Abstract
Background and Objectives:Rhododendron pulchrum Sweet (R. pulchrum) belongs to the genus Rhododendron (Ericaceae), a valuable horticultural and medicinal plant species widely used in Western Europe and the US. Despite its importance, this is the first member to have its cpGenome sequenced. Materials and Methods: In this study, the complete cp genome of R. pulchrum was sequenced with NGS Illumina HiSeq2500, analyzed, and compared to eight species in the Ericaceae family. Results: Our study reveals that the cp genome of R. pulchrum is 136,249 bp in length, with an overall GC content of 35.98% and no inverted repeat regions. The R. pulchrum chloroplast genome encodes 73 genes, including 42 protein-coding genes, 29 tRNA genes, and two rRNA genes. The synonymous (Ks) and nonsynonymous (Ka) substitution rates were estimated and the Ka/Ks ratio of R. pulchrum plastid genes were categorized; the results indicated that most of the genes have undergone purifying selection. A total of 382 forward and 259 inverted long repeats, as well as 221 simple-sequence repeat loci (SSR) were detected in the R. pulchrum cp genome. Comparison between different Ericaceae cp genomes revealed significant differences in genome size, structure, and GC content. Conclusions: The phylogenetic relationships among eight Ericaceae species suggested that R. pulchrum is closely related to Vaccinium oldhamii Miq. and Vaccinium macrocarpon Aiton. This study provides a theoretical basis for species identification and future biological research of Rhododendron resources.
1. Introduction
Rhododendron species belong to family Ericaceae and have been widely used as valuable horticultural and medicinal plants in China, Western Europe, the US, and Japan due to their beautiful vegetative forms and bright-colored flowers [1,2]. The family of Ericaceae consists of nine subfamilies, including exclusively autotrophic species, fully mycoheterotrophic (MH) species, and partially MH species [3]. The association between autotrophy and heterotrophy is related to drastic changes in plant morphology (such as the loss and/or reduction of vegetative organs) [4,5], physiology (for example, loss of chlorophyll and high stomatal conductivity) [5,6], genome (including rampant sequence divergence and gene loss) [7,8]. Chloroplast genome has been widely employed for studying the transition from autotrophy to heterotrophy due to its conserved size, gene content, linear gene order, and structure.
Rhododendron contains between 600–1000 species, and is among the largest genera of Ericaceae [9]. Previous Rhododendron classification was mainly based on phenotypic characteristics, and thclassification systems proposed by David Chamberlain (Edinburgh Botanic Garden, UK) and Sleumer (Germany) were the most popular. In recent years, the genetic diversity and structure of the nuclear and plastid genomes serves as another useful strategy to classify Ericaceae species [10,11]. The DNA sequences of nuclear genes have been adopted in studies of phylogeny, population genetics, molecular evolution, and genetic map construction in Rhododendrons [12].
According to the published data, the molecular marker technology and morphological analyses yield consistent results for Rhododendron classification [13]. Recently, complete plastid genome sequences have found their applications in plant studies, especially in the field of plant taxonomy; these include dissecting the diversity and phylogenetic relationships among different species [10,14], utilizing specific gene functions in agriculture and horticulture via genetic engineering [15], and estimating population genetics via DNA barcoding [16].
The plastid genome of higher plants is a circular molecule of double-stranded DNA, range from 72 to 217 kb in size and contain approximately 130 genes [17,18]. The plastid genomes (plastomes) of most photosynthetic land plants are between 140 to 160 kb in size and contain about 113 genes; in addition, these plastomes are conserved in size, gene content, gene order, gene structure, and genome structure [19,20]. Most plastomes contain four typical regions, including a large single-copy region (LSC) a small single-copy (SSC) region, and two identical inverted repeat regions (IR) [14]. Plastomes can provide valuable genetic information for studies on species identification, phylogeny, biology, and photosynthetic gene degradation [10,21,22,23] owing to their conserved structure and relatively high substitution rate [24].
The complete plastome sequences of eight Ericaceae species are currently available at the National Center for Biotechnology Information (NCBI); these include six fully MH species (Monotropoideae) and two exclusively autotrophic species (Vaccinioideae). By contrast, most previous studies on Rhododendron have focused on their biology and physiology, whereas their plastid genomes have not been reported [25]. As an important horticultural species in the genus Rhododendron, Rhododendron pulchrum (R. pulchrum) is widely distributed in the temperate regions of Europe, Asia and North America [26]. Herein, we report the exhaustively analyzed cp genome of R. pulchrum previously published as a brief note with minimal analysis [27]. The size and structure of the R. pulchrum cp genome were analyzed, and the Ka/Ks ratio was determined to study the relationships between Ericaceae species and their closest autotrophic relatives, as well as to infer their evolutionary patterns. The cp genome information reported here is valuable for future phylogenetic and evolution studies, molecular marker development, and genetic improvement through genetic engineering in Rhododendron.
2. Materials and Methods
2.1. Sampling, DNA Extraction and Sequencing
Fresh leaves were collected from a single R. pulchrum tree grown in the Jiyang College of Zhejiang A&F University, Zhejiang, China. The harvested tissues were stored at the Rhododendron Germplasm Resource nursery in Hangzhou Botanical Garden (N30°15’8.65’’, E120°7’11.09’’) under accession number HZ041286. Total genomic DNA was extracted using the modified CTAB method [28]. The integrity and quality of the DNA were evaluated using agarose gel electrophoresis and a one drop spectrophotometer (OD-1000, Shanghai Cytoeasy Biotech Co., Ltd., Shanghai, China). The whole cp genome of R. pulchrum was sequenced via 250 bp pair-ended sequencing on an Illumina Hiseq 2500 Platform (Manufacturer, Nanjing, China), and at least 3.68 GB of clean sequencing data were obtained.
2.2. Chloroplast Genome Assembly, Gene Annotation and Plastomes Analysis
The reads were de novo assembled into complete cp genome by the NOVOPlasty 3.7.2 using genomes of the Ericaceae species as references [29]. CpGAVAS 2 (http://www.herbalgenomics.org/ cpgavas) was used to annotate the sequences; DOGMA (http://dogma.ccbb.utexas.edu/) and BLAST were used to manually check the annotation results [30,31]. The complete and annotated cp genome sequences were deposited to GenBank (accession number MN182619). A physical map of the genome was generated by OGDRAWv1.2 (http://ogdraw.mpimp-golm.mpg.de/) and the online software REPuter [32,33] was used to identify repeat sequences.
Relative synonymous codon usage (RSCU) and codon usage were examined by CodonW 1.4.4 [34]. GC content of the complete cp genomes and the coding sequences (CDS) was analyzed. MISA [35] and REPuter [33] were used to visualize the simple sequence repeats (SSRs) and long repeats, respectively. (MISA. Available from: http://pgrc.ipk-gatersleben.de/misa/misa.html; REPuter. Available from: https://bibiserv.Cebitec.uni-bielefeld.de/reputer). The sequences were initially aligned using MAFFT version 5 [36], the pi value of each gene were calculated through alignment of each gene CDS sequences of different species using VCFtools [37], and the ratios of non-synonymous (Ka) to synonymous (Ks) substitutions (Ka/Ks) in protein-coding genes were determined by the KaKs_Calculator.
2.3. Whole Plastid Genome Comparison
To investigate the phylogenetic position of R. pulchrum, MUMmer [38] was used for pairing sequence alignment of the cp genomes, and the mVISTA (http://genome.lbl.gov/vista/mvista) [39] program was employed for comparing the complete cp genome of R. pulchrum to eight other related species whose cp genomes are sequenced. These eight species were divided into two groups at the genus level, including Vaccinieae (Vaccinium macrocarpon, NC_019616.1 and Vaccinium oldhamii, NC_042713.1) and Monotropeae (Monotropa hypopitys Linn., NC_029704.1; Allotropa virgata Torr. & A. Gray, NC_035580.1; Hemitomes congestum A. Gray, NC_035581.1; Monotropa uniflora L., NC_035582.1; Monotropsis odorata Schwein. ex Elliott, NC_035583.1; and Pityopus californicus (Eastw.) Copeland, NC_035584.1).
2.4. Phylogenetic Analysis
The Ericaceae cp genomes were obtained from the Organelle Genome and Nucleotide Resources database on NCBI. The sequences were initially aligned using MAFFT [36] (version 7, https://mat.cbrc.jp/alignment/software/) and the resulting multiple sequence alignment was visualized and manually adjusted in BioEdit [40]. Actinidia deliciosa C.F.Liang & A.R.Ferguson (NC_026691.1) and Actinidia chinensis Planch (NC_026690.1) were used as outgroups. The phylogenetic tree was constructed by the GTRGAMMA model implemented in RAxML.
3. Results
3.1. Features of the R. pulchrum cp Genome
A total of 3.68 Gb of clean data consisting 12.28 million pair-end reads were produced. All reads were deposited to NCBI Sequence Read Archive (SRA) under accession number MN182619. The complete R. pulchrum cp genome is 136,249 bp in length (Figure 1), and it does not take the form of a typical quadripartite structure due to the lack of inverted repeats (IR). The overall GC content of the R. pulchrum cp genome is 35.98% (Table 1) and we identified 73 functional genes, including two rRNA genes, 29 tRNA genes, and 42 protein-coding genes (Table 2). We then estimated codon usage frequency based on the protein-coding and tRNA genes. As shown in Figure 2, the cpGenome was composed of 8,693 codons (65 different types) encoding 20 amino acids, among which leucine (Leu) was the most frequently used amino acid (948 in number, 10.90%) and cysteine (Cys) was the least abundant (76 in number, 0.87%) (Table S1). The results suggest the R. pulchrum cp genome prefers synonymous codons ended with A or U with a relative synonymous codon usage value (RSCU) > 1.
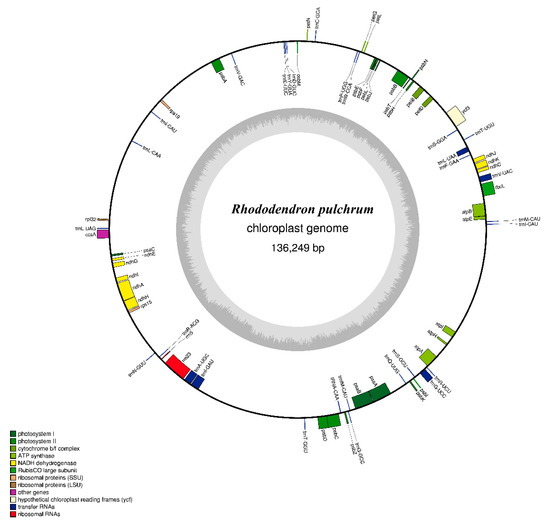
Figure 1.
Gene map of the R. pulchrum cp genome. (Color figure online). Genes reside in the inside and outside of the outer circle are in the forward and reverse directions, respectively. The dark and light gray bars in the inner circle denote G+C and A+T contents, respectively.

Table 1.
Base composition of the R. pulchrum cp genome.

Table 2.
Annotated genes of the R. pulchrum cp genome.
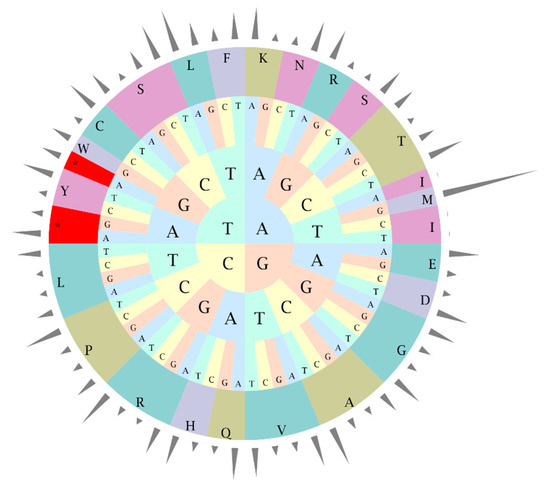
Figure 2.
Codon-anticodon recognition patterns and codon usage of the R. pulchrum cp genome. Height of the outer cylinder represents relative synonymous codon usage rate; the middle and inner layers show amino acids and codons, respectively.
Among the annotated genes in the R. pulchrum cp genome, seven have introns, two are protein-coding genes, and five are tRNA genes (Table 3). Six genes display one intron, these include the protein-coding gene ndhA and tRNA genes trnV-UAC, trnL-UAA, trnA-UGC, trnI-GAU, and trnG-UCC; one protein-coding gene (ycf3) contains two introns. Among all R. pulchrum genes, ycf3 has the largest intron (711 bp + 743 bp) and trnL-UAA has the smallest intron (504 bp).

Table 3.
Exon and intron lengths of R. pulchrum cp genes.
3.2. Ka/Ks Analysis of Base Variation
To test whether the remaining cp genes in R. pulchrum have undergone selection, we estimated the synonymous (Ks) and nonsynonymous (Ka) substitution rates (Table S2). The Ka/Ks ratios were then categorized, with Ka/Ks < 1, Ka/Ks = 1, and Ka/Ks > 1 denoting purifying, neutral, and positive selections, respectively, in the context of a codon substitution model. According to our results, only two genes, rps15 and psbZ, underwent positive selection compared with the eight Ericaceae species (Table S2). By contrast, most remaining genes were shown to have undergone purifying selection, which was evidenced by a Ka/Ks ratio below 1 and the presence of negatively selected sites within some genes. During the transformation of Ericaceae from exclusively autotrophic (R. pulchrum, V. macrocarpon and V. oldhamii) to heterotrophic (Monotropeae, six species). We found signs of purifying selection in rpl32, which is the only gene annotated in this transformation, in four heterotrophic species (Allotropa virgata, Hemitomes congestum, Monotropsis odorata, and Pityopus californicus); whereas the rpl32 gene was absent from two heterotrophic species, Monotropa hypopitys and Monotropa uniflora.
3.3. Long-Repeat and SSR Analysis
A total of 576 long repeats were identified in the R. pulchrum cp genome, including 382 forward (F) and 259 inverted repeats (I) (Table S3). The long repeats exhibited substantial variation in length—we found 460 (79.86%), 98 (17.01%), and 18 (3.13%) repeats of 15–30 bp, 30–100 bp and 100–1000 bp, respectively. The longest forward repeat was 951 bp in length and was identified in one spot involved the sequence of ycf3 gene and the intergenic space.
We also identified 221 SSRs from the R. pulchrum cp genome, including 140 mononucleotide (63.35%), six dinucleotide (2.71%), 58 trinucleotide (26.24%), nine tetranucleotide (4.07%), one pentanucleotide (0.45%), two hexanucleotide (0.90%), and five compound SSRs (2.26%) (Table S4). Fifty-two SSRs contained guanine (G) or cytosine (C), of which two were composed of tandem G or C repeats; whereas the remaining 169 SSRs had either polyadenine (poly A) or polythymine (poly T). Further, 34 SSRs were found in intragenic regions, and the rest were identified in intergenic regions.
3.4. Nucleotide Variability (pi) Values of Genes
Gene nucleotide variability (pi) values of R. pulchrum were compared with the eight Ericaceae species (Figure 3). The values of ndhK, rpl32, trnM-CAU, trnF-GAA, tRNA-CAA, trnC-GCA, trnN-GUU and trnL-UAG were between 0.03 and 0.05, while the values of trnfM-CAU, rrn23, trnS-GCU, trnV-GAC, rrn5 and rps19 were higher than 0.05. The results indicate that, in general, the nucleotide diversity among the nine Ericaceae species is high.
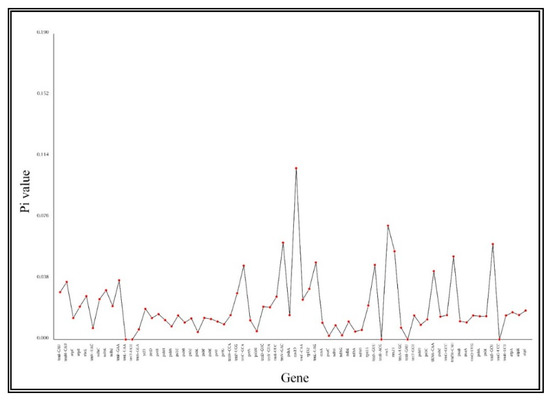
Figure 3.
Genes nucleotide variability (pi) values of R. pulchrum compared with eight Ericaceae species.
3.5. Comparative Analysis of Gene Content and Genome Structure
Differences in cp sequences can help to infer the gene flow between species [41]. Thus, the complete R. pulchrum cp genome was compared with those of exclusively autotrophic and mycoheterotrophic species of Ericaceae, respectively. As shown in Table S5, gene content of R. pulchrum is quite distinct from the other eight Ericaceae species. We determined that while 9–12 (rps) genes were found among the other eight species, only two (rps15 and rps 19) were found in R. pulchrum. rps15 was only annotated in the three exclusively autotrophic species (R. pulchrum, V. macrocarpon and V. oldhamii); rps19 was annotated in all nine Ericaceae species. There have 5-8 (rpl) genes were found among the other eight species, only one gene (rpl32) were found in R. pulchrum. There have 4 (rrn) genes were found among the other eight species, only two gene (rrn23 and rrn5) were found in R. pulchrum. However, gene content of R. pulchrum has common characteristics with the other eight species. For example, no rpo genes coding RNA polymerase subunits were found in R. pulchrum and six species of Monotropeae, while 4 genes were found in two species of Vaccinieae; 28-30 (trn) genes coding Transfer RNA were found in three exclusively autotrophic species (R. pulchrum, V. macrocarpon and V. oldhamii), while only 15–18 genes were found in six species of Monotropeae. Photosynthesis-related genes (psb, psa, pet, atp, rbc, ndh) were lost in the cp genomes of the six nonphotosynthetic species of Monotropeae. Moreover, 37, 41 and 44 photosynthesis-related genes were found in genome of R. pulchrum, V. macrocarpon and V. oldhamii, respectively, with 34 of these genes in common. Four genes (atpF, ndhG, ndhK and PsbZ) were lost in V. macrocarpon, and seven genes (atpF, ndhB, ndhD, ndhF, PetA, psal and psaJ) were lost in R. pulchrum. Besides self-replication and photosynthesis related genes, there have eight other genes (accD, ccsA, cemA, clpP, infA, lhbA, matK and rp3) were found in the other eight Ericaceae species. Among which, only one gene (ccsA) were found in genome of R. pulchrum, and three genes (accD, clpP and infA) were only found in six nonphotosynthetic species of Monotropeae.
As shown in (Figure 4, Figure 5 and Figure S1, the R. pulchrum cp genome is quite distinct from the eight Ericaceae species. Specifically, the R. pulchrum cp genome is more divergent than mycoheterotrophic Ericaceae species; sequence diversity is higher in the noncoding than coding regions (Figure 4 and Figure 5). Only one gene (trnL-CAU) in R. pulchrum exhibited higher similarity to that in the six mycoheterotrophic Ericaceae species (Figure 4A). By contrast, twenty R. pulchrum cp genes showed relatively higher similarity to those of exclusively autotrophic species of Ericaceae (Figure 4B). Together, these data revealed a high level of genetic variation among species of different genera within Ericaceae, especially between mycoheterotrophic (Monotropeae) and exclusively autotrophic species (Vaccinieae and Rhododendron).

Figure 4.
Comparison of the eight complete cp genomes of Ericaceae species. The grey arrows and thick black lines above the alignment denote gene orientations. The Y-axis represents sequence similarity. A. Comparison between the complete cp genomes of R. pulchrum and Monotropeae (Monotropa hypopitys, NC_029704.1; Allotropa virgata, NC_035580.1; Hemitomes congestum, NC_035581.1; Monotropa uniflora, NC_035582.1; Monotropsis odorata, NC_035583.1; and Pityopus californicus, NC_035584.1). B. Comparison between the complete R. pulchrum and Vaccinieae (Vaccinium macrocarpon, NC_019616.1 and Vaccinium oldhamii, NC_042713.1) cp genomes. The cp genome of Rhododendron pulchrum was used as the reference.

Figure 5.
Comparison between the cp genomes of R. pulchrum and related species. The two outermost circles describe gene length and the direction of genome; the two inner circles represent the similarity between the R. pulchrum cp genome and the reference genomes of Ericaceae species; the black circle shows GC content. A. The alignment of Vaccinium macrocarpon, Vaccinium oldhamii, and Rhododendron pulchrum cp genomes. B. The alignment of Rhododendron pulchrum cp genome to that of Monotropa hypopitys (NC_029704.1), Allotropa virgata (NC_035580.1), Hemitomes congestum (NC_035581.1), Monotropa uniflora (NC_035582.1), Monotropsis odorata (NC_035583.1), and Pityopus californicus (NC_035584.1).
3.6. The Phylogenetic Tree of Ericaceae
Phylogenetic analysis was performed based on an alignment of concatenated nucleotide sequences of all ten angiosperm cp genomes (Figure 6). A phylogenetic tree was built by using the Gtrgamma model and the Bayesian inference (BI) method based on RAxML, with Actinidia deliciosa and Actinidia chinensis as outgroups. All relationships inferred from these cp genomes received high supports with the support values ranging between 83 and 100. It is worth noticing that the nine species from family Ericaceae did not form a clade. Six heterotrophic species in genera Monotropeae clustered into one clade, and the three exclusively autotrophic Ericaceae species (R. pulchrum, V. oldhamii and V. macrocarpon) formed another (Figure 6).
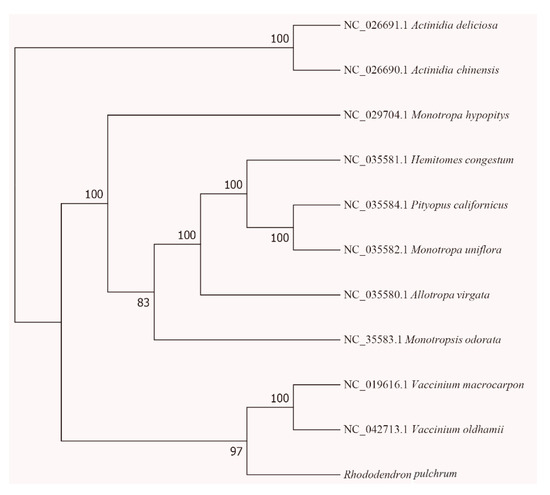
Figure 6.
Phylogenetic tree of the nine Ericaceae species based on complete cp genomes. Actinidia deliciosa (NC_026691.1) and the Actinidia chinensis (NC_026690.1) were used as outgroups.
4. Discussion
The complete cp genome of R. pulchrum differs significantly from those of the other eight Ericaceae species with regard to genome size, structure, GC content, genes structure, but was similar to those of the two Vaccinieae species. Compared with exclusively autotrophic Ericaceae species (photosynthetic, Vaccinieae and Rhododendron), the cp genomes of mycoheterotrophic species (nonphotosynthetic, Monotropeae) are substantially smaller in size (ca. 33–41 kb) and gene content [10,11,42]. The R. pulchrum cp genome was found to have 73 functional genes, 110 and 133 genes were annotated in those of Vaccinium macrocarpon and Vaccinium oldhamii, whereas 40–45 genes were annotated in species of Monotropeae respectively—suggesting that Ericaceae cp genomes are highly variable. Gene content of R. pulchrum is quite distinct from the eight Ericaceae species, such as most of the self-replication related genes (rps, rpl, rrn) being missing compared to the other eight species, but similar as it has maintained most of the trn self-replicating genes. Interestingly, both R. pulchrum and six Monotropeae species are missing the rpo genes that are present in the two Vaccinieae species. Photosynthesis-related genes (psb, psa, pet, atp, rbc, ndh) were lost in the cp genomes of nonphotosynthetic Ericaceae species [10], while 37, 41 and 44 photosynthesis-related genes were found in genome of R. pulchrum, V. macrocarpon and V. oldhamii, respectively. Thus, supporting the position of Rhododendron between Vaccinieae and Monotropeae during evolution. According to the published data, the plastid genomes of most photosynthetic land plants are conserved in size (140–160 kb) and display the typical quadripartite structure by showing the LSC, LSC, and two IRs [14,19,20]. However, our results indicate that the R. pulchrum cp genome lacks the IRs, which has also been reported in Monotropeae [10], Medicago [43,44] and Erodium plants (Erodium carvifolium HQ713469.1).
The R. pulchrum cp genome has an overall GC content of 35.98%, which is lower than exclusively autotrophic Ericaceae species including Vaccinium oldhamii (36.75%) and Vaccinium macrocarpon (36.80%) but higher than heterotrophic Ericaceae species (Monotropeae), including Pityopus californicus (34.37%), Monotropa hypopitys (34.31%), Hemitomes congestum (33.71%), Allotropa virgata (33.09%), Monotropsis odorata (31.20%), and Monotropa uniflora (28.47%). In plants, GC content is often associated with the degree of primitiveness of a taxon [45], whereas in MH species, it is often related to plastome degradation level [7]. Further, consistent with that observed with Q. acutissima [46], the ycf3 and trnL-UAA genes in R. pulchrum have the largest intron and the smallest intron, respectively. The ycf3 gene has been reported to be necessary for stabilizing the accumulation of photosystem I complexes [47], and the gain of intron is usually considered have closely relationship with the evolution of photosynthesis [46]. This result indicates that ycf3 is likely a key player in photosynthesis in R. pulchrum. Thus, the complete cp genomes of genera Vaccinieae, Monotropeae, and Rhododendron are useful for studying Ericaceae species evolution, photosynthetic gene degradation, and the phylogenetic relationships among Ericaceae species.
In this study, a total of 221 cp SSRs were identified in R. pulchrum, more than that has been reported for other Ericaceae species (62) [11]. This may be a result of the complex genetic background of Rhododendron. Meanwhile, the cpSSRs identified here rarely contain tandem G or C repeats, which is in line with the published data [48]. These cpSSR markers offer an alternative to morphological features and nuclear DNA markers in defining Rhododendron subsections. The cpSSRs yielded from this study can be employed for genetic structure determination, as well as the diversity, differentiation, and maternity studies of R. pulchrum and its related species.
Compared with the eight Ericaceae species, only two genes rps15 and psbZ—which encode the small subunit of ribosome and the core complex of photosystem II protein, respectively—showed signs of positive selection. Co-annotated genes were found in all the eight Ericaceae species during the transformation of Ericaceae from exclusively autotrophic (Rhododendron, R. pulchrum) to heterotrophic (Monotropeae, six species). In addition, we observed a high level of genetic diversity among Ericaceae genera, especially between mycoheterotrophic (Monotropeae) and exclusively autotrophic species (Vaccinieae and Rhododendron), which is consistent with the genomic rearrangement events in Ericaceae plants reported by previous studies [11]. In general, the cp genomes of species from the same family are conserved [23], and cp sequences have been successfully applied to the phylogenetic studies of angiosperms [49,50]. Our phylogenetic analysis clustered the nine Ericaceae species (from three genera) and two outgroup species into three distinct phylogenetic groups, including one outgroup (Actinidia deliciosa and Actinidia chinensis), one exclusively autotrophic group (R. pulchrum, Vaccinium macrocarpon, and V. oldhamii), and one heterotrophic group (Monotropa hypopitys, Allotropa virgata, Hemitomes congestum, Monotropa uniflora, Monotropsis odorata, and Pityopus californicus). In addition, the cp genome of R. pulchrum is closely related to those of Vaccinium oldhamii and Vaccinium macrocarpon species. Taken together, our results revealed the potential of Ericaceae family as suitable materials for studying plastid genome development and evolution.
5. Conclusions
In this study, we report the characterization of the complete cp genome of R. pulchrum, a horticultural and medicinal species cultivated worldwide. The R. pulchrum cp genome displays unique characteristics compared with species from other genera in Ericaceae, especially genus Monotropeae. The phylogenetic analysis revealed that R. pulchrum was closely related to species of Vaccinium oldhamii and Vaccinium macrocarpon. The complete cp genome assembly of R. pulchrum provided in this research is valuable for future population genomic and phylogenomic studies, and will benefit Rhododendron conservation and utilization.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/11/2/158/s1, Table S1. Codon-anticodon recognition patterns and codon usage of the R. pulchrum cp genome. Table S2. Ka/Ks ratios of the cp genes from R. pulchrum and related species (NA = not available). Table S3. Long repeats identified from the R. pulchrum cp genome (F = forward, I = inverted repeat; IGS = intergenic space). Table S4: Simple sequence repeats (SSRs) identified in the R. pulchrum cp genome. Table S5: Annotated genes of the nine species of Ericaceae cp genomes.
Author Contributions
The experiments were conceived and designed by S.J.; J.S., X.L., X.Z., and X.H. were involved in the collection of the study materials and participated in the DNA extraction and data analyses; J.S. wrote and S.J. revised the manuscript. All authors read and approved the final manuscript.
Funding
The work was funded by the Zhejiang Provincial Natural Science Foundation of China (grant number: LY16C160011), the National Natural Science Foundation of China (grant number: 31971641), Project of State Key Laboratory of Subtropical Silviculture, Zhejiang A & F University (grant number: ZY20180208, ZY20180308), and Jiyang College of Zhejiang A&F University under Grant (grant number: RQ1911B07).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Popescu, R.; Kopp, B. The genus Rhododendron: An ethnopharmacological and toxicological review. J. Ethnopharmacol. 2013, 147, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, D.; Hyam, R.; Argent, G.; Fairweather, G.; Walter, K.S. The Genus Rhododendron: Its Classification and Synonymy; Royal Botanic garden Edinburgh: Edinburgh, UK,, 1996; p. 181. [Google Scholar]
- Kron, K.A.; Judd, W.S.; Stevens, P.F.; Crayn, D.M.; Anderberg, A.A.; Gadek, P.A.; Quinn, C.J.; Luteyn, J.L. Phylogenetic Classification of Ericaceae: Molecular and Morphological Evidence. Bot. Rev. 2002, 68, 335–423. [Google Scholar] [CrossRef]
- Leake, J.R. The biology of myco-heterotrophic (’saprophytic’) plants. New Phytol. 1994, 127, 171–216. [Google Scholar] [CrossRef]
- Manen, J.-F.; Habashi, C.; Jeanmonod, D.; Park, J.-M.; Schneeweiss, G.M. Phylogeny and intraspecific variability of holoparasitic Orobanche (Orobanchaceae) inferred from plastid rbcL sequences. Mol. Phylogenetics Evol. 2004, 33, 482–500. [Google Scholar] [CrossRef]
- Stewart, G.R.; Press, M.C. The physiology and biochemistry of parasiticangiosperms. Annu. Rev. Plant Phys. 1990, 41, 127–151. [Google Scholar]
- Wicke, S.; Müller, K.F.; Depamphilis, C.W.; Quandt, D.; Bellot, S.; Schneeweiss, G.M. Mechanistic model of evolutionary rate variation en route to a nonphotosynthetic lifestyle in plants. Proc. Natl. Acad. Sci. USA 2016, 113, 9045–9050. [Google Scholar] [CrossRef]
- Graham, S.W.; Lam, V.K.Y.; Merckx, V.S.F.T. Plastomes on the edge: The evolutionary breakdown of mycoheterotroph plastid genomes. New Phytol. 2017, 214, 48–55. [Google Scholar] [CrossRef]
- Craven, L.; Dăneţ, F.; Veldkamp, J.; Goetsch, L.; Hall, B. Vireya Rhododendrons: Their monophyly and classification (Ericaceae, Rhododendron section Schistanthe). Blumea - Biodiversity, Evol. Biogeogr. Plants 2011, 56, 153–158. [Google Scholar] [CrossRef]
- Thomas, W.A.B.; Michael, B.B.; Sasa, S.; John, V.F. On the brink: The highly reduced plastomes of nonphotosynthetic Ericaceae. New Phytol. 2017, 216, 254. [Google Scholar]
- Fajardo, D.; Senalik, D.; Ames, M.; Zhu, H.Y.; Steffan, S.A.; Rebecca, H.; James, P.; Nicholi, V.; Emily, G.; Kathy, K.; et al. Complete plastid genome sequence of Vaccinium macrocarpon: Structure, gene content, and rearrangements revealed by next generation sequencing. Tree Genet. Genomes 2013, 9, 489–498. [Google Scholar] [CrossRef]
- Zheng, S.; Tian, X.; Huang, C.; Wang, L.; Feng, Y.; Zhang, J. Molecular and morphological evidence for natural hybridization between Rhododendron decorum and R. delavayi (Ericaceae). Biodivers. Sci. 2017, 25, 627–637. [Google Scholar] [CrossRef][Green Version]
- Tsai, C.; Huang, S.; Chen, C.; Tseng, Y.; Huang, P.; Tsai, S.; Hou, C. Genetic relationships of Rhododendron (Ericaceae) in Taiwan based on the sequence of the internal transcribed spacer of ribosomal DNA. J. Hortic. Sci. Biotechnol. 2003, 78, 234–240. [Google Scholar] [CrossRef]
- Jansen, R.K.; Saski, C.; Lee, S.; Hansen, A.K.; Daniell, H. Complete plastid genome sequences of three rosids (Castanea, Prunus, Theobroma): Evidence for at least two independent transfers of rpl22 to the nucleus. Mol. Biol. Evol. 2011, 28, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Maliga, P.; Svab, Z. Engineering the plastid genome of Nicotiana sylvestris, a diploid model species for plastid genetics. Plant Chromosom. Eng. Met. Protoc. 2011, 701, 37–50. [Google Scholar]
- Fazekas, A.J.; Burgess, K.S.; Kesanakurti, P.R.; Graham, S.W.; Newmaster, S.G.; Husband, B.C.; Percy, D.M.; Hajibabaei, M.; Barrett, S.C.H. Multiple Multilocus DNA Barcodes from the Plastid Genome Discriminate Plant Species Equally Well. PLoS One 2008, 3, e2802. [Google Scholar] [CrossRef]
- Sugiura, M. The chloroplast genome. Plant Mol. Boil. 1992, 19, 149–168. [Google Scholar] [CrossRef]
- Sugiura, M. The chloroplast genome. Essays Biochem. 1995, 30, 49–57. [Google Scholar]
- Ravi, V.; Khurana, J.P.; Tyagi, A.K.; Khurana, P. An update on chloroplast genomes. Plant Syst. Evol. 2008, 271, 101–122. [Google Scholar] [CrossRef]
- Wicke, S.; Schneeweiss, G.M.; DePamphilis, C.W.; M.€uller, K.F.; Quandt, D. The evolution of the plastid chromosome in land plants: Gene content, gene order, gene function. Plant Mol. Biol. 2011, 76, 273–297. [Google Scholar] [CrossRef]
- Zhu, Q.L. Comparative genomic research on the organellar genome of watermelon and melon. Ph.D. Thesis, Northeast Agricultural University: Harbin, China, 2018. [Google Scholar]
- Kang, H.I.; Lee, H.O.; Lee, I.H.; Kim, I.S.; Lee, S.W.; Yang, T.J.; Shim, D. Complete chloroplast genome of Pinus densiflora Siebold & Zucc. and comparative analysis with five pine trees. Forests 2019, 10, 600. [Google Scholar]
- Liu, X.; Chang, E.-M.; Liu, J.-F.; Huang, Y.-N.; Wang, Y.; Yao, N.; Jiang, Z.-P. Complete Chloroplast Genome Sequence and Phylogenetic Analysis of Quercus bawanglingensis Huang, Li et Xing, a Vulnerable Oak Tree in China. Forests 2019, 10, 587. [Google Scholar] [CrossRef]
- Wu, Z.-Q.; Ge, S. The phylogeny of the BEP clade in grasses revisited: Evidence from the whole-genome sequences of chloroplasts. Mol. Phylogenetics Evol. 2012, 62, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y. Study on cross compatibility of Rhododendron, L. Ph.D. Thesis, Nanjing Forestry University, Nanjing, China, 2012. [Google Scholar]
- Galle, F.C. Azaleas—Plants, Habits, Flowers and Leaves; Timber Press: Portland, OR, USA, 1985. [Google Scholar]
- Shen, J.S.; Li, X.Q.; Zhu, X.T.; Huang, X.L.; Jin, S.H. Complete chloroplast genome of Rhododendron pulchrum, an ornamental medicinal and food tree. Mitochondrial DNA Part B 2019, 4, 3527–3528. [Google Scholar] [CrossRef]
- Doyle, J.J. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. Novoplasty: De novo assembly of organelle genomes from whole genome DNA. Nucleic Acids Res. 2016, 45, e18. [Google Scholar]
- Liu, C.; Shi, L.; Zhu, Y.; Chen, H.; Zhang, J.; Lin, X.; Guan, X. CpGAVAS, an integrated web server for the annotation, visualization, analysis, and GenBank submission of completely sequenced chloroplast genome sequences. BMC Genom. 2012, 13, 715. [Google Scholar] [CrossRef]
- Wyman, S.K.; Jansen, R.K.; Boore, J.L. Automatic annotation of organellar genomes with DOGMA. Bioinform. 2004, 20, 3252–3255. [Google Scholar] [CrossRef]
- Kurtz, S.; Schleiermacher, C. REPuter: Fast computation of maximal repeats in complete genomes. Bioinform. 1999, 15, 426–427. [Google Scholar] [CrossRef]
- Kurtz, S. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001, 29, 4633–4642. [Google Scholar] [CrossRef]
- Peden, J.F. Analysis of codon usage. Biosystems. 1999, 5, 45–50. [Google Scholar]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinform. 2017, 33, 2583–2585. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Kuma, K.-I.; Toh, H.; Miyata, T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005, 33, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; Depristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinform. 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Kurtz, S.; Phillippy, A.; Delcher, A.L.; Smoot, M.; Shumway, M.; Antonescu, C.; Salzberg, S.L. Versatile and open software for comparing large genomes. Genome Boil. 2004, 5, R12. [Google Scholar] [CrossRef] [PubMed]
- Mayor, C.; Brudno, M.; Schwartz, J.R.; Poliakov, A.; Rubin, E.M.; Frazer, K.A.; Pachter, L.S.; Dubchak, I. VISTA: Visualizing global DNA sequence alignments of arbitrary length. Bioinform. 2000, 16, 1046–1047. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Cavender Bares, J.; González Rodríguez, A.; Eaton, D.A.R.; Hipp, A.A.L.; Beulke, A.; Manos, P.S. Phylogeny and biogeography of the American live oaks (Quercus subsection Virentes): A genomic and population genetics approach. Mol Ecol. 2015, 24, 3668–3687. [Google Scholar] [CrossRef]
- Kim, S.-C.; Baek, S.-H.; Lee, J.-W.; Hyun, H.J. Complete chloroplast genome of Vaccinium oldhamii and phylogenetic analysis. Mitochondrial DNA Part B 2019, 4, 902–903. [Google Scholar] [CrossRef]
- Tao, X.; Ma, L.; Zhang, Z.; Liu, W.; Liu, Z. Characterization of the complete chloroplast genome of alfalfa (Medicago sativa ) (Leguminosae). Gene Rep. 2017, 6, 67–73. [Google Scholar] [CrossRef]
- Yang, C.; Wu, X.; Guo, X.; Bao, P.; Chu, M.; Zhou, X.; Liang, Z.; Ding, X.; Yan, P. The complete chloroplast genome of Medicago sativa cv. Hangmu No.1, a plant of space mutation breeding. Mitochondrial DNA Part B 2019, 4, 603–604. [Google Scholar] [CrossRef]
- Schmid, P.A.; Flegel, W. Codon usage in vertebrates is associated with a low risk of acquiring nonsense mutations. J. Transl. Med. 2011, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Zang, M.; Li, M.; Fang, Y. Complete Chloroplast Genome Sequence and Phylogenetic Analysis of Quercus acutissima. Int. J. Mol. Sci. 2018, 19, 2443. [Google Scholar] [CrossRef]
- Boudreau, E.; Takahashi, Y.; Lemieux, C.; Turmel, M.; Rochaix, J. The chloroplast ycf3 and ycf4 open reading frames of Chlamydomonas reinhardtii are required for the accumulation of the photosystem I complex. EMBO J. 1997, 16, 6095–6104. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wuyun, T.-N.; Du, H.; Wang, D.; Cao, D. Complete chloroplast genome sequences of Eucommia ulmoides: Genome structure and evolution. Tree Genet. Genomes 2016, 12, 12. [Google Scholar] [CrossRef]
- Jansen, R.K.; Cai, Z.; Raubeson, L.A.; Daniell, H.; Depamphilis, C.W.; Leebens-Mack, J.; Müller, K.F.; Guisinger-Bellian, M.; Haberle, R.C.; Hansen, A.K.; et al. Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proc. Natl. Acad. Sci. 2007, 104, 19369–19374. [Google Scholar] [CrossRef]
- Goremykin, V.V.; Holland, B.; Hirsch-Ernst, K.I.; Hellwig, F.H. Analysis of Acorus calamus Chloroplast Genome and Its Phylogenetic Implications. Mol. Boil. Evol. 2005, 22, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).