Comparative Analysis Reveals the Metabolic Characteristics of Astringent Seeds of Chinese Fir (Cunninghamia lanceolata (Lamb) Hook) during Astringent Compounds Accumulation Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Collection, Processing, and Storage
2.2. Samples Preparation and Extraction
2.3. HPLC and ESI-Q TRAP-MS/MS Conditions
2.4. Qualitation and Quantitation of Metabolites
2.5. Identification and Verification of Different Expressive Metabolites
2.6. KEGG Enrichment Analysis
3. Results
3.1. Status of Astringent and Germinable Seeds
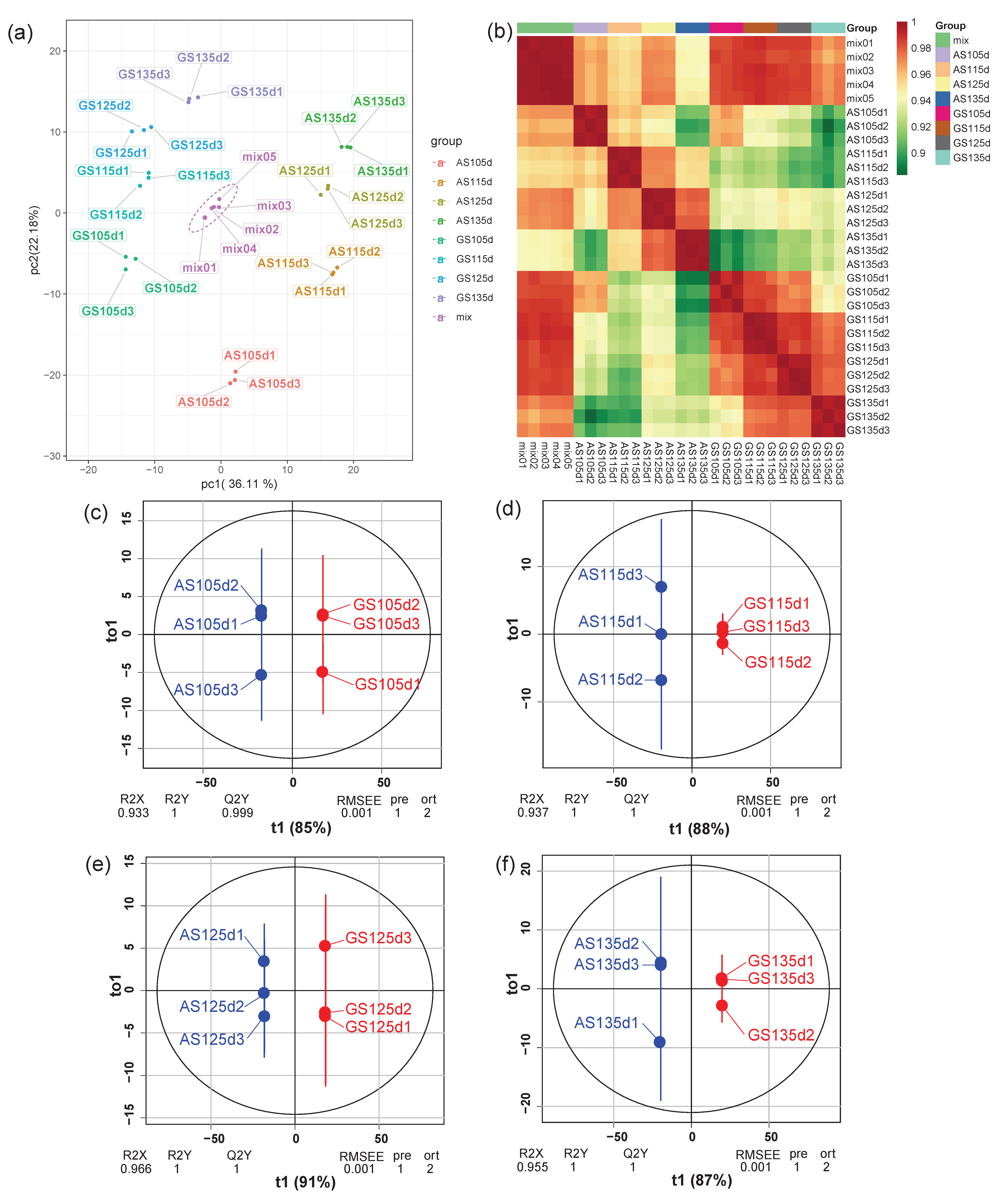
3.2. Samples Reliability Analysis
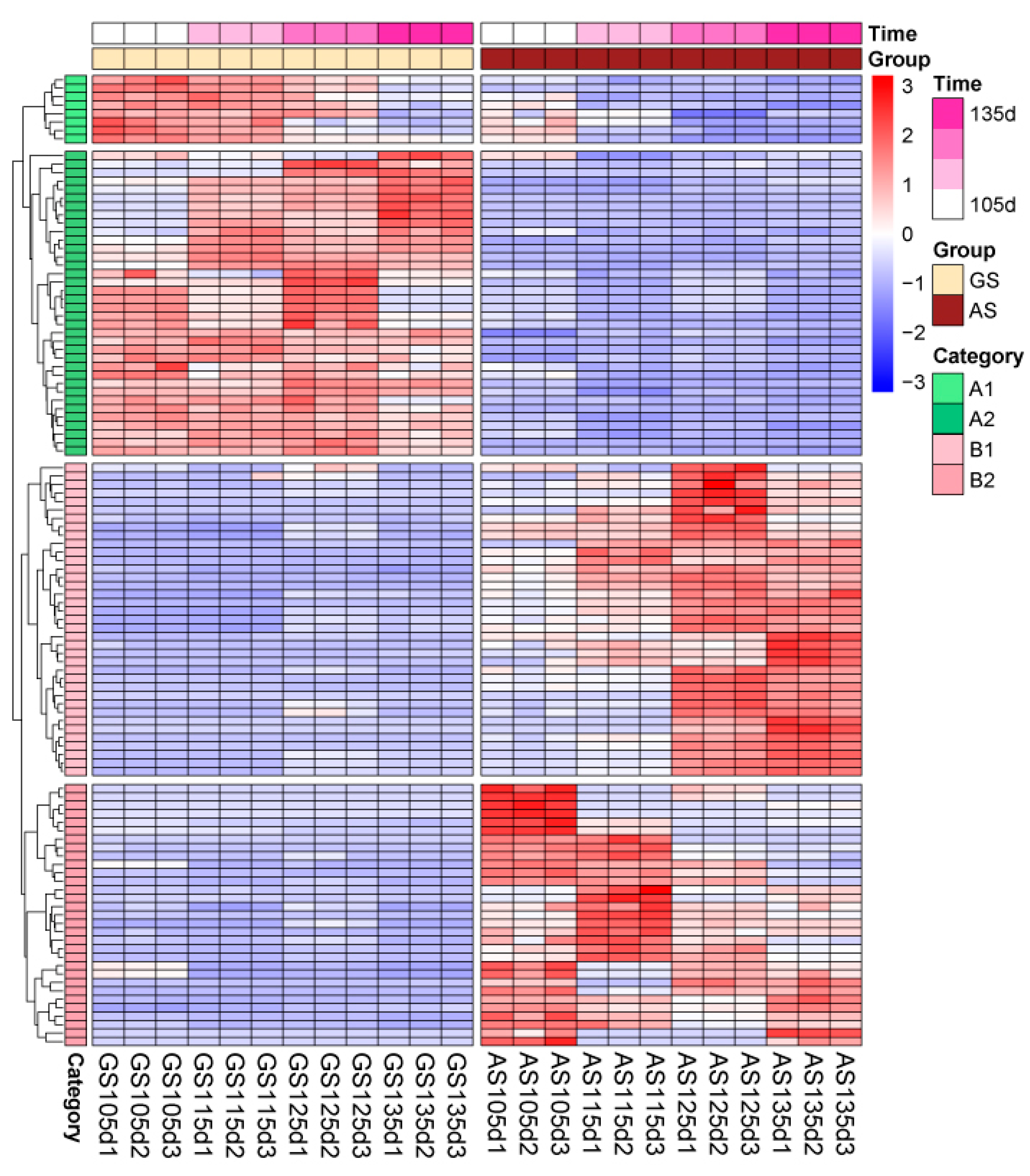
3.3. Selection and Classification of Different Expressive Metabolites
3.4. Expressive and Accumulative Change of Different Expressive Metabolites
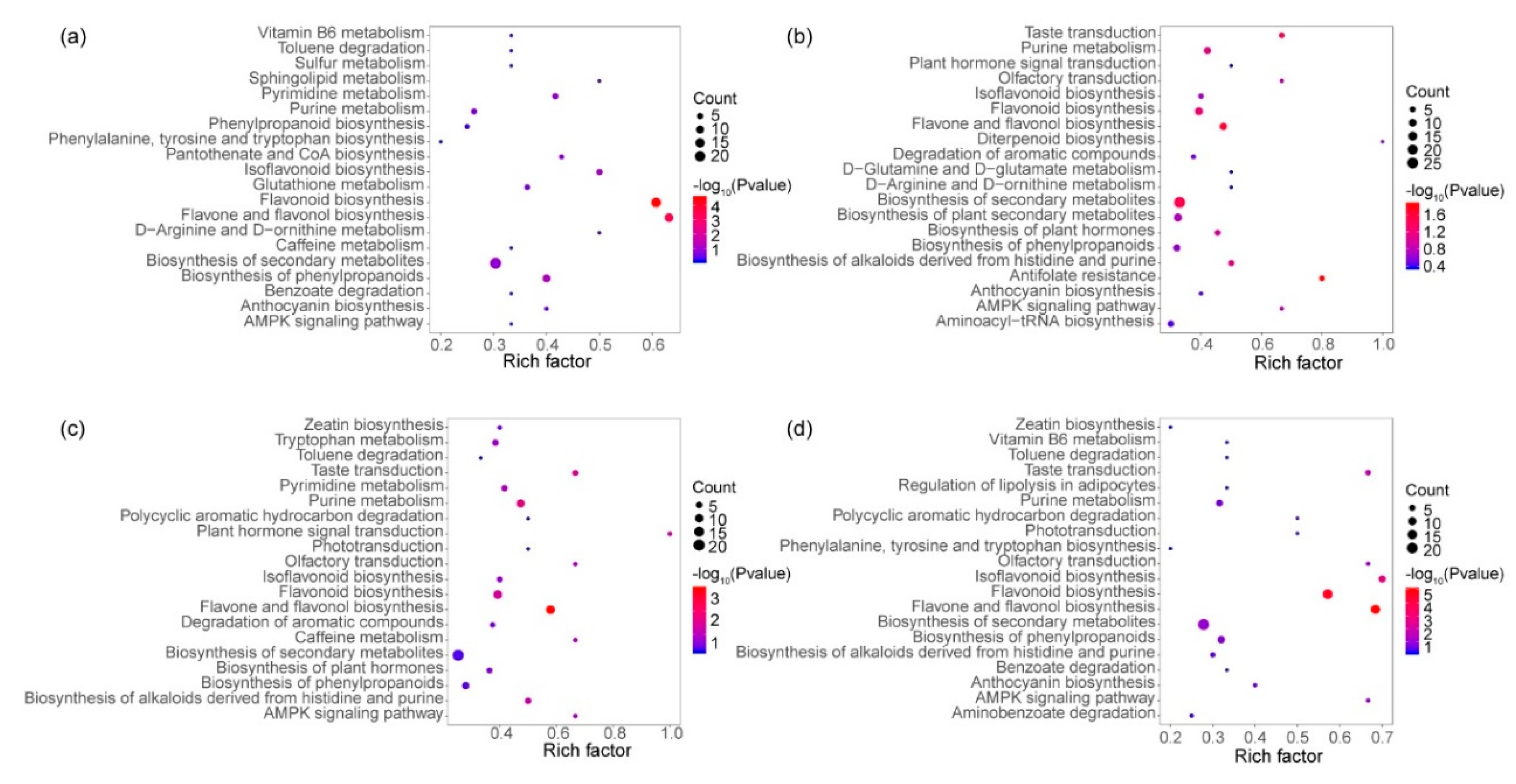
3.5. KEGG Enrichment of Different Expressive Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bian, L.M.; Zheng, R.H.; Su, S.D.; Lin, H.Z.; Xiao, H.; Wu, X.M.; Shi, J. Spatial analysis increases efficiency of progeny testing of Chinese fir. J. For. Res. 2017, 28, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.S. The present situation of Chinese fir genetic improvement in Fujian Province and the technical countermeasures of developing it. J. Fujian For. Sci. Technol. 1994, 21, 28–31, (Chinese with English abstract). [Google Scholar]
- Zhang, Z.W. Studies on the pollination characteristics and pollination level of Chinese fir seed orchard. Silvae Genet. 2004, 53, 7–11. [Google Scholar]
- Zheng, R.H.; Shi, J.S.; Su, S.D.; Lin, H.Z.; Lin, J.C.; Fang, L.M.; Ye, D.Q. The establishment technique and application of the third generation seed orchard of Chinese fir. J. For. Environ. 2018, 38, 406–413, (Chinese with English abstract). [Google Scholar]
- Zhang, Y.; Zuo, D.D.; Chen, Y.; Xiong, S.; Wang, Z.; Lin, S.Z. Anatomical observation of ovule of Cunninghamia lanceolata. J. For. Environ. 2017, 37, 142–147, (Chinese with English abstract). [Google Scholar]
- Guan, K.L.; Huang, J.Q.; He, F.J. Cone seed-extracting percentage and full seed percentage for seed orchard of Chinese fir. J. Zhejiang For. Coll. 1997, 14, 127–133, (Chinese with English abstract). [Google Scholar]
- Chang, Z.Y.; Chen, Z.F.; Wang, N.; Xie, G.; Lu, J.W.; Yan, W.; Zhou, J.; Tang, X.; Deng, X.W. Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene. Proc. Natl. Acad. Sci. USA 2016, 113, 14145–14150. [Google Scholar] [CrossRef] [Green Version]
- Sander, P.M.; Bui, A.Q.; Weterings, K.; Mcintire, K.N.; Hsu, Y.C.; Lee, P.Y. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex. Plant Reprod. 1999, 11, 297–322. [Google Scholar] [CrossRef]
- Suzuki, K.; Takeda, H.; Tsukaguchi, T.; Egawa, Y. Ultrastructural study on degeneration of tapetum in anther of snap bean (Phaseolus vulgaris L.) under heat stress. Sex. Plant Reprod. 2001, 13, 293–299. [Google Scholar] [CrossRef]
- Kong, X.K.; Khan, A.; Li, Z.L.; You, J.Y.; Munsif, F.; Kang, H.D.; Zhou, R. Identification of chalcone synthase genes and their expression patterns reveal pollen abortion in cotton. Saudi J. Biol. Sci. 2020. [Google Scholar] [CrossRef]
- Pavlova, D.K.; Bani, A. Pollen biology of the serpentine-endemic Orobanche nowackiana (Orobanchaceae) from Albania. Aust. J. Bot. 2019. [Google Scholar] [CrossRef]
- Ku, S.; Yoon, H.; Suh, H.S.; Chung, Y.Y. Male-sterility of thermosensitive genic male-sterile rice is associated with premature programmed cell death of the tapetum. Planta 2003, 217, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Pacini, E.; Franchi, G.G.; Hesse, M. The tapetum: Its form, function, and possible phylogeny in Embryophyta. Plant Syst. Evol. 1985, 149, 155–185. [Google Scholar] [CrossRef]
- Pettitt, J. Developmental mechanisms in heterospory: I. Megasporocyte degeneration in Selaginella. Bot. J. Linn. Soc. 2010, 64, 237–246. [Google Scholar] [CrossRef]
- Pannell, J.; Voillemot, M. Plant mating systems: Female sterility in the driver’s seat. Curr. Biol. 2015, 25, R511–R514. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Olivencia, A.; Paredes, J.A.; Rodríguez-Riaño, T.; Alcaraz, J.A. Modes of self-pollination and absence of cryptic self-incompatibility in Drosophyllum (Droseraceae). Plant Biol. 2015, 111, 474–480. [Google Scholar] [CrossRef]
- Butcher, A. The relationship between sexual outcrossing and heterokaryon incompatibility in Aspergillus nidulans. Heredity 1968, 23, 443–452. [Google Scholar] [CrossRef]
- Liu, K.W.; Liu, Z.J.; Huang, L.Q.; Li, L.Q.; Chen, L.J.; Tang, G.D. Self-fertilization strategy in an orchid. Nature 2006, 441, 945–946. [Google Scholar] [CrossRef]
- Zhang, S.S. Insights on seed abortion (endosperm and embryo development failure) from the transcriptome analysis of the wild type plant species Paeonia lutea. Bioinformation 2020, 16, 638–651. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Okubo, H.; Fujieda, K. Pollen tube growth, fertilization and embryo development of camellia japonica L.*C. chrysantha (Hu) tuyama. J. Jpn. Soc. Hortic. Sci. 2008, 60, 955–961. [Google Scholar] [CrossRef]
- Bennell, M.R.; Cleugh, H.; Leys, J.; Hein, D. The effect of hot dry wind on the pod set of faba bean (Vicia faba) cv. Fiord: A preliminary wind tunnel study. Aust. J. Exp. Agric. 2007, 47. [Google Scholar] [CrossRef]
- Meinke, D. Genome-wide identification of EMBRYO—DEFECTIVE (EMB) genes required for growth and development in Arabidopsis. New Phytologist. 2019, 226, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, K.L. Comprehensive analysis on causes for hollow, kernel and woody seeds of Chinese fir cones. J. Zhejiang For. Coll. 1997, 14, 88–93, (Chinese with English abstract). [Google Scholar]
- Lin, S.Z.; Song, S.X. A study on the trend of geographic epidemic of Chinese fir sterile seed. Acta Phytoecol. Geobot. Sin. 1990, 14, 40–45, (Chinese with English abstract). [Google Scholar]
- Wu, C.Z.; Hong, W.; Lin, S.Z. Study on spatial variability of Chinese fir sterile seeds. Chin. J. Appl. Environ. Biol. 1998, 4, 15–19, (Chinese with English abstract). [Google Scholar]
- Huang, L.Z. The peroxidase isozymes activity and lipid peroxidation in relation to aborted embryo in aborted seed of Chinese-fir. Acta Bot. Boreal. Occident. Sin. 1996, 16, 83–88, (Chinese with English abstract). [Google Scholar]
- Lin, S.Z.; Zhuang, Z.R.; Yu, X.T. Analysis of the epidemic of the Chinese fir sterile seed in the zones and its application on choice of the habital of the seed garden. J. Fujian Coll. For. 1989, 9, 310–314, (Chinese with English abstract). [Google Scholar]
- Lin, X.Q.; Ma, Z.H.; He, Z.M.; Chen, Y.; Peng, Y.R.; Lin, S.Z. Optimization of ultrasonic-assisted extraction process for polyphenols from the seeds of Chinese fir. J. For. Environ. 2017, 37, 47–53, (Chinese with English abstract). [Google Scholar]
- Zürcher, P.; Sokolov, M.; Brühlmann, D.; Ducommun, R.; Stettler, M.; Souquet, J.; Jordan, M.; Broly, H. Cell culture process metabolomics together with multivariate data analysis tools opens new routes for bioprocess development and glycosylation prediction. Biotechnol. Prog. 2020. [Google Scholar] [CrossRef]
- Clarke, J.D.; Alexander, D.C.; Ward, D.P.; Ryals, J.A.; Mitchell, M.W.; Wulff, J.E.; Guo, L.N. Assessment of Genetically Modified Soybean in Relation to Natural Variation in the Soybean Seed Metabolome. Sci. Rep. 2013, 3, 3082. [Google Scholar] [CrossRef]
- Khakimov, B.; Rasmussen, M.A.; Kannangara, R.M.; Jespersen, B.M.; Munck, L.; Engelsen, S.B. From metabolome to phenotype: GC-MS metabolomics of developing mutant barley seeds reveals effects of growth, temperature and genotype. Sci. Rep. 2017, 7, 8195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onda, Y.; Inoue, K.; Sawada, Y.; Shimizu, M.; Takahagi, K.; Uehara-Yamaguchi, Y.; Hirai, M.Y.; Garvin, D.F. Genetic variction for seed metabolite levels in Brachypodium distachyon. Int. J. Mol. Sci. 2019, 20, 2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazlan, O.; Aizat, W.M.; Baharum, S.N.; Azizan, K.A.; Noor, N.M. Metabolomics anaylsis of developing Garcinia mangostana seed reveals modulated levels of suguars, organic acids and phenylpropanoid compounds. Sci. Hortic. 2018, 233, 323–330. [Google Scholar] [CrossRef]
- Wang, L.; Fu, J.L.; Li, M.; Fragner, L.; Weckwerth, W.; Yang, P.F. Metabolomic and proteomic profiles reveal the dynamics of primary metabolism during seed development of Lotus (Nelumbo nucifera). Front. Plant Sci. 2016, 7, 750. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.L.; Xie, Q.J.; Xiang, X.E.; Li, J.Q.; Zheng, S.N.; Xu, X.Y.; Guo, H.; Ye, W. Dynamic metabolic profiles and tissue-specific source effects on the metabolome of developing seeds of Brassica napus. PLoS ONE 2015, 10, e0124794. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Gong, L.; Guo, Z.L.; Wang, W.S.; Zhang, H.Y.; Liu, X.Q.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography-mass spectrometry, XCMS, and chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef]
- Amri, N.E.; Errachidi, F.; Bour, A.; Bouhaddaoui, S.; Chabir, R. Morphological and nutritional properties of Moroccan Capparis spinosa Seeds. Sci. World J. 2019. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.Y.; Li, P.C.; Xu, Y.; Liu, J.; Yang, T.T.; Wei, J.; Xu, S.; Yu, J.; Fang, H.; Xue, L.; et al. Genetic and genomic analysis of the seed-filling process in maize based on a logistic model. Heredity 2020, 124, 122–134. [Google Scholar] [CrossRef]
- Morton, K.J.; Jia, S.G.; Zhang, C.; Holding, D.R. Proteomic profiling of maize opaque endosperm mutants reveals selective accumulation of lysine-enriched proteins. J. Exp. Bot. 2015, 67, 1381–1396. [Google Scholar] [CrossRef] [Green Version]
- Rousserie, P.; Rabot, A.; Geny-Denis, L. From Flavanols Biosynthesis to Wine Tannins: What Place for Grape Seeds? J. Agric. Food Chem. 2019, 67, 1325–1343. [Google Scholar] [CrossRef] [PubMed]
- L’hadj, I.; Azzi, R.; Lahfa, F.; Koceir, E.; Omari, N. The nutraceutical potential of Lepidium sativum L. seed flavonoid-rich extract in managing metabolic syndrome components. J. Food Biochem. 2018, 42, e12725. [Google Scholar] [CrossRef] [PubMed]
- De Jesús Uribe-Gómez, J.; Zamora-Natera, J.F.; Bañuelos-Pineda, J.; Kachlicki, P.; Stobiecki, M.; García-López, P.M. Flavonoid profile of Lupinus mexicanus germinated seed extract and evaluation of its neuroprotective effect. Histol. Histopathol. 2014, 29, 1415–1421. [Google Scholar] [CrossRef]
- Song, Y.E.; Wang, X.; Shen, Z.W.; Xu, Y.; Li, J.Y. Expressing the maize anthocyanin regulatory gene Lc increased flavonoid content in the seed of white pericarp rice and purple pericarp rice. Genetika 2013, 49, 1127–1133. [Google Scholar] [CrossRef]
- Ogo, Y.; Ozawa, K.; Ishimaru, T.; Murayama, T.; Takaiwa, F. Transgenic rice seed synthesizing diverse flavonoids at high levels: A new platform for flavonoid production with associated health benefits. Plant Biotechnol. J. 2013, 11, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef]
- Chen, W.; Lu, L.X. Relationship between Litchi embryo abortion and phenolic inhibitors. Acta Bot. Sin. 2002, 44, 168–172. [Google Scholar]
- Zhan, X.Q.; Shen, Q.W.; Wang, X.M.; Hong, Y.Y. The Sulfoquinovosyltransferase-like enzyme SQD2.2 is involved in flavonoid glycosylation, regulating sugar metabolism and seed setting in rice. Sci. Rep. 2017, 7, 4685. [Google Scholar] [CrossRef]
- Zažímalová, E.; Křeček, P.; Skůpa, P.; Petrášek, J. Polar transport of the plant hormone auxin—the role of PIN-FORMED (PIN) proteins. Cell. Mol. Life Sci. 2007, 64, 1621–1637. [Google Scholar] [CrossRef]
- Bayer, M.; Slane, D.; Jurgens, G. Early plant embryogenesis-dark ages or dark matter? Curr. Opin. Plant Biol. 2017, 35, 30–36. [Google Scholar] [CrossRef]
- Robert, H.S.; Park, C.; Gutièrrez, C.L.; Barbara, W.; Pěnčík, A.; Novák, O.; Chen, J.; Grunewald, W.; Dresselhaus, T.; Friml, J.; et al. Maternal auxin supply contributes to early embryo patterning in Arabidopsis. Nat. Plants 2018, 4, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zachgo, S. TCP3 interacts with R2R3-MYB proteins, promotes flavonoid biosynthesis and negatively regulates the auxin response in arabidopsis thaliana. Plant J. 2013, 76, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Ashikari, M.; Sakakibara, H.; Lin, S.Y.; Yamamoto, T.; Takashi, T.; Nishimura, A.; Angeles, E.R.; Qian, Q.; Kitano, H.; Matsuoka, M. Cytokinin oxidase regulates rice grain production. Science 2005, 309, 741–745. [Google Scholar] [CrossRef]
- Bhushan, P.B.; Sudhanshu, S.; Kumar, D.S.; Lamboder, B.; Prasad, S.B. Biochemical and molecular characterisation of exogenous cytokinin application on grain filling in rice. BMC Plant Biol. 2018, 18, 89. [Google Scholar] [CrossRef] [Green Version]
- Vondrakova, Z.; Dobrev, P.I.; Pesek, B.; Fischerova, L.; Vagner, M.; Motyka, V. Profiles of endogenous phytohormones over the course of norway spruce somatic embryogenesis. Front. Plant Sci. 2018, 9, 1283. [Google Scholar] [CrossRef]
- Wang, L.P.; Shen, W.Y.; Kazachkov, M.; Chen, G.Q.; Chen, Q.L.; Carlsson, A.S.; Stymne, S.; Weselake, R.J.; Zou, J. Metabolic interactions between the lands cycle and the Kennedy pathway of glycerolipid synthesis in Arabidopsis developing seeds. Plant Cell 2012, 24, 4652–4669. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.L.; Yu, X.M.; Chen, H.Y.; Li, W.Q. Turnover of glycerolipid metabolite pool and seed viability. Int. J. Mol. Sci. 2018, 19, 1417. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.K.; Liu, C.Y.; Huang, X.B.; Zhang, H.Y.; Yuan, Z.H. Land-plant phylogenomic and pomegranate transcriptomic analyses reveal an evolutionary scenario of cyp75 genes subsequent to whole genome duplications. J. Plant Biol. 2019, 62, 48–60. [Google Scholar] [CrossRef]
- Xie, P.; Shi, J.Y.; Tang, S.Y.; Chen, C.X.; Khan, A.; Zhang, F.X.; Xiong, Y.; Li, C.; He, W.; Wang, G.; et al. Control of bird feeding behavior by Tannin1 through modulating the biosynthesis of polyphenols and fatty acid-derived volatiles in sorghum. Mol. Plant 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]


| Metabolites Class | Amount | Percentage (%) | Amount in Different Categories 1 | |||
|---|---|---|---|---|---|---|
| A1 | A2 | B1 | B2 | |||
| Alkaloids | 1 | 0.89 | 1 | 0 | 0 | 0 |
| Amino acid derivatives | 10 | 8.93 | 1 | 8 | 1 | 0 |
| Amino acids | 2 | 1.79 | 0 | 1 | 0 | 1 |
| Anthocyanins | 3 | 2.68 | 0 | 0 | 0 | 3 |
| Benzoic acid derivatives | 1 | 0.89 | 0 | 0 | 1 | 0 |
| Catechin derivatives | 2 | 1.79 | 0 | 0 | 1 | 1 |
| Coumarins | 3 | 2.68 | 1 | 0 | 1 | 1 |
| Flavanone | 7 | 6.25 | 1 | 0 | 3 | 3 |
| Flavone | 18 | 16.07 | 0 | 2 | 9 | 7 |
| Flavone C-glycosides | 5 | 4.46 | 0 | 2 | 1 | 2 |
| Flavonol | 15 | 13.39 | 0 | 1 | 10 | 4 |
| Flavonolignan | 1 | 0.89 | 0 | 0 | 1 | 0 |
| Hydroxycinnamoyl derivatives | 3 | 2.68 | 0 | 0 | 1 | 2 |
| Indole derivatives | 1 | 0.89 | 0 | 1 | 0 | 0 |
| Isoflavone | 4 | 3.57 | 0 | 0 | 3 | 1 |
| Lipids_Fatty acids | 2 | 1.79 | 0 | 0 | 2 | 0 |
| Lipids_Glycerolipids | 2 | 1.79 | 0 | 1 | 0 | 1 |
| Lipids_Glycerophospholipids | 9 | 8.04 | 0 | 9 | 0 | 0 |
| Nucleotide and its derivatives | 8 | 7.14 | 0 | 5 | 1 | 2 |
| Organic acids | 2 | 1.79 | 0 | 0 | 0 | 2 |
| Others | 3 | 2.68 | 1 | 1 | 1 | 0 |
| Phenolamides | 1 | 0.89 | 0 | 1 | 0 | 0 |
| Phytohormones | 4 | 3.57 | 2 | 1 | 1 | 0 |
| Pyridine derivatives | 1 | 0.89 | 0 | 0 | 0 | 1 |
| Tryptamine derivatives | 2 | 1.79 | 0 | 2 | 0 | 0 |
| Vitamins | 2 | 1.79 | 1 | 1 | 0 | 0 |
| Grand total | 112 | 100.00 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wu, Y.; Wu, C.; Lin, S. Comparative Analysis Reveals the Metabolic Characteristics of Astringent Seeds of Chinese Fir (Cunninghamia lanceolata (Lamb) Hook) during Astringent Compounds Accumulation Stages. Forests 2020, 11, 1206. https://doi.org/10.3390/f11111206
Chen Y, Wu Y, Wu C, Lin S. Comparative Analysis Reveals the Metabolic Characteristics of Astringent Seeds of Chinese Fir (Cunninghamia lanceolata (Lamb) Hook) during Astringent Compounds Accumulation Stages. Forests. 2020; 11(11):1206. https://doi.org/10.3390/f11111206
Chicago/Turabian StyleChen, Yu, Yihan Wu, Chao Wu, and Sizu Lin. 2020. "Comparative Analysis Reveals the Metabolic Characteristics of Astringent Seeds of Chinese Fir (Cunninghamia lanceolata (Lamb) Hook) during Astringent Compounds Accumulation Stages" Forests 11, no. 11: 1206. https://doi.org/10.3390/f11111206
APA StyleChen, Y., Wu, Y., Wu, C., & Lin, S. (2020). Comparative Analysis Reveals the Metabolic Characteristics of Astringent Seeds of Chinese Fir (Cunninghamia lanceolata (Lamb) Hook) during Astringent Compounds Accumulation Stages. Forests, 11(11), 1206. https://doi.org/10.3390/f11111206




