Abstract
Since the 1990s, a decline of riparian black alder (Alnus glutinosa Gaertn.) has been observed over Europe. The fungus-like eukaryotic pathogen Phytophthora alni subsp. alni is thought to be a causal agent of this process; however, abiotic factors may also be involved. Previous studies suggest that climate conditions and, especially, depletion of groundwater level may be among the most important factors that trigger this phenomenon. We investigated the radial growth and wood vessel diameter of black alder trees of various vigour classes as well as their response to groundwater level changes to search for the link between soil water resources availability and mortality related to alder dieback. Samples were collected in the natural stand located near Sieraków village in the Kampinoski National Park, central Poland, in the area where alder dieback has been recently observed. Based on the crown defoliation level, three vigour classes (healthy, weakened, and dead trees) were distinguished. Cross sections were prepared with a sliding microtome, and Cell P image analysis software was used for the measurements. Tree-ring width (TRW) and vessel diameter (VD) were determined and correlated with the monthly values of precipitation and groundwater level. Alders of the analysed vigour classes exhibited similar patterns of TRW and VD changes over the analysis time. The narrowest tree rings were observed in weakened alders, while the largest vessels were noted in healthy trees. In the case of TRW and VD chronologies, the weakest, and hence insignificant, resemblance was found for healthy and dead trees. TRW and VD of the analysed alders were not correlated with the monthly sum of precipitation, but a negative influence of rainfall in April was observed. In turn, groundwater level had an impact on the radial growth and wood anatomical features of the analysed trees. A negative effect of the highest water table level was found for TRW of weakened and dead trees as well as for VD of healthy and weakened alders. The lowest groundwater level and the amplitude of the water table positively affected VD of the dead trees. Alder decline has a polyetiological nature, and groundwater level fluctuations are one of many factors contributing to disease development.
1. Introduction
Black alder (Alnus glutinosa Gaertn.) covers Europe as well as large parts of North America and Asia and constitutes an important component of riverine ecosystems, which form a buffer zone along many streams and fulfil a crucial role in the filtration and purification of water [1]. Alders contribute to the stability of riverbanks and, together with willows, are considered a pioneer species because of their quick initial growth, wind pollination, seed dispersal by wind and water, fast colonisation of naked ground, intolerance of shade, and a comparatively short lifespan [2]. They are tolerant of wet habitats, especially those with spring gain in a river water table. Their characteristic adventitious roots form an ecological niche for fish and other organisms. Black alder is also a dual mycorrhizal tree and possesses the capability to fix nitrogen by means of endophytic microorganisms [3].
Since the 1990s, in several European countries, a decline of riparian alders has been observed [4,5,6]. Phytophthora alni subsp. alni, a fungus-like eukaryotic pathogen, is thought to be a causal agent of this dieback [7,8,9,10]. The important role of rivers in the spread of the pathogen is well documented [2,11]. When this microorganism is present in watercourses, its further dissemination is inevitable as a result of its inherent connectivity [12]. However, the weather conditions also seem to be important for disease development. As described by Aguayo et al. [13], the most favourable conditions for disease spread include mild air temperature of above 3.5 °C in winter and of 21–22 °C in July and August in the year prior to ring formation.
The development of vegetation (especially riparian) is driven by various hydrological processes. Trees may suffer from water access limitations caused by lack of water uptake in case of table dropdown. On the contrary, when the groundwater level rises, they fulfil their water demands and undergo substantial growth. However, waterlogging and resulting anaerobic conditions caused by too high groundwater levels may shift this water supply effect from a positive to a negative one [14,15]. Flood (or waterlogging) events, their duration, frequency and spatial extent, as well as the amount and quality of the carried sediments, affect the distribution, age and morphology, as well as the anatomy of riparian tree species [16,17]. Kozlowski [18] pinpointed that groundwater table fluctuation and long-term waterlogging are primary abiotic causes of damage to several tree species. It is known that flooding modifies the structure of soil, diminishes its oxygen level and increases its carbon dioxide content [19,20]. This, in turn, creates anoxia/hypoxia, which inhibits tree growth, leads to the decay of root systems and promotes mortality [18]. A tree’s response to flooding depends on many factors, among which, genotype, plant age, as well as time and duration of the flooding events [21]. Vyhlidková et al. [22] stated that alder is not tolerant of groundwater fluctuations and long-term flooding by stagnant water. However, alders possess important morphological adaptations to flooding, which include the formation of aerenchyma and hypertrophied lenticels that allow them to increase gas exchanges and to get rid of toxic compounds associated with hypoxia, as well as support the generation of new roots [23,24].
A retrospective analysis of the severity of disease symptoms can be provided by the study of wood structure [25,26,27], as this tissue has proved to be an informative proxy record of environmental events that took place in the past.
Our research aimed to: (i) investigate the modification of the wood structure in trees differing in health conditions and (ii) determine the impact of groundwater level fluctuations as well as precipitation on the radial growth of black alders with visible symptoms of decline. Considering that both tree-ring width and vessel size chronologies have been successfully introduced to detect unusual environmental phenomena, we set forward the hypothesis that these parameters would reflect the effect of climatic factors or water table changes on the deterioration of the health conditions of the investigated alders.
2. Material and Methods
2.1. Study Site
The material was collected in the natural black alder (A. glutinosa Gaertn.) stand located near Sieraków village in the Kampinoski National Park (central Poland), in an area where an extensive alder dieback has been recently observed. The investigated stand grows on a wetland at the northern foothill of an inland dune covered with 110-year-old Scots pines, on peat-murshic soils shallowly overlying sands. The abundance of the Wilcza Struga stream in the northern part of the stand leads to periodic flooding. The forest habitat was defined as very mesic eutrophic (swampy). The stand is characterised by low quality and productivity, corresponding to the 3rd site index class. The average height of the trees was 14 m, while the mean breast height diameter equalled to 12 cm (data from the last inventory held in 2002).
According to Kottek et al. [28], the study site is located within the Cfb Köppen–Geiger climate zone. The mean annual temperature in the period 1971–2016 equalled 8.4 °C, while the mean temperature in the vegetation season of April–September amounted to 15.0 °C. The average annual precipitation was rather low, reaching merely 487 mm, with 314 mm (i.e., 65% of annual amount) in April–September (Figure 1).
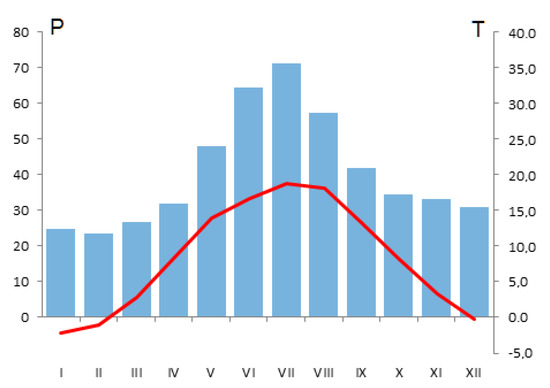
Figure 1.
Mean monthly temperature (T, °C, line) and precipitation (p, mm, bars) in 1971–2016 at the Sieraków site.
2.2. Sampling and Data Acquisition
We collected the research material in September 2016, when the investigated trees were at the age of 45–46 years. Prior to tree-felling, we distinguished tree vigour classes based on the crown vitality and the occurrence of cambium lesions. The trees were classified into one of three groups [27]. The first group (H) represented healthy trees with normally developed crown, the second one consisted of weakened individuals with cambium necrosis and retarded crown (W), while the last one included dead alders (D). Each class was represented by 5 trees that were felled. From their diameter at breast height, we cut out cross sections that served for the acquisition of the samples for wood anatomy analyses.
Small samples of wood including all tree rings from the pith towards the bark were obtained. Next, standard protocols were applied to prepare wood sections [29]. After a softening process, 30 µm-thick cross sections were cut with the use of a Microm HM 440E sliding microtome (GMI Inc., Ramsey, MN, USA). These sections were transferred onto slides, sealed with glycerol and then studied under an OLYMPUS BX61 light microscope coupled with an OLYMPUS Color View digital microscope camera (Olympus, Tokyo, Japan) and the OLYMPUS Cell P software (ver. 3.4, Olympus Soft Imaging System GmbH, Münster, Germany) for image acquisition and measurement. We measured the width of annual rings (TRW in mm) as well as the diameter of each vessel (VD in µm) within an individual ring. The latter feature was acquired in the radial plane at 10× magnification.
The Kampinoski National Park provided the data about the groundwater level in 1999–2016 (Figure 2). A piezometer was located in close vicinity of the investigated black alder stand (52.32° N, 20.82° E; 79.3 m a.s.l.). It recorded the groundwater table level (meters below ground) in two-week intervals. Missing values for June 2009–May 2010 were filled on the basis of data from a neighbouring device, according to the standard hydrological procedures. Climate data were obtained from the E-OBS 17.0 database [30] and consisted of monthly values of precipitation.
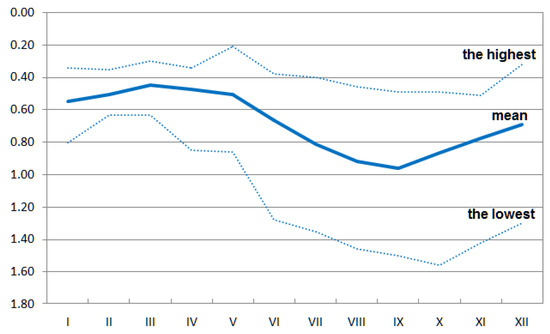
Figure 2.
Highest, mean, and the lowest monthly averages of the groundwater level (meters below ground) at the Sieraków site in 1999–2016.
2.3. Data Transformation and Analyses
Individual TRW series were cross-dated visually and statistically with the CDendro 8.1 (Cybis Elektronik & Data, Saktsjöbaden, Sweden; www.cybis.se) software. To eliminate the influence of tree’s age and to emphasize the short-term inter-annual variability in the series of analysed attribute, individual TRW sequences were detrended using the spline function with frequency response 0.50 at a wavelength of 0.67 of the series length. Then, the indices were prewhitened using an autoregressive model and averaged across all series within a vigour group using a biweight robust mean estimation to produce mean residual chronologies of tree-ring indices (TRI). For each tree, all VDs within individual tree rings were averaged, so that a sequence of mean vessel diameters was constructed. In the next step, these series were averaged within a vigour group also using a biweight robust mean estimation to produce mean VD chronologies. No detrending or autoregression removal was applied for this parameter. Calculations and TRW, TRI and VD chronologies construction were carried out using the dplR package [31,32] developed for the R environment (www-r-project.org).
Because of the relative short length of the analysed period (43 years), we decided not to calculate standard indices of the representativeness and part of ‘common signal’ expressed by the analysed sequences. Similarity and synchronicity of the chronologies were assessed using Gleichläufigkeit (Glk) and Pearson correlation (r) coefficients [33].
The mean values of TRW and VD were tested for the significance of the difference among distinct tree vigour classes. We compared the means for the whole common interval (e.g., 1978–2013) as well as for 10, 5 and 3 years before the death of some of the analysed alders. As the distribution of TRW was consistent with a normal one (Shapiro–Wilk test, p > 0.05), we used ANOVA followed by Tuckey post-hoc test with p = 0.05 as the significance threshold level for the comparisons. The PAST3.18 software [34] was used for the calculations.
On the basis of the obtained information about the groundwater, we determined its mean and the highest and the lowest levels in each month as well as for the whole vegetation period (mid-April–mid-September) and expected period of the highest cambium activity (mid-May–end of July). We also calculated the groundwater level amplitudes (difference between the highest and the lowest values) for a whole year as well as for the above-mentioned vegetation period and time of the most intensive cambium activity. The influence of the groundwater level and amount of precipitation on the radial increment and on the mean vessel diameter of the analysed alders was investigated using simple Pearson correlation coefficients. Taking into consideration the shortness of the data time span, no temporal stability of the observed relationships was analysed.
3. Results
Alders from all three vigour classes had diffuse-porous wood. Among uniseriate rays, false rays were observed as well (Figure 3). Vessel elements were connected with each other by scalariform perforation plates.

Figure 3.
Cross section of the wood of a weakened alder with a false ray marked with arrows.
In general, alders from the analysed vigour classes exhibited a similar course of radial increment over time, which was confirmed by significant r and Glk values (Table 1, Figure 4). The weakest and insignificant (p = 0.177) resemblance was found between healthy and dead trees. The series of mean VD showed a rather good correlation; however, it was lower for TRW. Again, an insignificant relationship was observed between healthy and dead alders (p = 0.124). In turn, the synchronicity between series of VD from distinct vigour classes was much lower than for TRW (Table 1, Figure 4).

Table 1.
Similarity (Pearson’s correlation coefficient—below the diagonal, Gleichläufigkeit coefficient—above the diagonal) of the tree-ring width (TRW) and mean vessel diameter (VD) in healthy (H), weakened (W) and dead (D) alders from Sieraków.
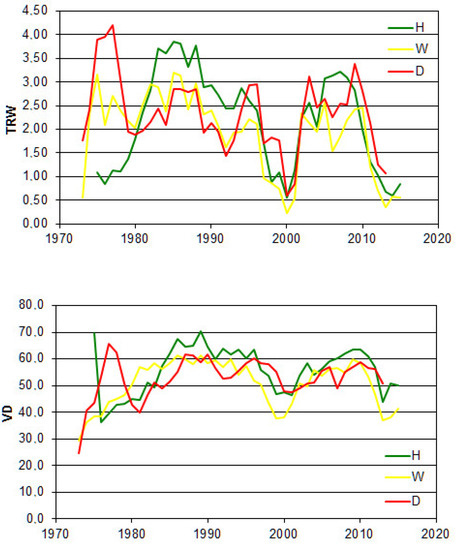
Figure 4.
TRW (mm) and VD (μm) chronologies for healty (H), weakened (W) and dead (D) alders from Sieraków.
We found no significant difference in TRW between distinct vigour classes in the whole common period as well as in the 3, 5 and 10 years prior to dieback (Table 2). In all analysed variants, weakened trees appeared to have the lowest mean TRW, while dead trees were characterised by the highest TRW values. For VD, significant differences were found 5 and 10 years prior to dieback. In these cases, healthy trees had a significantly higher mean vessel diameter than weakened and dead alders (Table 2).

Table 2.
Mean with standard deviation (in parentheses) TRW (mm) and VD (μm) in the common period (CP, 1975–2013) as well as 10, 5 and 3 years prior to alder dieback (10, 5 and 3, respectively) for healthy (H), weakened (W) and dead (D) trees from Sieraków.
For the investigated alders, VD was significantly positively related to TRW and TRI (Figure 5). The higher correlation coefficient value was found for the latter parameter, indicating a slight influence of tree age on the observed relationship (rTRW = 0.513, p < 0.001 vs. rTRI = 0.550, p < 0.001). Health status also affected these relations, since for the dead trees, we found much lower correlation coefficients (rTRW = 0.290, p = 0.066 vs. rTRI = 0.404, p = 0.009) compared to the healthy (rTRW = 0.593, p < 0.001 vs. rTRI = 0.581, p < 0.001) and weakened trees (rTRW = 0.683, p < 0.001 vs. rTRI = 0.404, p < 0.001).
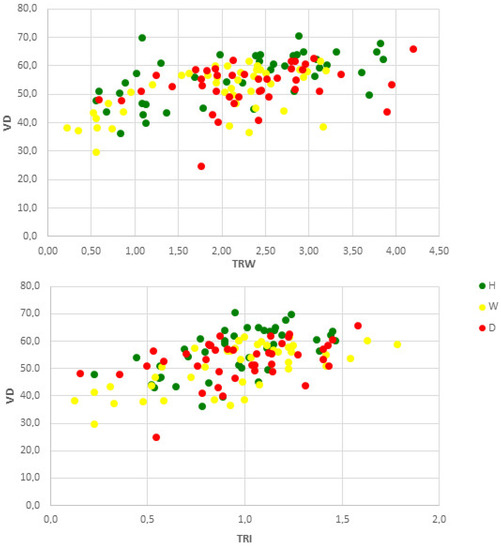
Figure 5.
Relationship between TRW (mm) or tree-ring index TRI (dimensionless) and VD (μm) for healthy (H), weakened (W) and dead (D) alders from Sieraków.
TRW and VD of the analysed alders were not correlated with the monthly sum of precipitation, except for April, which exhibited a negative influence of rainfall. Both investigated attributes had quite high values of correlation coefficient in relation to April precipitation, but the correlation was significant only for healthy trees (r = −0.514, p = 0.036 for TRW, and r = −0.481, p = 0.050 for VD). In turn, the groundwater level seemed to largely influence the radial growth and wood anatomical features of the analysed trees (Table 3 and Table 4). Significant or close to significant values of correlation coefficients were found for the relationship between the TRW of weakened or dead trees and the highest groundwater level of the whole year, the vegetation period and the period of the highest cambium activity. As far as the monthly resolution of the groundwater level is concerned, a significant correlation was found for April and May as well as for the period between September and December in the year proceeding tree-ring formation (Table 3). Both weakened and dead trees responded negatively to the highest, the lowest and the mean level of groundwater. Higher coefficient values were observed for the dead trees. No influence of the amplitude of the water table level was detected for the analysed vigour classes. In the case of VD, a significant negative correlation was found for the relationship with the highest groundwater level of a whole year for healthy and weakened alders, whereas a positive correlation was found for the mean and the lowest monthly groundwater level and the amplitude of the water table level in a whole year, in the vegetation period and in the period of highest cambium activity for the dead trees (Table 4). As far as the monthly resolution of the groundwater level is concerned, the picture is more complex and rather unclear. However, one can distinguish a negative influence of the highest and mean groundwater level in April on VD in healthy and dead trees, as well as a positive effect of these features in June–July for VD in dead alders. Again, no significant relationship with the amplitude of the water table level was detected for healthy and weakened trees.

Table 3.
Pearson correlation coefficients between the highest, the lowest and the mean monthly groundwater level as well as its amplitude and the tree-ring width of healthy (H), weakened (W) and dead (D) alders from Sieraków.

Table 4.
Pearson correlation coefficients between the highest, the lowest and the mean monthly groundwater level as well as its amplitude and the vessel diameter of healthy (H), weakened (W) and dead (D) alders from Sieraków.
4. Discussion
The soil water resources have a significant impact on tree health and survival. Their shortage or surplus may be a chronic stressor for trees. Permanent or seasonal changes in water supply affect individual trees or stands, their structure and growth, as well as the functioning of the whole forest ecosystem [35,36]. In the presented study, we compared TRW and VD of healthy, weakened and dead black alder (A. glutinosa Gaertn.) trees as well as analysed the dependence of these parameters on groundwater level and amount of precipitation, to investigate the causes of the significant dieback of this species that has been observed in the Kampinoski National Park (central Poland) for some time.
It was not possible to establish the health condition of black alder trees in the basis of the sole TRW. The investigated individuals exhibited rather similar growth patterns and paces, regardless of their vigour status. A similar effect was noted by Worrall et al. [37] who studied the dieback of thinleaf alder (Alnus incana ssp. tenuifolia) associated with cytospora canker expansion, as well as by Tulik et al. [38] who analysed the diminishing European ash (Fraxinus excelsior L.). In both cases, the severity of crown damage displayed no relationship with the average radial increment rate. In turn, Tulik and Bijak [27] found that healthy oaks (Quercus sp.) were characterised by a significantly larger TRW than weakened or dead trees; however, the course of the radial increment of the analysed oaks was similar within all distinct health classes.
The vessel diameter indicates the health condition of alders in a better way than TRW, as the lumen of the vessels of healthy alders is significantly larger than that of weakened or dead trees. A large size of the conducting elements allows more efficient water transport in healthy alders. Cavitation in wood can contribute to a major reduction in hydraulic conductivity, seriously threatening photosynthesis and plant survival. The largest lumen diameter in healthy alder trees reached 70 µm, which seems to be enough to secure a low risk of cavitation, especially in comparison to oak, false acacia or elm, whose lumen is visible to the naked eye [39,40]. A similar pattern as in the present study was observed for European ash [38] or oak [27].
For the analysed features, one can spot two periods of deep stress (remarkably reduced TRW and VD) in 2000 and 2013 (Figure 4). In the case of the first stress event, all trees in distinct vigour groups recovered, whereas for the latter event, analysed in this paper, a group of trees did not manage to rebuild their growth potential and died. The standard dendrochronological methods used in the paper did not allow obtaining a clear explanation of this phenomenon. Perhaps, the application of genetic studies or more sophisticated wood anatomy analyses could be helpful.
The groundwater level is considered as one of the most important environmental factors that affect the annual radial increment in black alder [41,42]. The importance of this feature is also stressed by the results of our study. The vessel diameter of the analysed alders also depends on the availability of water, and this relationship is bound to the health status of the trees. This result itself is not surprising, as the process of vessel differentiation is, among others, ruled by turgor pressure [43], but indicates that the health condition of alders affects the intensity of vessel growth during cell differentiation. Copini et al. [44] proved that in oak, stem flooding leads to the cessation of earlywood vessel development. It is a fact that, due to small size of the vessels, the hydraulic conductivity is reduced. Then, we can assume that limited hydraulic conductivity resulting from narrower vessels in weakened and dead alders could contribute to diminishing their radial growth after short-term flooding events and/or during fluctuations of the groundwater level and hampered their recovery.
Trees are more prone to flooding that occurs in the vegetative season than to those happening during dormancy [21]. Batzli and Dawson [45] stated that water logging in the growing season may change the nitrogen budget in alders and in whole alder-dominated ecosystems. Hence, the deficiency of nitrogen as a macro-element may modify metabolic pathways in trees and contribute to the weakening of the vigour of trees. These observations may support our findings on the correlation in weakened or dead trees between TRW and VD and the highest groundwater level in a whole year, in the vegetation period and in the period of the highest cambium activity. As reported by Ballesteros et al. [46], flash floods caused a significant reduction in the anatomy features of black alder wood. Astrade and Bégin [47] related the presence of smaller and fewer vessels in Populus tremula L. and Quercus robur L. to floods at the start of the growing season.
Short-lasting water logging within the growing season seems to be tolerated by most of the tree species, but when it is repeated or uninterrupted, serious damage to trees may appear. Strnadová et al. [48], while studying the effects of flooding and Phytophthora alni infection on black alder, showed that both of these factors could contribute to the alder decline. An increase of groundwater level and summer flooding in August proved to be significantly correlated with the damage to alder stands. In turn, flooding, during which the root system and lower part of the trunks stay water-logged over longer time, results in the cessation of nutrient and hormones transport caused by the lack of soil aeration [18]. Douda et al. [49] reported the negative influence of the increased water table caused by the clogging of drainage ditches on the growth of black alder. According to Vyhlidková et al. [22], groundwater fluctuations and long-term flooding by stagnant water are not tolerated by alders.
Previous research reported a rather straightforward correlation between the groundwater level and rainfall (i.e., when precipitation increases, the water table rises too), which was utilised in ecological modelling based on tree-ring proxies [50]. We found a significant positive relationship between the amount of rainfall in the vegetation period and the mean or the lowest groundwater level (r = 0.633, p = 0.006 and r = 0.648, p = 0.005, respectively). A similar observation was made for precipitation in the period of the highest cambium activity (r = 0.484, p = 0.049 for mean, and r = 0.503, p = 0.040 for the lowest groundwater level). However, surprisingly, these relationships did not translate into a significance dependence of TRW and VD of the analysed alders on precipitation in the case of a substantial influence of the water table level on the radial growth and wood anatomical features of the analysed trees. The analysed alders exhibited a rather small dependence of TRW or VD on precipitation, regardless of their health status. This observation contrasts with the findings of Vacek et al. [51] regarding mountains in the Czech Republic but, in turn, is supported by the statement of Laganis et al. [41], who recorded that the radial growth of the black alder is relatively independent of temperature and precipitation fluctuations. In addition, Elferts et al. [42] found a weak precipitation influence on alder tree-rings width in Latvia. Perhaps, the processes of tree-ring and vessels formation at the investigated site re more strongly controlled by endogenous rather than exogenous factors [52].
We did not study the effect of abundance of Phytophthora spp. On the dieback of the analysed alder trees directly, because of the lack of long-term data. However, regarding the observed climate changes, one can assume that the threat caused by this pathogen will increase [2], as it prefers milder winters [13,53]. On the other hand, reduced precipitation or lowering of the groundwater level may limit the negative effect caused by increased temperature [2].
5. Conclusions
Increased mortality of black alder at the analysed site in Kampinoski National Park (central Poland) is undoubtedly reflected by tree-ring width and vessel diameter of the investigated trees, which in turn are largely related to the groundwater level fluctuations (especially in the vegetation period). Hence, there seems to be a quite straightforward connection between decrease of trees vigour and changes in the amount of available water. Perhaps, the abundance of Phytophthora spp., which was not analysed because of the lack of historical data, plays an additional important role. The findings of the present study support the thesis that tree species dieback is a rather complex process of polyetiological nature.
Author Contributions
Study design, M.T.; Field data collection, A.G.; Laboratory work and data acquisition, M.T.; Statistical analysis, S.B.; Writing—Review and Editing, M.T., J.J.-M., S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank the Directorate of Kampinoski National Park for permission to conduct studies and for providing access to groundwater level monitoring data and Aleksandra Jasińska for technical support during wood sections preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Claessens, H.; OOsterbaan, A.; Savill, P.; Rondeux, J. A review of the characteristics of black alder (Alnus glutinosa (L.) Gaertn.) and their implications for silvicultural practices. Forestry 2010, 83, 163–175. [Google Scholar] [CrossRef]
- Bjelke, U.; Boberg, J.; Oliva, J.; Tattersdill, K.; Mckie, B.G. Dieback of riparian alder caused by the Phytophthora alni complex: Projected consequences for stream ecosystems. Freshw. Biol. 2016, 61, 565–579. [Google Scholar] [CrossRef]
- Kaelke, C.M.; Dawson, J.O. Seasonal flooding regimes influence survival, nitrogen fixation, and the partitioning of nitrogen and biomass in Alnus incana ssp. Rugosa. Plant Soil 2003, 254, 167–177. [Google Scholar] [CrossRef]
- Gibbs, J.N.; Van Dijk, C.; Webber, J. Phytophthora disease of alder in Europe. Bulletin 2003, 126, 82. [Google Scholar]
- Oszako, T. Zamieranie olszy w Europie. Lesn. Pr. Badaw. 2005, 1, 53–63. [Google Scholar]
- Belbahri, L.; Moralejo, E.; Calmin, G.; Oszako, T.; Garcia, J.A.; Descals, E.; Lefort, F. Phytophthora polonica, a new species isolated from declining Alnus glutinosa stands in Poland. FEMS Microbiol. Lett. 2006, 261, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Brasier, C.M.; Kirk, S.A.; Delcan, J.; Cooke, D.E.L.; Jung, T.; Manin’t Veld, W.A. Phytophthora alni sp. Nov. and its variants: Designation of emerging heteroploid hybrid pathogens spreading on Alnus trees. Mycol. Res. 2004, 108, 1172–1184. [Google Scholar] [CrossRef]
- Thoirain, B.; Husson, C.; Marcais, B. Risk factors for the Phytophthora-induced decline of alder in northeastern France. Phytopathology 2007, 97, 99–105. [Google Scholar] [CrossRef]
- Černy, K.; Gregorová, B.; Strnadová, V.; Holub, V.; Tomsovsky, M.; Cervenka, M. Phytophthora alni causing decline of black alders in Czech Republic. Plant Pathol. 2008, 57, 370. [Google Scholar] [CrossRef]
- Husson, C.; Aguayo, J.; Revellin, C.; Frey, P.; Ioos, R.; Marçais, B. Evidence for homoploid speciation in Phytophthora alni supports taxonomic reclassification in this species complex. Fungal Genet. Biol. 2015, 77, 12–21. [Google Scholar] [CrossRef]
- Černy, K.; Strnadová, V. Phytophthora alni decline: Disease symptoms, causal agent and its distribution in Czech Republic. Plant Protect. Sci. 2010, 46, 12–18. [Google Scholar] [CrossRef]
- Leuven, R.E.W.; Van Der Velde, G.; Baijens, I.; Snijders, J.; Van der Zwart, C.; Lenders, H.J.R.; de Vaate, A. The river Rhine: A global highway for dispersal of aquatic invasive species. Biol. Invasions 2009, 11, 1989–2008. [Google Scholar] [CrossRef]
- Aguayo, J.; Elegbede, F.; Husson, C.; Saintonge, F.X.; Marcais, B. Modelling climate impact on an emerging disease, the Phytophthora alni—Induced alder decline. Glob. Chang. Biol. 2014, 20, 3209–3221. [Google Scholar] [CrossRef] [PubMed]
- Jobbagy, E.G.; Jackson, R.B. Groundwater use and salinization with grassland afforestation. Glob. Chang. Biol. 2004, 10, 1299–1312. [Google Scholar] [CrossRef]
- Nosetto, M.D.; Jobbagy, E.G.; Jackson, R.B.; Sznaider, G.A. Reciprocal influence of crops and shallow ground water in sandy landscapes of the Inland Pampas. Field Crop Res. 2009, 113, 138–148. [Google Scholar] [CrossRef]
- Oliviera-Filho, A.T.; Vilela, E.A.; Gavilanes, M.L.; Carvalho, D.A. Effect of flooding regime and understorey bamboos on the physiognomy and tree species composition of a tropical semideciduous forest in southeastern Brazil. Vegetatio 1994, 113, 99–124. [Google Scholar]
- Kozlowski, T.T.; Pallardy, S.G. Physiology of Woody Plants; Academic Press: New York, NY, USA, 1997. [Google Scholar]
- Kozlowski, T.T. Responses of woody plants to flooding and salinity. Tree Physiol. 1997, 1, 1–29. [Google Scholar] [CrossRef]
- Rose, M.; Ghulam, M.; Waseem, T. A Review: Effects of climate change on agriculture, food resources and forestry. Int. J. Environ. Eco. Res. 2019, 1, 33–37. [Google Scholar]
- Parent, C.; Capelli, N.; Berger, A.; Crévecoeur, M.; Dat, J.F. An overiew of plant responses to soil waterlogging. Plant Stress 2008, 2, 20–27. [Google Scholar]
- Kozlowski, T.T. Plant responses to flooding of soil. BioScience 1984, 34, 162–167. [Google Scholar] [CrossRef]
- Vyhlidková, D.; Palovčiková, M.; Rybniček, P.; Čermak, P.; Janovský, L. Some aspects of alder decline along the Lužnice River. J. For. Sci. 2005, 51, 381–391. [Google Scholar] [CrossRef]
- Glenz, C.; Schlaepfer, R.; Iorgulescu, I.; Kienast, F. Flooding tolerance of Central European tree and shrub species. For. Ecol. Manag. 2006, 235, 1–13. [Google Scholar] [CrossRef]
- Wang, G.B.; Cao, F.L. Formation and function of aerenchyma in baldcypress (Taxodium distichym (L.) Rich.) and Chinese tallow tree (Sapium sebiferum (L.) Rocb.) under flooding. S. Afr. J. Bot. 2012, 81, 71–78. [Google Scholar] [CrossRef][Green Version]
- Tulik, M.; Rusin, A. Microfibril angle in wood of Scots pine trees (Pinus sylvestris) after Chernobyl accident. Environ. Pollut. 2005, 134, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Tulik, M. The anatomical traits of trunk wood and their relevance to oak (Quercus robur L.) vitality. Eur. J. For. Res. 2014, 133, 845–855. [Google Scholar] [CrossRef]
- Tulik, M.; Bijak, S. Are climatic factors responsible for the process of oak decline in Poland? Dendrochronologia 2016, 38, 18–25. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Gärtner, H.; Schweingruber, F.H. Microscopic Preparation Techniques for Plant Stem Analysis; Kessel Publishing House: Remagen-Oberwinter, Germany, 2013; p. 78. [Google Scholar]
- Haylock, M.R.; Hofstra, N.; Klein Tank, A.M.G.; Klok, E.J.; Jones, P.D.; New, M.A. European daily high-resolution gridded data set of surface temperature and precipitation for 1950–2006. J. Geophys. Res. 2008, 113, D20119. [Google Scholar] [CrossRef]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Bunn, A.G. Statistical and visual crossdating in R using the dplR library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- Cook, E.R.; Kairiūkštis, L.A. Methods of Dendrochronology. Applications in the Environmental Sciences; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1990. [Google Scholar]
- Hammar, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Rodríguez-González, P.M.; Campelo, F.; Albuquerque, A.; Rivaes, R.; Ferreira, T.; Pereira, J.S. Sensitivity of black alder (Alnus glutinosa [L.] Gaerth.) growth to hydrological changes in wetland forests at the rear edge of the species distribution. Plant Ecol. 2014, 215, 233–245. [Google Scholar] [CrossRef]
- Worral, J.J.; Adams, G.C.; Tharp, S.C. Summer heat and an epidemic of Cytospora canker of Alnus. Can. J. Plant Pathol. 2010, 32, 376–386. [Google Scholar] [CrossRef]
- Tulik, M.; Marciszewska, K.; Adamczyk, J. Diminished vessel diameter as apossible factor in the decline of European ash (Fraxinus excelsior L.). Ann. For. Sci. 2010, 67, 103–110. [Google Scholar] [CrossRef]
- Sperry, J.S.; Nicholas, K.L.; Sullivan, J.E.M.; Eastlack, S.E. Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of northern Utah and interior Alaska. Ecology 1994, 75, 1736–1752. [Google Scholar] [CrossRef]
- Sperry, J.S.; Meinzer, F.C.; McCulloh, K.A. Safety and efficiency conflicts in hydraulic architecture: Scaling from tissues to trees. Plant Cell Environ. 2008, 31, 632–645. [Google Scholar] [CrossRef]
- Laganis, J.; Peckov, A.; Debeljak, M. Modelling radial growth increment of black alder (Alnus glutinosa [L.] Gaerth.) tree. Ecol. Model. 2008, 215, 180–189. [Google Scholar] [CrossRef]
- Elferts, D.; Dauškane, I.; Ūsele, G.; Treimane, A. Effect of water level and climatic factors on the radial growth of black alder. Proc. Latv. Acad. Sci. Sect. B 2011, 65, 164–169. [Google Scholar]
- Zimmermann, M.H. Xylem Structure and the Ascent of Sap; Springer: New York, NY, USA, 1983. [Google Scholar]
- Copini, P.; Ouden, J.; Robert, E.M.R.; Tardif, J.C.; Loesberg, W.A.; Goudzwaard, L.; Sass-Klaassen, U. Flood-ring formation and root development in response to experimental flooding of young Quercus robur trees. Front. Plant Sci. 2016, 7, 775–788. [Google Scholar] [CrossRef]
- Batzli, J.M.; Dawson, J.O. Physiological and morphological responses of red alder and sitka alder to flooding. Physiol. Plant. 1999, 99, 653–663. [Google Scholar] [CrossRef]
- Ballesteros, J.A.; Stoffel, M.; Bollschweiler, M.; Bodoque, J.M.; Dıez-Herrero, A. Flash-flood impacts cause changes in wood anatomy of Alnus glutinosa, Fraxinus angustifolia and Quercus pyrenaica. Tree Physiol. 2010, 30, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Astrade, L.; Bégin, Y. Tree-ring response of Populus tremula L. and Quercus robur L. to recent spring floods of the Saône River, France. Écoscience 1997, 4, 232–239. [Google Scholar] [CrossRef]
- Strnadova, V.; Černy, K.; Holub, V.; Gregorová, B. The effects of flooding and Phytophthora alni infection on black alder. J. For. Sci. 2010, 56, 41–46. [Google Scholar] [CrossRef]
- Douda, J.; Čejková, A.; Douda, K.; Kochánková, J. Development of alder carr after the abandonment of wet grasslands during the last 70 years. Ann. For. Sci. 2009, 66, 712–725. [Google Scholar] [CrossRef]
- Gholami, V.; Fadaie, F.; Torkaman, J.; Ghaffari, A. Modeling of groundwater level fluctuations using dendrochronology in alluvial aquifers. J. Hydrol. 2015, 529, 1060–1069. [Google Scholar] [CrossRef]
- Vacek, Z.; Vacek, S.; Podrázský, V.; Král, J.; Bulušek, D.; Putalová, T.; Baláš, M.; Kalousková, I.; Schwarz, O. Structural diversity and production of alder stands on former agricultural land at high altitudes. Dendrobiology 2016, 75, 31–44. [Google Scholar] [CrossRef]
- Schmitz, N.; Verheyden, A.; Beckman, H.; Kairo, J.G.; Koedam, N. Influence of a salinity gradient on the vessel characters of the mangrove species Rhizophora mucronata. Ann. Bot. 2006, 98, 1321–1330. [Google Scholar] [CrossRef]
- Redondo, M.; Boberg, J.; Olsson, C.; Oliva, J. Winter Conditions Correlate with Phytophthora alni Subspecies Distribution in Southern Sweden. Phytopathology 2015. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).