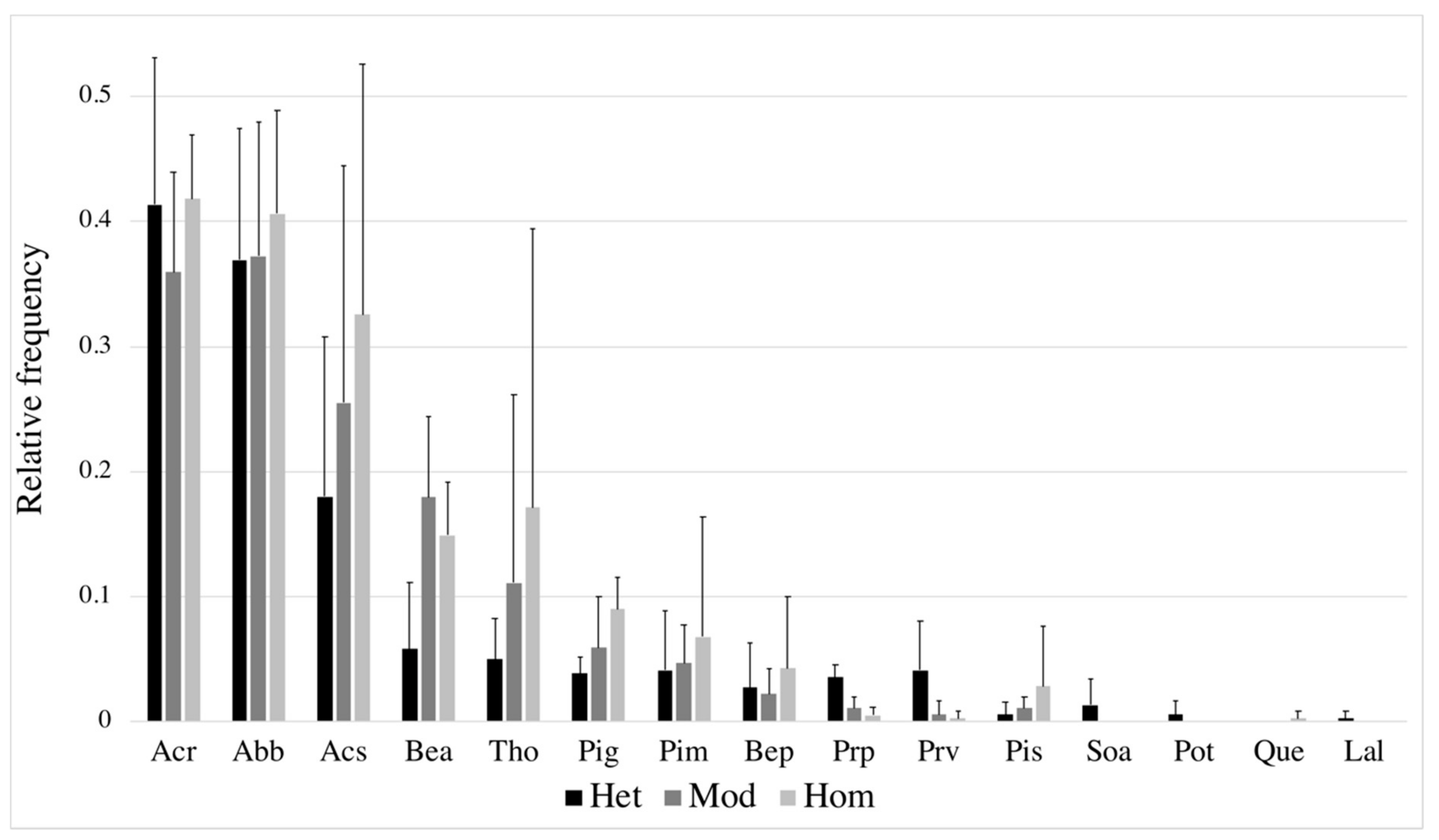
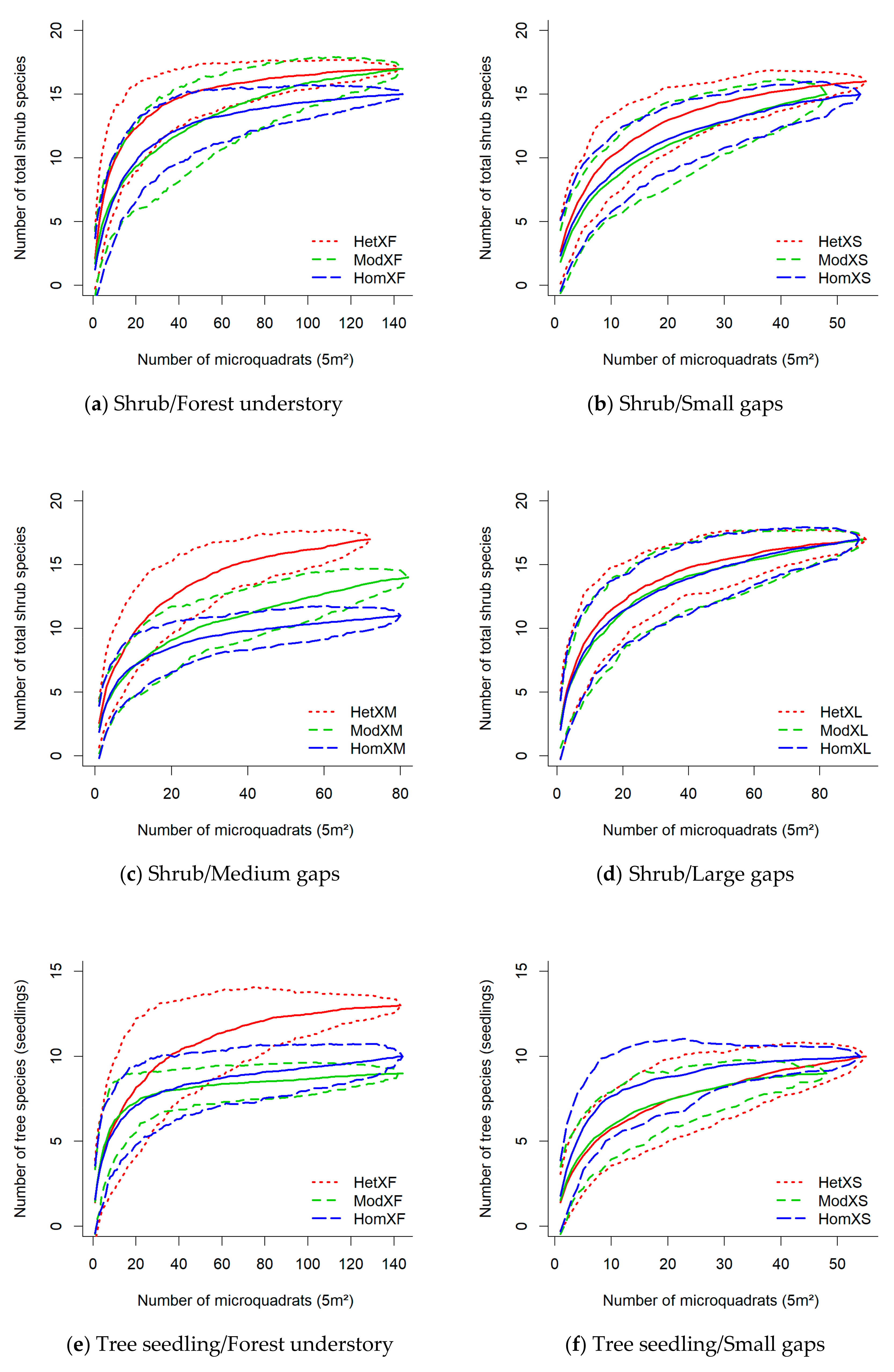
| Landscape No. | Heterogeneity Level | Stand Type Shannon Diversity Index | Stand Type Richness | Area-Weighted Cover Density | Average Stand Size | Spatial Landscape Heterogeneity Index |
|---|---|---|---|---|---|---|
| 1 | Heterogeneous | 0.61 | 0.78 | 0.77 | 0.47 | 0.66 |
| 27 | Heterogeneous | 0.67 | 0.81 | 0.99 | 0.61 | 0.77 |
| 72 | Heterogeneous | 0.69 | 0.57 | 0.61 | 0.76 | 0.66 |
| 82 | Heterogeneous | 0.99 | 1.00 | 0.26 | 0.37 | 0.66 |
| Mean (StDev) | 0.74 a (0.17) | 0.74 a (0.18) | 0.66 a (0.31) | 0.55 a (0.17) | 0.69 a (0.06) | |
| 2 | Moderate | 0.18 | 0.24 | 0.57 | 0.52 | 0.38 |
| 50 | Moderate | 0.45 | 0.37 | 0.83 | 0.80 | 0.61 |
| 60 | Moderate | 0.36 | 0.63 | 0.94 | 0.35 | 0.57 |
| 89 | Moderate | 0.38 | 0.37 | 0.18 | 0.74 | 0.42 |
| Mean (StDev) | 0.34 b (0.12) | 0.40 b (0.17) | 0.63 a (0.34) | 0.60 a (0.21) | 0.50 b (0.11) | |
| 10 | Homogenous | 0.25 | 0.16 | 0.70 | 0.23 | 0.34 |
| 70 | Homogenous | 0.51 | 0.44 | 0.50 | 0.05 | 0.37 |
| 81 | Homogenous | 0.18 | 0.27 | 0.23 | 0.05 | 0.18 |
| 86 | Homogenous | 0.29 | 0.20 | 0.35 | 0.35 | 0.30 |
| Mean (StDev) | 0.31 b (0.14) | 0.27 c (0.12) | 0.45 b (0.20) | 0.17 b (0.15) | 0.30 c (0.08) |
| Landscape No. | Heterogeneity Level | LiDAR Coverage Area (ha) | 4–40 m2 (%) | 40–200 m2 (%) | 200–600 m2 (%) | 600–800 m2 (%) | Total (%) |
|---|---|---|---|---|---|---|---|
| 1 | Heterogeneous | 131.3 | 1.33 | 1.05 | 0.37 | 0.25 | 2.99 |
| 27 | Heterogeneous | 110.0 | 2.30 | 1.80 | 0.87 | 0.43 | 5.40 |
| 72 | Heterogeneous | 133.4 | 0.84 | 0.76 | 0.31 | 0.21 | 2.12 |
| 82 | Heterogeneous | 134.8 | 1.12 | 0.99 | 0.13 | 0.47 | 2.71 |
| Mean (StDev) | 1.40 (0.63) | 1.15 (0.45) | 0.42 (0.32) | 0.34 a (0.13) | 3.30 (1.44) | ||
| 2 | Moderate | 83.0 | 0.70 | 0.40 | 0.08 | 0.10 | 1.28 |
| 50 | Moderate | 86.8 | 1.18 | 0.91 | 0.30 | 0.22 | 2.60 |
| 60 | Moderate | 115.4 | 0.80 | 0.45 | 0.27 | 0.18 | 1.71 |
| 89 | Moderate | 130.2 | 1.04 | 0.83 | 0.22 | 0.07 | 2.16 |
| Mean (StDev) | 0.93 (0.22) | 0.65 (0.26) | 0.22 (0.10) | 0.14 b (0.07) | 1.94 (0.57) | ||
| 10 | Homogenous | 103.9 | 1.25 | 0.54 | 0.32 | 0.05 | 2.15 |
| 70 | Homogenous | 128.8 | 1.19 | 0.86 | 0.30 | 0.13 | 2.48 |
| 81 | Homogenous | 128.1 | 0.94 | 0.60 | 0.37 | 0.16 | 2.07 |
| 86 | Homogenous | 135.0 | 1.26 | 1.04 | 0.30 | 0.14 | 2.73 |
| Mean (StDev) | 1.16 (0.15) | 0.76 (0.23) | 0.32 (0.03) | 0.12 b (0.05) | 2.36 (0.31) |
| Shannon | |||
|---|---|---|---|
| F | P | DF | |
| Tree seedlings | |||
| GE | 1.87 | 0.14 | 3 |
| LH | 3.70 | 0.03 | 2 |
| GE × LH | 1.19 | 0.31 | 6 |
| Shrubs | |||
| GE | 7.29 | <0.01 | 3 |
| LH | 6.74 | <0.01 | 2 |
| GE × LH | 1.30 | 0.26 | 6 |
| Shannon | |||
|---|---|---|---|
| Z | P | ||
| Tree seedlings | |||
| GE | NA | NA | |
| LH | Hom > Het | 2.61 | 0.02 |
| Shrubs | |||
| GE | L > F | 3.02 | 0.01 |
| M > F | 2.90 | 0.02 | |
| S > F | 3.96 | <0.01 | |
| LH | Het > Hom | 3.55 | <0.01 |
| Het > Mod | 2.49 | 0.03 | |
| Landscape Heterogeneity Level | |||||
|---|---|---|---|---|---|
| Species Group | Gap/Forest Environment | Hom | Mod | Het | P(F) |
| Shrub | Forest | 0.115 a | 0.138 ab | 0.164 b | <0.01 |
| Large gap | 0.179 | 0.179 | 0.206 | 0.16 | |
| Medium gap | 0.158 | 0.166 | 0.192 | 0.18 | |
| Small gap | 0.196 | 0.211 | 0.227 | 0.47 | |
| Tree seedling | Forest | 0.081 | 0.096 | 0.100 | 0.26 |
| Large gap | 0.163 | 0.146 | 0.179 | 0.40 | |
| Medium gap | 0.184 | 0.158 | 0.136 | 0.12 | |
| Small gap | 0.170 | 0.111 | 0.156 | 0.067 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markgraf, R.; Doyon, F.; Kneeshaw, D. Forest Landscape Heterogeneity Increases Shrub Diversity at the Expense of Tree Seedling Diversity in Temperate Mixedwood Forests. Forests 2020, 11, 160. https://doi.org/10.3390/f11020160
Markgraf R, Doyon F, Kneeshaw D. Forest Landscape Heterogeneity Increases Shrub Diversity at the Expense of Tree Seedling Diversity in Temperate Mixedwood Forests. Forests. 2020; 11(2):160. https://doi.org/10.3390/f11020160
Chicago/Turabian StyleMarkgraf, Rudiger, Frédérik Doyon, and Daniel Kneeshaw. 2020. "Forest Landscape Heterogeneity Increases Shrub Diversity at the Expense of Tree Seedling Diversity in Temperate Mixedwood Forests" Forests 11, no. 2: 160. https://doi.org/10.3390/f11020160
APA StyleMarkgraf, R., Doyon, F., & Kneeshaw, D. (2020). Forest Landscape Heterogeneity Increases Shrub Diversity at the Expense of Tree Seedling Diversity in Temperate Mixedwood Forests. Forests, 11(2), 160. https://doi.org/10.3390/f11020160




