Effects of Arbuscular Mycorrhizal Fungi on Growth and Physiological Performance of Catalpa bungei C.A.Mey. under Drought Stress
Abstract
1. Introduction
2. Materials and Methods
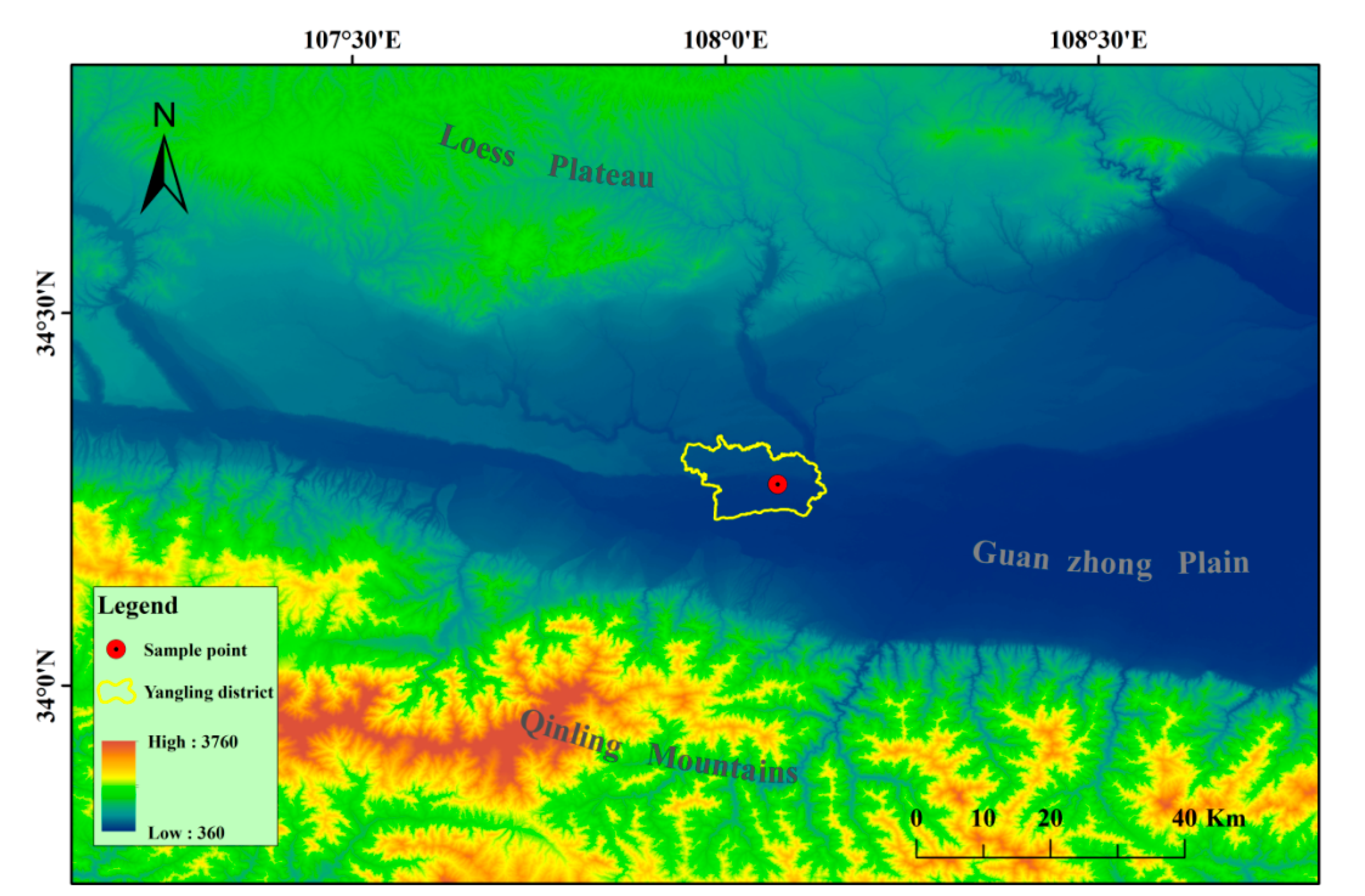
2.1. Arbuscular Mycorrhizal Inoculum, Plant Material, and Cultivation Substrate
2.2. Experimental Design
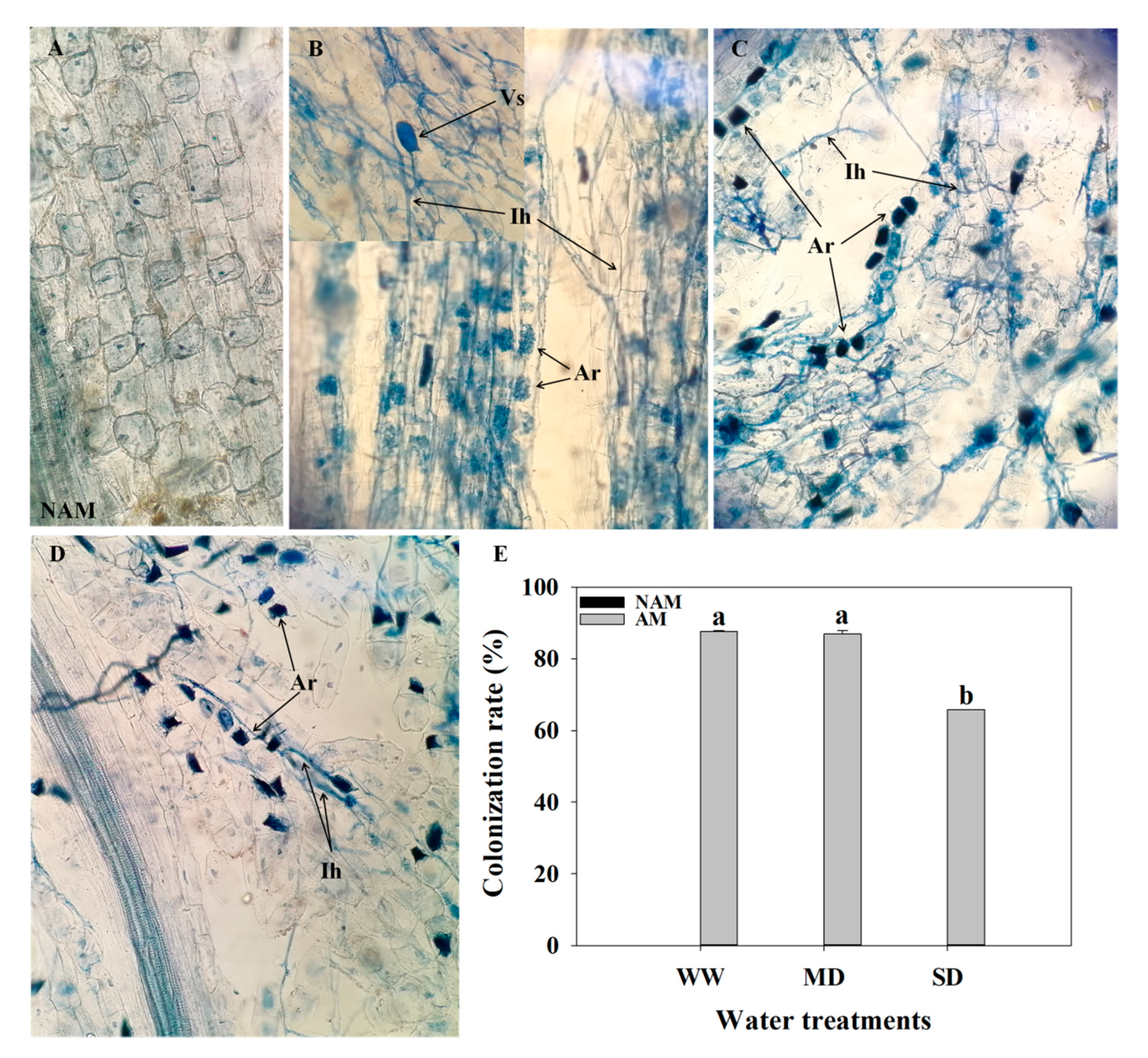
2.3. Measurement of AMF Colonization
2.4. Measurement of Gas Exchange Parameters and Photosynthetic Pigments
2.5. Determination of Plant Growth Parameters and Biomass Allocation
2.6. Analysis of Nutrient Absorption and Distribution
2.7. Determination of Specific Leaf Area and Specific Leaf Weight
2.8. Estimation of the Root System Architecture
2.9. Determination of Phytohormone Levels
2.10. Determination of ROS
2.11. Determination of GRSP Content
2.12. Analysis of Soil Aggregate Distribution
2.13. Statistical Analysis
3. Results
3.1. AMF Root Colonization
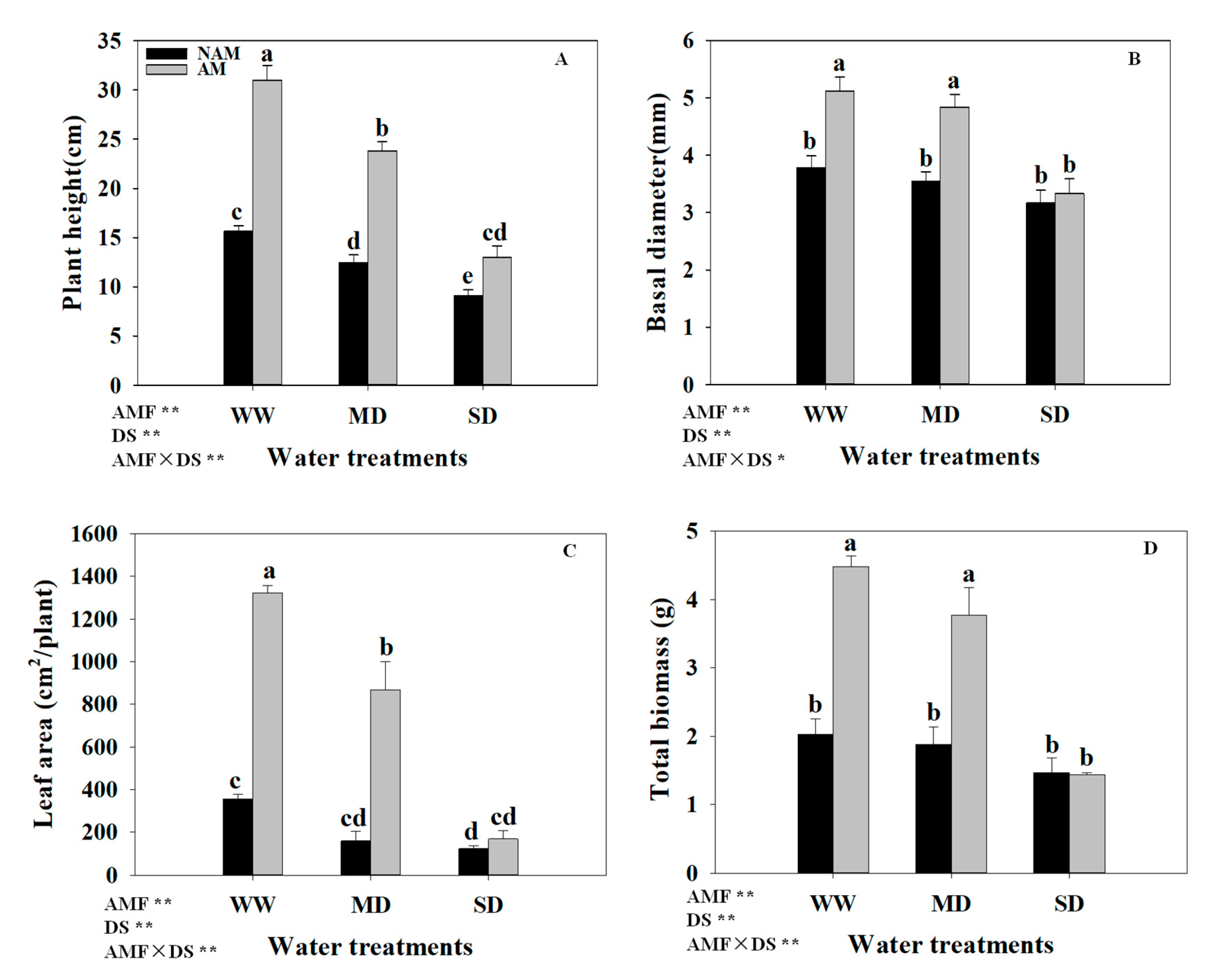
3.2. Growth Parameters
3.3. Biomass Allocation
3.4. Photosynthetic Gas Exchange Capacity
3.5. Photosynthetic Pigment Concentrations
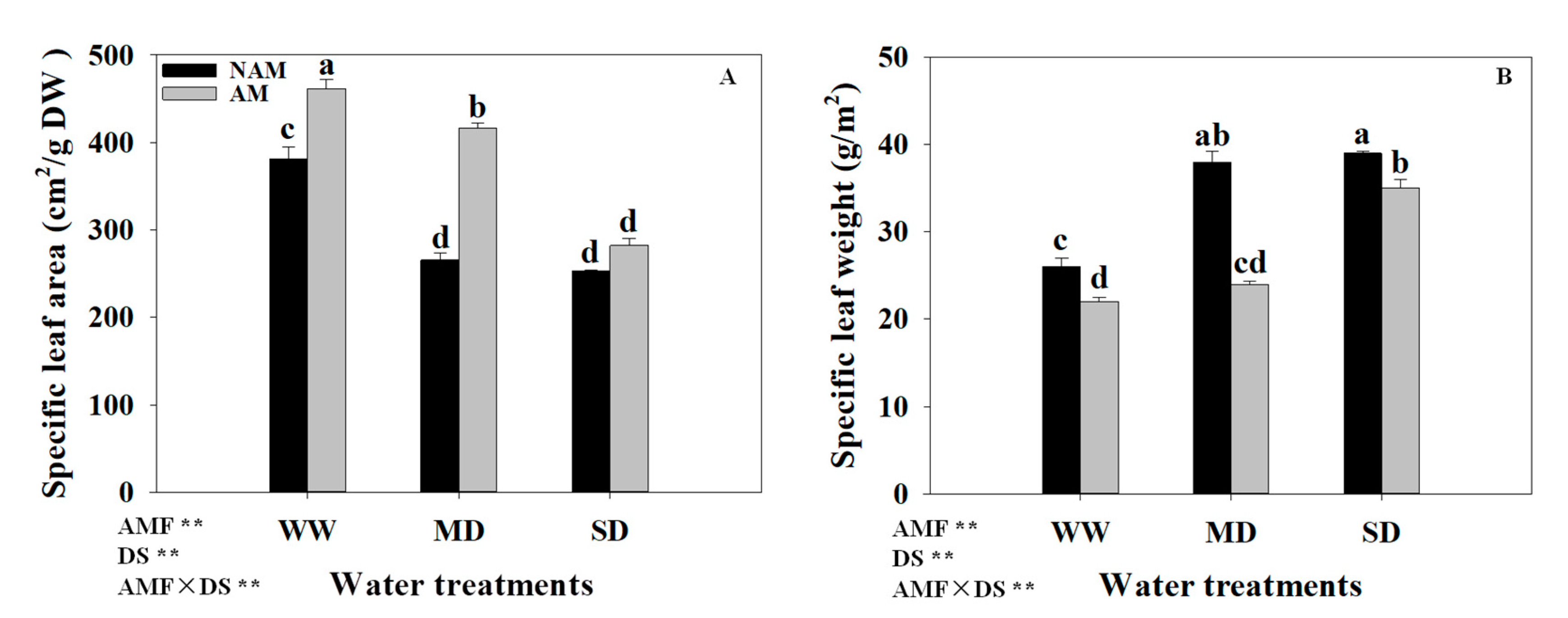
3.6. SLA and SLW
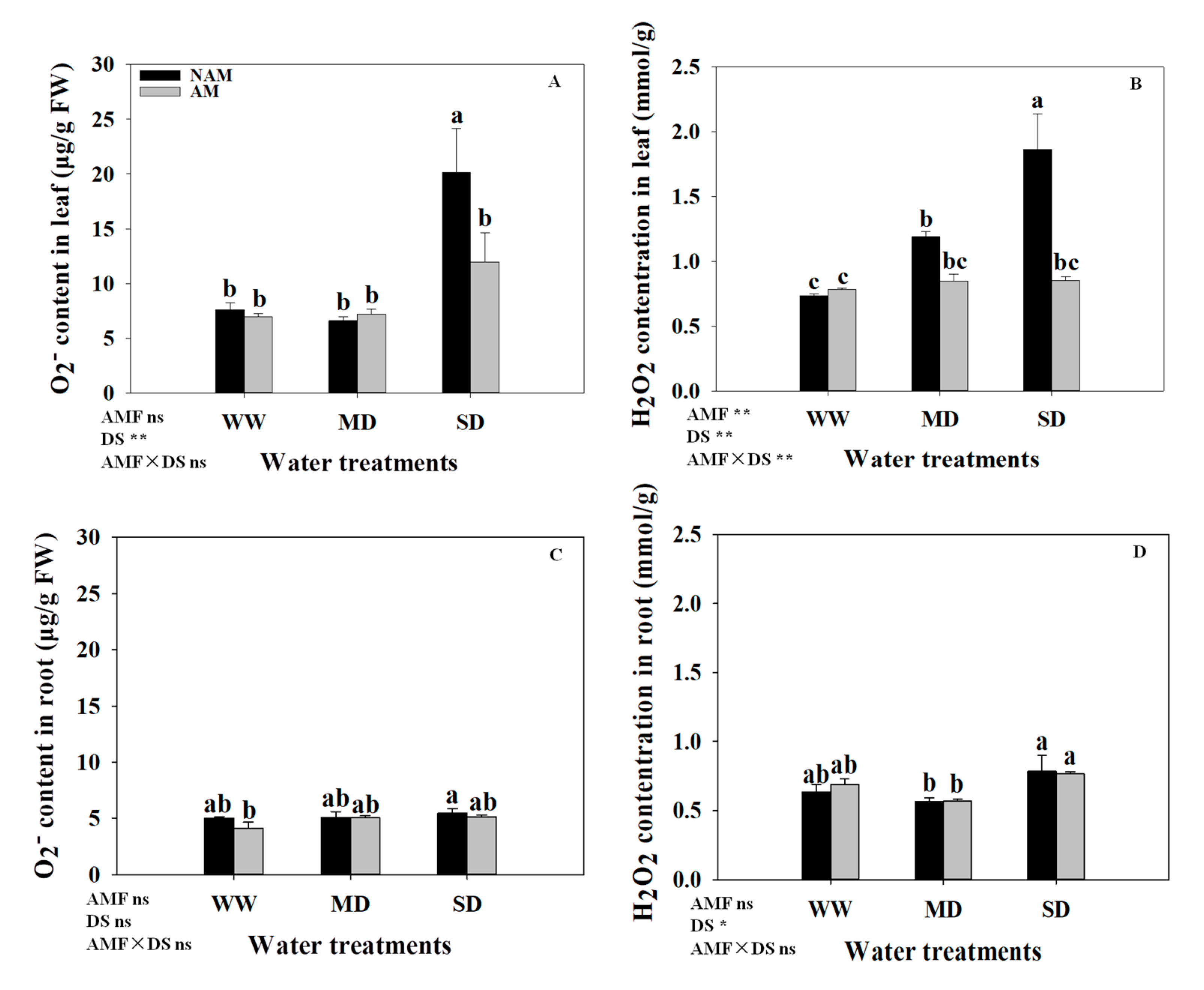
3.7. ROS (H2O2 and O2−) Contents
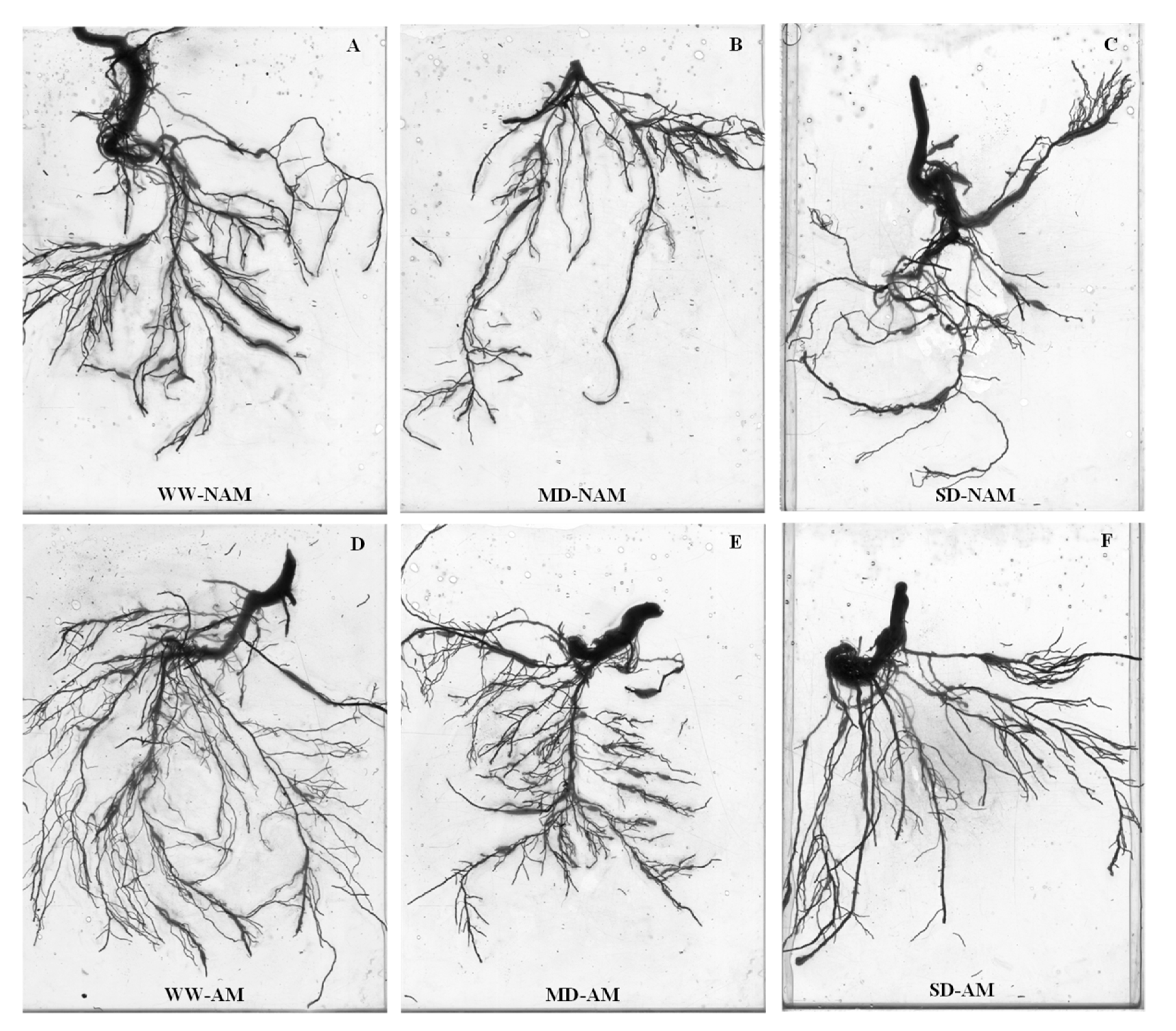
3.8. Root System Architecture
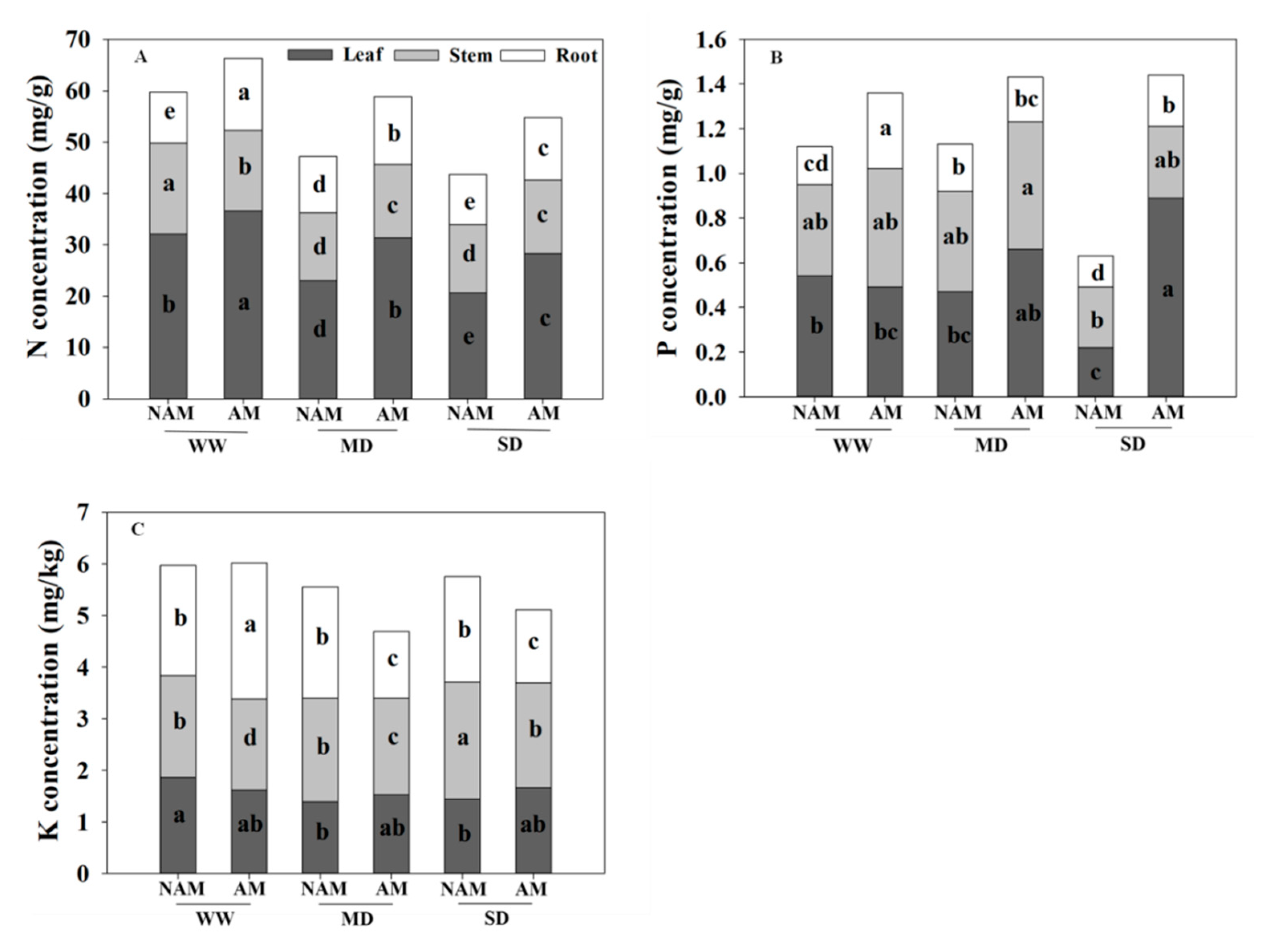
3.9. Nutrient Absorption and Distribution
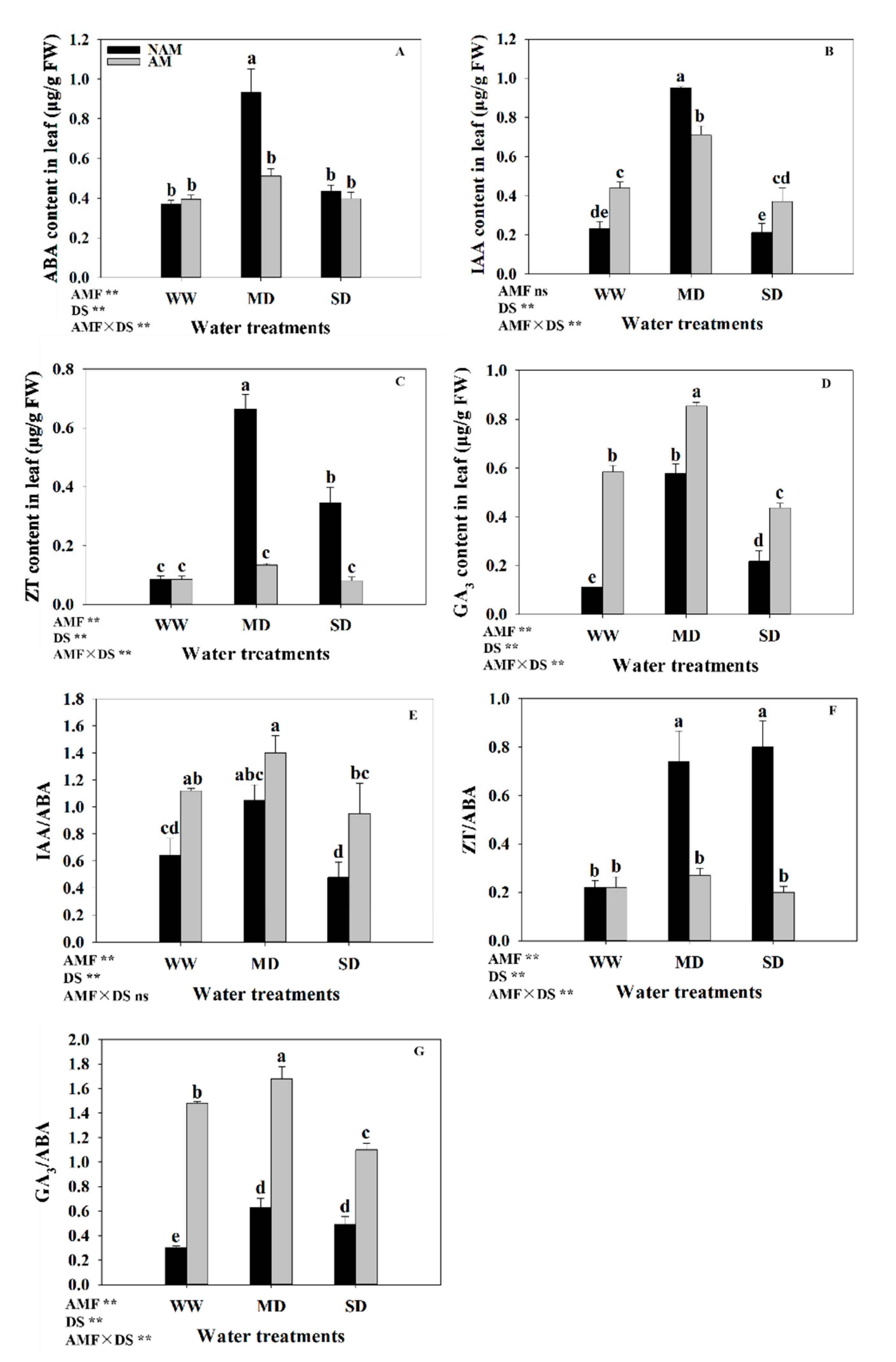
3.10. Phytohormones Levels
3.11. GRSP Content
3.12. Soil Aggregates
3.13. Correlation Between AMF Colonization and Other Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amiri, R.; Nikbakht, A.; Etemadi, N. Alleviation of drought stress on rose geranium [Pelargonium graveolens (L.) Herit.] in terms of antioxidant activity and secondary metabolites by mycorrhizal inoculation. Sci. Hortic. 2015, 197, 373–380. [Google Scholar] [CrossRef]
- Behrooz, A.; Vahdati, K.; Rejali, F.; Lotfi, M.; Sarikhani, S.; Leslie, C.A. Arbuscular mycorrhiza and plant growth-promoting bacteria alleviate drought stress in walnut. Hortscience 2019, 54, 1087–1092. [Google Scholar] [CrossRef]
- Huang, D.; Ma, M.; Wang, Q.; Zhang, M.; Jing, G.; Li, C.; Ma, F. Arbuscular mycorrhizal fungi enhanced drought resistance in apple by regulating genes in the MAPK pathway. Plant Physiol. Biochem. 2020, 149, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Ma, K.M.; Qu, L.Y. Inoculation with arbuscular mycorrhizal fungi enhances the root system of Bauhinia faberi var. microphylla seedlings under drought stress conditions. Acta Ecol. Sin. 2017, 37, 2611–2619. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, X.; Li, J.; Yang, J.; Jiang, Y.; Wang, R. Effects of arbuscular mycorrhizal fungi on the photosynthetic characteristics and fluorescence parameters of Tectona grandis seedlings under drought stress. J. Forest Environ. 2019, 39, 608–615. (In Chinese) [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Zhang, L. AMF inoculation and phosphorus supplementation alleviates drought induced growth and photosynthetic decline in Nicotiana tabacum by up-regulating antioxidant metabolism and osmolyte accumulation. Environ. Exp. Bot. 2020, 104088. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N. Arbuscular mycorrhizal fungi and tolerance of drought stress in plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Springer: Singapore, 2017; pp. 25–41. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, M.; Sulpice, R.; Chen, H.; Tian, S.; Ban, Y.H. Arbuscular mycorrhizal fungi alter fractal dimension characteristics of Robinia pseudoacacia L. seedlings through regulating plant growth, leaf water status, photosynthesis, and nutrient concentration under drought stress. J. Plant Growth Regul. 2014, 33, 612–625. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal symbiosis. Q. Rev. Biol. 2008, 3, 273–281. [Google Scholar] [CrossRef]
- Bi, Y.; Xiao, L.; Sun, J. An arbuscular mycorrhizal fungus ameliorates plant growth and hormones after moderate root damage due to simulated coal mining subsidence: A microcosm study. Environ. Sci. Pollut. Res. 2019, 26, 11053–11061. [Google Scholar] [CrossRef]
- Gupta, M.M. Arbuscular Mycorrhizal Fungi: The Potential Soil Health Indicators. In Soil Health; Springer: Cham, Switzerlan, 2020; pp. 183–195. [Google Scholar] [CrossRef]
- Hashem, A.; Kumar, A.; Al-Dbass, A.M.; Alqarawi, A.A.; Al-Arjani, A.B.F.; Singh, G.; Farooq, M.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J. Biol. Sci. 2019, 26, 614–624. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Xu, G.; Zhou, L.; Li, Y. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Zenia insignis seedlings under drought stress. New For. 2019, 50, 593–604. [Google Scholar] [CrossRef]
- MacLean, A.M.; Bravo, A.; Harrison, M.J. Plant signaling and metabolic pathways enabling arbuscular mycorrhizal symbiosis. Plant Cell 2017, 29, 2319–2335. [Google Scholar] [CrossRef]
- Santander, C.; Aroca, R.; Ruiz-Lozano, J.M.; Olave, J.; Cartes, P.; Borie, F.; Cornejo, P. Arbuscular mycorrhiza effects on plant performance under osmotic stress. Mycorrhiza 2017, 27, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Aalipour, H.; Nikbakht, A.; Etemadi, N.; Rejali, F.; Soleimani, M. Biochemical response and interactions between arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria during establishment and stimulating growth of Arizona cypress (Cupressus arizonica G.) under drought stress. Sci. Hortic. 2020, 261, 108923. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, C.L.; Chen, Y.; Chen, Z.; Jiang, Q.B.; Wu, C.; Pinyopusarerk, K. Improving drought tolerance of Casuarina equisetifolia seedlings by arbuscular mycorrhizas under glasshouse conditions. New For. 2010, 40, 261–271. [Google Scholar] [CrossRef]
- Ren, A.T.; Zhu, Y.; Chen, Y.L.; Ren, H.X.; Li, J.Y.; Abbott, L.K.; Xiong, Y.C. Arbuscular mycorrhizal fungus alters root-sourced signal (abscisic acid) for better drought acclimation in Zea mays L. seedlings. Environ. Exp. Bot. 2019, 167, 103824. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, P.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Effects of mycorrhizal fungi on root-hair growth and hormone levels of taproot and lateral roots in trifoliate orange under drought stress. Arch. Agron. Soil Sci. 2019, 65, 1316–1330. [Google Scholar] [CrossRef]
- Rydlová, J.; Püschel, D. Arbuscular mycorrhiza, but not hydrogel, alleviates drought stress of ornamental plants in peat-based substrate. Appl. Soil Ecol. 2020, 146, 103394. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Miller, R.M. Carbon cycling by arbuscular mycorrhizal fungi in soil–plant systems. Trends Plant Sci. 2003, 8, 407–409. [Google Scholar] [CrossRef]
- Al-Arjani, A.B.F.; Hashem, A.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi modulates dynamics tolerance expression to mitigate drought stress in Ephedra foliata Boiss. Saudi J. Biol. Sci. 2020, 27, 380–394. [Google Scholar] [CrossRef]
- Wang, P.; Ma, L.; Li, Y.; Wang, S.; Li, L.; Yang, R.; Ma, Y.; Wang, Q. Transcriptome profiling of indole-3-butyric acid-induced adventitious root formation in softwood cuttings of the Catalpa bungei variety ‘YU-1’ at different developmental stages. Genes Genom. 2016, 38, 145–162. [Google Scholar] [CrossRef]
- Zhang, E.; Ma, L.; Yang, R.; Li, L.; Wang, Q.; Li, Y.; Wang, P. Transcriptome profiling of IBA-induced adventitious root formation in softwood cuttings of Catalpa bungei ‘Yu-1’. Sci. Silvae Sin. 2018, 54, 48–61. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Ma, Q.; Du, K.; Li, Z.; Chen, H. Rapid propagation system of Catalpa bungei and C. fargesii Bur. F. duclouxii. J. Northeast For. Univ. 2016, 44, 51. (In Chinese) [Google Scholar] [CrossRef]
- Meng, L.; Liu, Y.; Wang, X.P.; Li, J.Y.; He, G.X.; Xue, D.M.; Xing, L.X.; Li, S.A. Comparison study on rapid reproductive capacity of four cultivars of Catalpa bungei in tissue culture. J. Cent. South Univ. For. Technol. 2020, 40, 82–88. (In Chinese) [Google Scholar] [CrossRef]
- Qiu, Q.; Li, J.; Wang, J.; He, Q.; Dong, L.; Ma, J.; Bai, J.; Wu, J. Coupling effects of water and fertilizer on the growth characteristics of Catalpa bungei seedlings. Pak. J. Bot. 2015, 47, 889–896. [Google Scholar]
- Wu, J.W.; Su, Y.; Wang, J.H.; He, Q.; Qiu, Q.; Ma, J.W.; Li, J.Y. Morphological and physiological acclimation of Catalpa bungei plantlets to different light conditions. Photosynthetica 2018, 56, 537–548. [Google Scholar] [CrossRef]
- Shi, H.; Ma, W.; Song, J.; Lu, M.; Rahman, S.U.; Bui, T.T.X.; Vu, D.D.; Zheng, H.; Wang, J.; Zhang, Y. Physiological and transcriptional responses of Catalpa bungei to drought stress under sufficient- and deficient-nitrogen conditions. Tree Physiol. 2017, 37, 1457–1468. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, X.; Ma, W.; Song, J.; Rahman, S.U.; Wang, J.; Zhang, Y. Morphological and physiological responses to cyclic drought in two contrasting genotypes of Catalpa bungei. Environ. Exp. Bot. 2017, 138, 77–87. [Google Scholar] [CrossRef]
- Wu, J.W.; He, Q.; Li, J.Y.; Wang, J.H.; Su, Y.; Wang, L.P.; Dong, J.L.; Bai, J.J. Dynamic changes of foliage growth of Catalpa bungei clones under different nitrogen exponential fertilizations. J. Beijing For. Univ. 2015, 37, 19–28. (In Chinese) [Google Scholar] [CrossRef]
- Yan, Y.H.; Zheng, J.Y.; Zhang, X.C.; Li, S.Q. Impact of biochar addition into typical soils on field capacity in Loess Plateau. J. Soil Water Conserv. 2013, 27, 120–124. (In Chinese) [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular-Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Gao, J.F. Experimental Guidance for Plant Physiology; Higher Education Press: Beijing, China, 2006; ISBN 978-7-04-019170-7. (In Chinese) [Google Scholar]
- Yang, B.; Wang, J.C.; Zhang, Y.B. Effect of long-term warming on growth and biomass allocation of Abies faxoniana seedlings. Acta Ecol. Sin. 2010, 30, 5994–6000. (In Chinese) [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000; ISBN 978-7-109-06644-1. (In Chinese) [Google Scholar]
- Chu, H.L.; Wang, C.Y.; Li, Z.M.; Wang, H.H.; Xiao, Y.G.; Chen, J.; Tang, M. The dark septate endophytes and ectomycorrhizal fungi effect on Pinus tabulaeformis carr. seedling growth and their potential effects to pine wilt disease resistance. Forests 2019, 10, 16. [Google Scholar] [CrossRef]
- Cao, X.; Jia, J.; Zhang, C.; Li, H.; Liu, T.; Jiang, X.; Polle, A.; Peng, C.; Luo, Z.B. Anatomical, physiological and transcriptional responses of two contrasting poplar genotypes to drought and re-watering. Physiol. Plant. 2014, 151, 480–494. [Google Scholar] [CrossRef]
- Tarkowski, P.; Ge, L.; Yong, J.W.H.; Tan, S.N. Analytical methods for cytokinins. Trends Anal. Chem. 2009, 28, 323–335. [Google Scholar] [CrossRef]
- Bedini, S.; Pellegrino, E.; Avio, L.; Pellegrini, S.; Bazzoffi, P.; Argese, E.; Giovannetti, M. Changes in soil aggregation and glomalin-related soil protein content as affected by the arbuscular mycorrhizal fungal species Glomus mosseae and Glomus intraradices. Soil Biol. Biochem. 2009, 41, 1491–1496. [Google Scholar] [CrossRef]
- Bieganowski, A.; Ryzak, M.; Witkowska-Walczak, B. Determination of soil aggregate disintegration dynamics using laser diffraction. Clay Min. 2010, 45, 23–34. [Google Scholar] [CrossRef]
- Song, F.M.; Liu, J.H.; Liu, D.B.; Shi, Z.J.; Wang, W.M. Effects of 3 kinds of arbuscular mycorrhizal fungi on the growth and drought resistance of Zenia insignis. J. Southwest Forest. Univ. (Nat. Sci.) 2018, 38, 97–105. (In Chinese) [Google Scholar] [CrossRef]
- Bi, Y.; Zhang, Y.; Jiang, B.; Qiu, L. Effects of AM fungi and phosphate-solubilizing bacteria inoculation on maize growth and soil fertility under water stress. J. China Coal Soc. 2019, 44, 3655–3661. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Huang, Y. Effects of arbuscular mycorrhizal fungi on the drought tolerance of Cyclobalanopsis glauca seedlings under greenhouse conditions. New For. 2014, 45, 545–556. [Google Scholar] [CrossRef]
- He, F.; Sheng, M.; Tang, M. Effects of Rhizophagus irregularis on photosynthesis and antioxidative enzymatic system in Robinia pseudoacacia L. under drought stress. Front. Plant Sci. 2017, 183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Arbuscular mycorrhizas modulate root polyamine metabolism to enhance drought tolerance of trifoliate orange. Environ. Exp. Bot. 2020, 171, 103926. [Google Scholar] [CrossRef]
- Chen, J.; Xie, J.; Tang, M. Effects of arbuscular mycorrhizal fungi on the growth and drought resistance of Amorpha fruticosa under water stress. J. Beijing Forest. Univ. 2014, 36, 142–148. (In Chinese) [Google Scholar] [CrossRef]
- Liu, T.; Sheng, M.; Wang, C.Y.; Chen, H.; Li, Z.; Tang, M. Impact of arbuscular mycorrhizal fungi on the growth, water status, and photosynthesis of hybrid poplar under drought stress and recovery. Photosynthetica 2015, 53, 250–258. [Google Scholar] [CrossRef]
- Ji, L.; Tan, W.; Chen, X. Arbuscular mycorrhizal mycelial networks and glomalin-related soil protein increase soil aggregation in Calcaric Regosol under well-watered and drought stress conditions. Soil Tillage Res. 2019, 185, 1–8. [Google Scholar] [CrossRef]
- Golubkina, N.; Logvinenko, L.; Novitsky, M.; Zamana, S.; Sokolov, S.; Molchanova, A.; Shevchuk, O.; Sekara, A.; Tallarita, A.; Caruso, G. Yield, essential oil and quality performances of Artemisia dracunculus, Hyssopus officinalis and Lavandula angustifolia as affected by arbuscular mycorrhizal fungi under organic management. Plants 2020, 9, 375. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, Y.S.; Fu, W.; Wu, Z.X.; Xie, W.; Zhang, X.; Hao, Z.P.; Chen, B.D. Effects of arbuscular mycorrhizal fungi Rhizophagus irregularis and super absorbent polymers on growth and drought tolerance of Medicago sativa. Mycosystema 2019, 11, 1976–1991. (In Chinese) [Google Scholar] [CrossRef]
- Gong, M.; Tang, M.; Chen, H.; Zhang, Q.; Feng, X. Effects of two Glomus species on the growth and physiological performance of Sophora davidii seedlings under water stress. New For. 2013, 44, 399–408. [Google Scholar] [CrossRef]
- Fagbola, O.; Osonubi, O.; Mulongoy, K.; Odunfa, S.A. Effects of drought stress and arbuscular mycorrhiza on the growth of Gliricidia sepium (Jacq). Walp, and Leucaena leucocephala (Lam.) de Wit. in simulated eroded soil conditions. Mycorrhiza 2001, 11, 215–223. [Google Scholar] [CrossRef]
- Stevens, K.J.; Wall, C.B.; Janssen, J.A. Effects of arbuscular mycorrhizal fungi on seedling growth and development of two wetland plants, Bidens frondosa L., and Eclipta prostrata (L.) L., grown under three levels of water availability. Mycorrhiza 2011, 21, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.C.; Copeland, P.J.; Crookston, R.K.; Pfleger, F.L. Mycorrhizae: Possible explanation for yield decline with continuous corn and soybean. Agron. J. 1992, 84, 387–390. [Google Scholar] [CrossRef]
- Schmidt, C.S.; Mrnka, L.; Frantík, T.; Motyka, V.; Dobrev, P.I.; Vosátka, M. Combined effects of fungal inoculants and the cytokinin-like growth regulator thidiazuron on growth, phytohormone contents and endophytic root fungi in Miscanthus × giganteus. Plant Physiol. Biochem. 2017, 120, 120–131. [Google Scholar] [CrossRef]
- Fakhech, A.; Manaut, N.; Ouahmane, L.; Hafidi, M. Contributions of indigenous arbuscular mycorrhizal fungi to growth of Retama monosperma and Acacia gummifera under water stress (case study: Essaouira sand dunes forest). J. Sustain. For. 2019, 38, 686–696. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Ma, K.M.; Qu, L.Y. Arbuscular mycorrhizal fungi (AMF) promotes Bauhinia faberi var. microphylla seedling growth under drought stress conditions. Acta Ecol. Sin. 2016, 36, 3329–3337. (In Chinese) [Google Scholar] [CrossRef]
- Song, H.X.; Zhong, Z.C. Effects of Glomus etunicatum inoculation on root morphology of Broussonetia papyrifera seedlings under water stress. For. Res. 2007, 20, 79–83. (In Chinese) [Google Scholar] [CrossRef]
- Pankoke, H.; Höpfner, I.; Matuszak, A.; Beyschlag, W.; Müller, C. The effects of mineral nitrogen limitation, competition, arbuscular mycorrhiza, and their respective interactions, on morphological and chemical plant traits of Plantago lanceolata. Phytochemistry 2015, 118, 149–161. [Google Scholar] [CrossRef]
- Yang, Y.H.; Li, W.H.; Chen, Y.N.; Zhu, C.G.; Ma, J.X. Response of Populus euphratica seedling inoculated Glomus mosseae to progressive soil water deficit. Arid Land Geogr. 2015, 38, 60–66. (In Chinese) [Google Scholar] [CrossRef]
- Ouledali, S.; Ennajeh, M.; Zrig, A.; Gianinazzi, S.; Khemira, H. Estimating the contribution of arbuscular mycorrhizal fungi to drought tolerance of potted olive trees (Olea europaea). Acta Physiol. Plant. 2018, 40, 81. [Google Scholar] [CrossRef]
- Barros, V.; Frosi, G.; Santos, M.; Ramos, D.G.; Falcão, H.M.; Santos, M.G. Arbuscular mycorrhizal fungi improve photosynthetic energy use efficiency and decrease foliar construction cost under recurrent water deficit in woody evergreen species. Plant Physiol. Biochem. 2018, 127, 469–477. [Google Scholar] [CrossRef]
- Essahibi, A.; Benhiba, L.; Babram, M.A.; Ghoulam, C.; Qaddoury, A. Influence of arbuscular mycorrhizal fungi on the functional mechanisms associated with drought tolerance in carob (Ceratonia siliqua L.). Trees 2018, 32, 87–97. [Google Scholar] [CrossRef]
- Wang, W.X.; Zhang, F.; Chen, Z.L.; Liu, J.; Guo, C.; He, J.D.; Zou, Y.N.; Wu, Q.S. Responses of phytohormones and gas exchange to mycorrhizal colonization in trifoliate orange subjected to drought stress. Arch. Agron. Soil Sci. 2017, 63, 14–23. [Google Scholar] [CrossRef]
- Jördens, C.; Scheller, M.; Breitenstein, B.; Selmar, D.; Koch, M. Evaluation of leaf water status by means of permittivity at terahertz frequencies. J. Biol. Phys. 2009, 35, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.S.; Xia, R.X. Effects of arbuscular mycorrhizal fungi on leaf solutes and root absorption areas of trifoliate orange seedlings under water stress conditions. Front. For. China 2006, 1, 312–317. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiong, J.L.; Lü, G.C.; Asfa, B.; Wang, Z.B.; Li, P.F.; Xiong, Y.C. Effects of arbuscular mycorrhizal fungi and plant symbiosis on plant water relation and its mechanism. Acta Ecol. Sin. 2015, 35, 2419–2427. (In Chinese) [Google Scholar] [CrossRef]
- Doubková, P.; Vlasáková, E.; Sudová, R. Arbuscular mycorrhizal symbiosis alleviates drought stress imposed on Knautia arvensis plants in serpentine soil. Plant Soil 2013, 370, 149–161. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Y.; Zhang, K.; Tian, C.; Guo, J. Arbuscular mycorrhizal fungi improve plant growth of Ricinus communis by altering photosynthetic properties and increasing pigments under drought and salt stress. Ind. Crop. Prod. 2018, 117, 13–19. [Google Scholar] [CrossRef]
- Manoharan, P.T.; Shanmugaiah, V.; Balasubramanian, N.; Gomathinayagam, S.; Sharma, M.P.; Muthuchelian, K. Influence of AM fungi on the growth and physiological status of Erythrina variegata Linn. grown under different water stress conditions. Eur. J. Soil Biol. 2010, 46, 151–156. [Google Scholar] [CrossRef]
- Huang, D.; Sang, W.G.; Zhu, L.; Song, Y.Y.; Wang, J.P. Effects of nitrogen and carbon addition and arbuscular mycorrhiza on alien invasive plant Ambrosia artemisiifolia. Chin. J. Appl. Ecol. 2010, 21, 3056–3062. (In Chinese) [Google Scholar]
- Zhang, B.; Zhang, H.; Wang, H.; Wang, P.; Wu, Y.; Wang, M. Effect of phosphorus additions and arbuscular mycorrhizal fungal inoculation on the growth, physiology, and phosphorus uptake of wheat under two water regimes. Commun. Soil Sci. Plant Anal. 2018, 49, 862–874. [Google Scholar] [CrossRef]
- Tian, Y.H.; Lei, Y.B.; Zheng, Y.L.; Cai, Z.Q. Synergistic effect of colonization with arbuscular mycorrhizal fungi improves growth and drought tolerance of Plukenetia volubilis seedlings. Acta Physiol. Plant. 2013, 35, 687–696. [Google Scholar] [CrossRef]
- Hernández-Ortega, H.A.; Alarcón, A.; Ferrera-Cerrato, R.; Zavaleta-Mancera, H.A.; López-Delgado, H.A.; Mendoza-López, M.R. Arbuscular mycorrhizal fungi on growth, nutrient status, and total antioxidant activity of Melilotus albus during phytoremediation of a diesel-contaminated substrate. J. Environ. Manag. 2012, 95, S319–S324. [Google Scholar] [CrossRef]
- Busquets, M.; Calvet, C.; Camprubí, A.; Estaún, V. Differential effects of two species of arbuscular mycorrhiza on the growth and water relations of Spartium junceum and Anthyllis cytisoides. Symbiosis 2010, 52, 95–101. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; Xia, R.X. Effects of arbuscular mycorrhizal fungi on reactive oxygen metabolism of Citrus tangerine leaves under water stress. Chin. J. Appl. Ecol. 2007, 18, 825–830. (In Chinese) [Google Scholar]
- Gong, M.; You, X.; Zhang, Q. Effects of Glomus intraradices on the growth and reactive oxygen metabolism of foxtail millet under drought. Ann. Microbiol. 2015, 65, 595–602. [Google Scholar] [CrossRef]
- Benhiba, L.; Fouad, M.O.; Essahibi, A.; Ghoulam, C.; Qaddoury, A. Arbuscular mycorrhizal symbiosis enhanced growth and antioxidant metabolism in date palm subjected to long-term drought. Trees 2015, 29, 1725–1733. [Google Scholar] [CrossRef]
- Berta, G.; Trotta, A.; Fusconi, A.; Hooker, J.E.; Munro, M.; Atkinson, D.; Giovannetti, M.; Morini, S.; Fortuna, P.; Tisserant, B.; et al. Arbuscular mycorrhizal induced changes to plant growth and root system morphology in Prunus cerasifera. Tree Physiol. 1995, 15, 281–293. [Google Scholar] [CrossRef]
- Zou, Y.N.; Wang, P.; Liu, C.Y.; Ni, Q.D.; Zhang, D.J.; Wu, Q.S. Mycorrhizal trifoliate orange has greater root adaptation of morphology and phytohormones in response to drought stress. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Bi, Y.; Zhang, Y.; Zou, H. Plant growth and their root development after inoculation of arbuscular mycorrhizal fungi in coal mine subsided areas. Int. J. Coal Sci. Technol. 2018, 5, 47–53. [Google Scholar] [CrossRef]
- Abdelmalik, A.M.; Alsharani, T.S.; Al-Qarawi, A.A.; Ahmed, A.I.; Aref, I.M. Response of growth and drought tolerance of Acacia seyal Del. seedlings to arbuscular mycorrhizal fungi. Plant Soil Environ. 2020, 66, 264–271. [Google Scholar] [CrossRef]
- Gamalero, E.; Trotta, A.; Massa, N.; Copetta, A.; Martinotti, M.G.; Berta, G. Impact of two fluorescent pseudomonads and an arbuscular mycorrhizal fungus on tomato plant growth, root architecture and P acquisition. Mycorrhiza 2004, 14, 185–192. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Zhang, J.C.; Huang, Y.Q.; Guo, X.P.; Yang, H.; Deng, Y. Effects of water stress and mycorrhizal fungi on root morphology of Cyclobalanopsis glauca seedlings. Chin. J. Ecol. 2015, 34, 1198–1204. (In Chinese) [Google Scholar] [CrossRef]
- Shao, Y.D.; Zhang, D.J.; Hu, X.C.; Wu, Q.S.; Jiang, C.J.; Xia, T.J.; Gao, X.B.; Kuča, K. Mycorrhiza-induced changes in root growth and nutrient absorption of tea plants. Plant Soil Environ. 2018, 64, 283–289. [Google Scholar] [CrossRef]
- Chen, Y.L.; Luo, J.; Cai, K.L.; Chen, W.Y.; Mai, Z.T.; Hong, W.J. Effects of inoculation with arbuscular mycorrhizal fungi on the absorption of nutrients of Aquilaria sinensis and Dalbergia odorifera. Chin. J. Trop. Crops 2018, 39, 2355–2362. (In Chinese) [Google Scholar] [CrossRef]
- Ma, Q.L.; Wang, Y.L.; Sun, T.; Li, Y.K.; Jin, H.J.; Song, D.W.; Zhu, G.Q. Allocation, accumulation, and output characteristics of nutrient elements of Lycium barbarum grown on secondary saline land. Acta Ecol. Sin. 2017, 37, 6111–6119. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, Q.; Li, J.; Hu, Y.; Chen, J. Growth response and nutrient uptake of Eriobotrya japonica plants inoculated with three isolates of arbuscular mycorrhizal fungi under water stress condition. J. Plant Nutr. 2014, 37, 690–703. [Google Scholar] [CrossRef]
- Zarik, L.; Meddich, A.; Hijri, M.; Hafidi, M.; Ouhammou, A.; Ouahmane, L.; Duponnois, R.; Boumezzough, A. Use of arbuscular mycorrhizal fungi to improve the drought tolerance of Cupressus atlantica G. C. R. Biol. 2016, 339, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Shaul-Keinan, O.; Gadkar, V.; Ginzberg, I.; Grünzweig, J.M.; Chet, I.; Elad, Y.; Wininger, S.; Belausov, E.; Eshed, Y.; Atzmon, N.; et al. Hormone concentrations in tobacco roots change during arbuscular mycorrhizal colonization with Glomus intraradices. New Phytol. 2002, 154, 501–507. [Google Scholar] [CrossRef]
- Miransari, M.; Abrishamchi, A.; Khoshbakht, K.; Niknam, V. Plant hormones as signals in arbuscular mycorrhizal symbiosis. Crit. Rev. Biotechnol. 2014, 34, 123–133. [Google Scholar] [CrossRef]
- Ouledali, S.; Ennajeh, M.; Ferrandino, A.; Khemira, H.; Schubert, A.; Secchi, F. Influence of arbuscular mycorrhizal fungi inoculation on the control of stomata functioning by abscisic acid (ABA) in drought-stressed olive plants. S. Afr. J. Bot. 2019, 121, 152–158. [Google Scholar] [CrossRef]
- He, L.; Li, C.; Liu, R. Indirect interactions between arbuscular mycorrhizal fungi and Spodoptera exigua alter photosynthesis and plant endogenous hormones. Mycorrhiza 2017, 27, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, Q.; Li, J.; Wang, Y.; Liu, X.; Hu, Y.; Chen, J. Contributions of an arbuscular mycorrhizal fungus to growth and physiology of loquat (Eriobotrya japonica) plants subjected to drought stress. Mycol. Prog. 2015, 14, 84. [Google Scholar] [CrossRef]
- Estrada-Luna, A.A.; Davies, F.T., Jr. Arbuscular mycorrhizal fungi influence water relations, gas exchange, abscisic acid and growth of micropropagated chile ancho pepper (Capsicum annuum) plantlets during acclimatization and post-acclimatization. J. Plant Physiol. 2003, 160, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Bompadre, M.J.; Bidondo, L.F.; Silvani, V.A.; Colombo, R.P.; Pérgola, M.; Pardo, A.G.; Godeas, A.M. Combined effects of arbuscular mycorrhizal fungi and exogenous cytokinins on pomegranate (Punica granatum) under two contrasting water availability conditions. Symbiosis 2015, 65, 55–63. [Google Scholar] [CrossRef]
- Liu, C.Y.; Zhang, F.; Zhang, D.J.; Srivastava, A.K.; Wu, Q.S.; Zou, Y.N. Mycorrhiza stimulates root-hair growth and IAA synthesis and transport in trifoliate orange under drought stress. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Huang, G.T.; Ma, S.L.; Bai, L.P.; Zhang, L.; Ma, H.; Jia, P.; Liu, J.; Zhong, M.; Guo, Z.F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012, 39, 969–987. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef]
- Ye, J.S.; Li, T.; Hu, Y.J.; Hao, Z.P.; Gao, Y.Z.; Wang, Y.S.; Chen, B.D. Influences of AM fungi on plant growth and water-stable soil aggregates under drought stresses. Acta Ecol. Sin. 2013, 33, 1080–1090. (In Chinese) [Google Scholar] [CrossRef][Green Version]
- Wu, Q.S. Study on the Effect and Mechanism of Arbuscular Mycorrhizal Fungi in Drought Resistence of Citrus. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2006. (In Chinese). [Google Scholar]
- Zhang, H.; Liu, Z.; Chen, H.; Tang, M. Symbiosis of arbuscular mycorrhizal fungi and Robinia pseudoacacia L. improves root tensile strength and soil aggregate stability. PLoS ONE 2016, 11, e0153378. [Google Scholar] [CrossRef]
- Wu, Q.S.; Xia, R.X.; Zou, Y.N. Improved soil structure and citrus growth after inoculation with three arbuscular mycorrhizal fungi under drought stress. Eur. J. Soil Biol. 2008, 44, 122–128. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
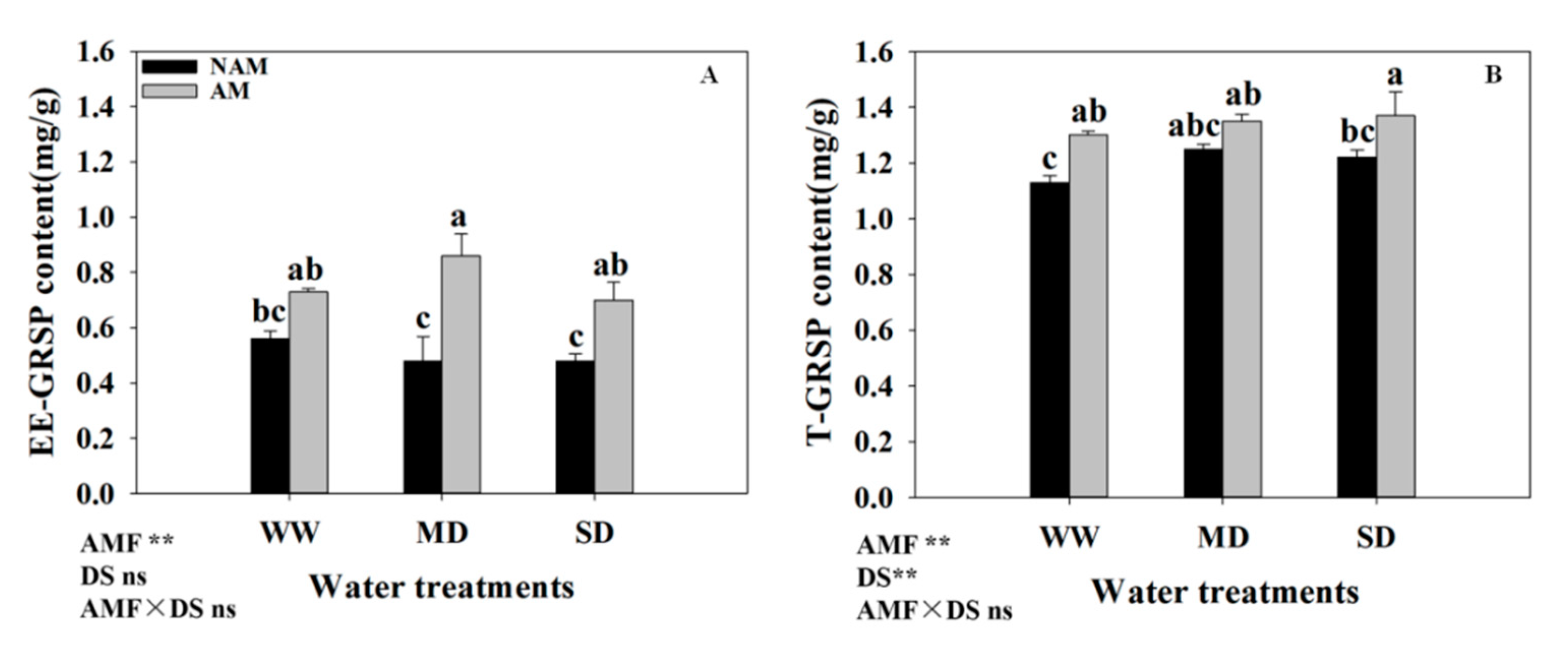
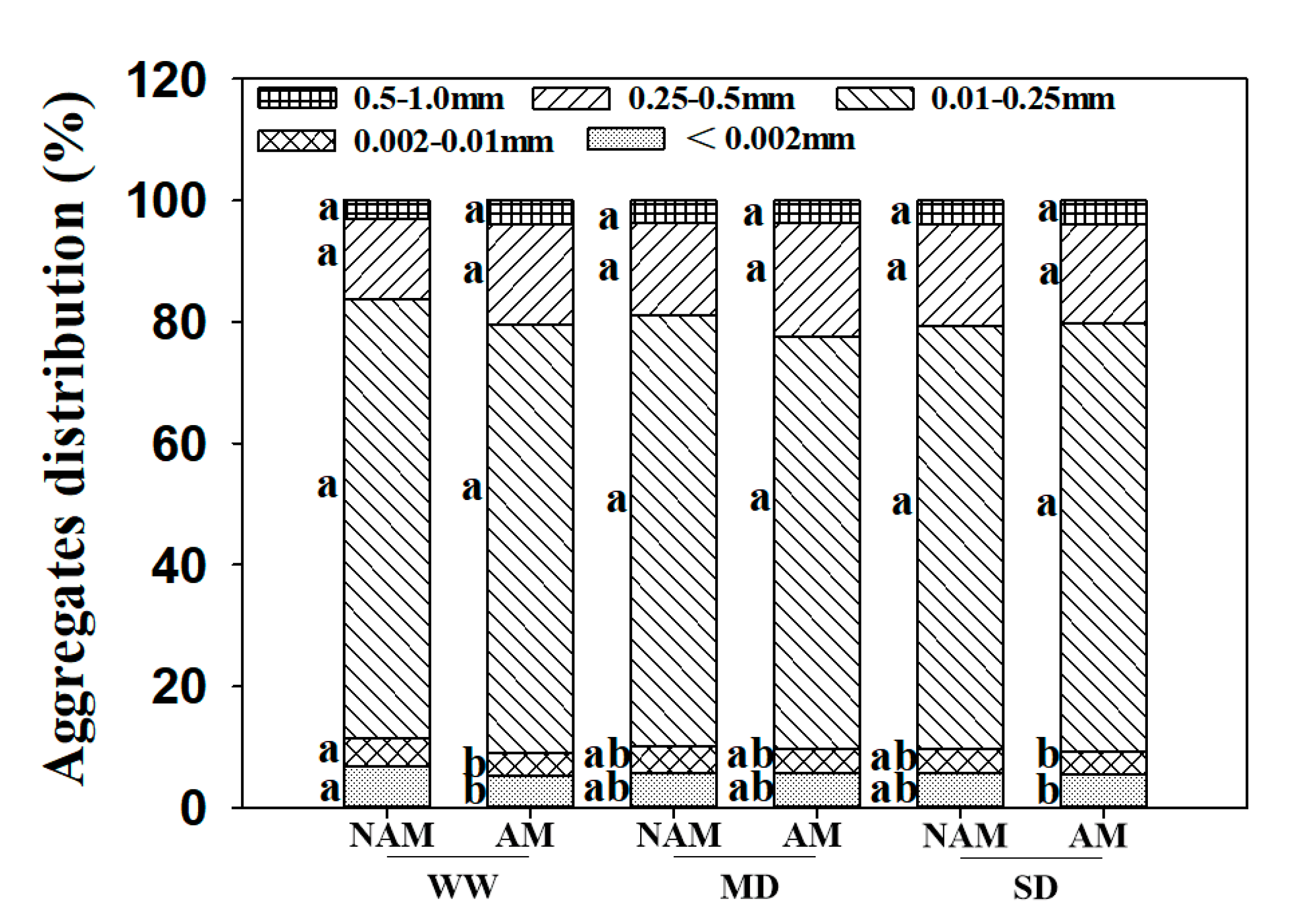
| Water Status | AMF Status | Leaf Biomass(g) | Stem Biomass(g) | Root Biomass(g) | Leaf Mass Ratio | Stem Mass Ratio | Root Mass Ratio | Root/Shoot Ratio |
|---|---|---|---|---|---|---|---|---|
| WW | NAM | 0.94 ± 0.07 c | 0.36 ± 0.02 b | 0.72 ± 0.15 a | 0.47 ± 0.03 abc | 0.18 ± 0.01 a | 0.35 ± 0.04 ab | 0.55 ± 0.10 bc |
| AM | 2.87 ± 0.06 a | 0.99 ± 0.25 a | 0.62 ± 0.15 a | 0.64 ± 0.01 a | 0.22 ± 0.05 a | 0.14 ± 0.04 c | 0.17 ± 0.05 c | |
| MD | NAM | 0.60 ± 0.17 c | 0.32 ± 0.02 b | 0.97 ± 0.18 a | 0.31 ± 0.06 c | 0.17 ± 0.02 a | 0.52 ± 0.06 a | 1.14 ± 0.27 a |
| AM | 2.10 ± 0.34 b | 0.85 ± 0.12 a | 0.83 ± 0.06 a | 0.55 ± 0.04 ab | 0.22 ± 0.01 a | 0.23 ± 0.04 bc | 0.31 ± 0.08 bc | |
| SD | NAM | 0.49 ± 0.06 c | 0.32 ± 0.03 b | 0.66 ± 0.18 a | 0.34 ± 0.01 c | 0.23 ± 0.05 a | 0.43 ± 0.06 a | 0.79 ± 0.20 ab |
| AM | 0.60 ± 0.15 c | 0.34 ± 0.04 b | 0.50 ± 0.12 a | 0.42 ± 0.10 bc | 0.24 ± 0.02 a | 0.34 ± 0.08 ab | 0.58 ± 0.21 bc | |
| Significance | AMF | ** | ** | ns | ** | ns | ** | ** |
| DS | ** | * | ns | * | ns | ns | ns | |
| AMF × DS | ** | * | ns | ns | ns | ns | ns |
| Water Status | AMF Status | Pn (μmol.m−2s−1) | Gs (mol·m−2·s−1) | Ci (µmol·mol−1) | Tr (mmol·m−2·s−1) | WUE (μmol/mmol) |
|---|---|---|---|---|---|---|
| WW | NAM | 6.01 ± 0.25 a | 0.11 ± 0.01 ab | 312.15 ± 5.21 a | 3.41 ± 0.24 a | 1.79 ± 0.06 a |
| AM | 6.52 ± 0.26 a | 0.12 ± 0.01 a | 241.89 ± 16.82 bc | 3.63 ± 0.29 a | 1.85 ± 0.10 a | |
| MD | NAM | 3.78 ± 0.21 b | 0.06 ± 0.01 c | 277.79 ± 24.51 ab | 2.54 ± 0.10 b | 1.50 ± 0.09 b |
| AM | 4.40 ± 0.45 b | 0.09 ± 0.01 b | 225.08 ± 15.72 c | 2.53 ± 0.23 b | 1.72 ± 0.05 a | |
| SD | NAM | 1.81 ± 0.19 c | 0.03 ± 0.00 d | 203.35 ± 12.91 c | 1.30 ± 0.13 c | 1.39 ± 0.05 b |
| AM | 2.47 ± 0.13 c | 0.04 ± 0.00 cd | 202.75 ± 9.82 c | 1.76 ± 0.11 c | 1.41 ± 0.03 b | |
| Significance | AMF | ** | ** | ** | ns | ns |
| DS | ** | ** | ** | ** | ** | |
| AMF × DS | ns | ns | ns | ns | ns |
| Water Status | AMF Status | Chlorophyll a(mg/g) | Chlorophyll b(mg/g) | Chlorophyll a/b | Carotenoid(mg/g) |
|---|---|---|---|---|---|
| WW | NAM | 1.66 ± 0.08 ab | 0.51 ± 0.03 ab | 3.28 ± 0.07 a | 0.34 ± 0.01 a |
| AM | 1.84 ± 0.07 a | 0.58 ± 0.04 a | 3.18 ± 0.10 a | 0.34 ± 0.01 a | |
| MD | NAM | 1.00 ± 0.46 bcd | 0.31 ± 0.17 abc | 3.51 ± 0.32 a | 0.21 ± 0.08 ab |
| AM | 1.41 ± 0.23 abc | 0.42 ± 0.09 ab | 3.44 ± 0.18 a | 0.30 ± 0.02 a | |
| SD | NAM | 0.52 ± 0.16 d | 0.14 ± 0.04 c | 3.75 ± 0.13 a | 0.12 ± 0.04 b |
| AM | 0.84 ± 0.00 cd | 0.24 ± 0.00 bc | 3.55 ± 0.03 a | 0.19 ± 0.06 ab | |
| Significance | AMF | ns | ns | ns | ns |
| DS | ** | ** | ns | ** | |
| AMF × DS | ns | ns | ns | ns |
| Water Status | AMF Status | Total Root Length/(cm) | Total Root Surface Area/(cm2) | Root Projected Area/(cm2) | Total Root Volume/(cm3) | Mean Root Diameter/(mm) | Root Tips | Branching Number | LF (cm) | SAF (cm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| WW | NAM | 602.81 ± 13.10 b | 179.67 ± 14.81 b | 57.98 ± 3.97 b | 603.66 ± 12.31 bc | 1.05 ± 0.09 a | 2861.67 ± 73.26 b | 3142.00 ± 177.69 b | 189.71 ± 4.43 bc | 14.65 ± 0.11 c |
| AM | 1022.21 ± 136.34 a | 234.84 ± 23.94 a | 74.74 ± 7.62 a | 1022.54 ± 136.31 a | 0.74 ± 0.04 b | 3645.33 ± 183.39 a | 4288.00 ± 397.20 a | 473.60 ± 85.65 a | 45.84 ± 8.30 a | |
| MD | NAM | 548.39 ± 39.65 b | 165.64 ± 7.74 b | 52.72 ± 2.46 b | 548.39 ± 39.65 c | 0.99 ± 0.07 a | 1844.67 ± 104.97 c | 2635.33 ± 153.78 b | 200.70 ± 25.40 bc | 17.98 ± 3.08 c |
| AM | 692.37 ± 43.32 b | 191.82 ± 3.97 b | 64.08 ± 2.36 ab | 774.15 ± 71.81 b | 0.89 ± 0.06 ab | 2882.67 ± 71.39 b | 3823.67 ± 61.43 a | 315.47 ± 49.39 b | 33.92 ± 2.77 b | |
| SD | NAM | 302.77 ± 44.66 c | 94.07 ± 11.30 c | 29.66 ± 3.53 c | 316.10 ± 47.65 d | 1.00 ± 0.03 a | 1296.33 ± 138.36 d | 970.50 ± 43.60 d | 127.02 ± 16.68 c | 12.06 ± 0.26 c |
| AM | 315.67 ± 6.44 c | 87.62 ± 7.35 c | 27.89 ± 2.34 c | 315.67 ± 6.44 d | 0.88 ± 0.06 ab | 1144.67 ± 28.98 d | 1787.00 ± 17.90 c | 137.35 ± 17.98 c | 11.79 ± 1.04 c | |
| Significance | AMF | ** | * | * | ** | ** | ** | ** | ** | ** |
| DS | ** | ** | ** | ** | ns | ** | ** | ** | ** | |
| AMF×DS | * | ns | ns | * | ns | ** | ns | * | ** |
| Variable | AMF Colonization | Variable | AMF Colonization | ||
|---|---|---|---|---|---|
| Growth parameters | Plant height | 0.906 ** | Root morphological parameters | Total root length | 0.828 ** |
| Basal diameter | 0.728 * | Surface area | 0.912 ** | ||
| Leaf area | 0.917 ** | Projected area | 0.922 ** | ||
| SLA | 0.954 ** | Root volume | 0.851 ** | ||
| SLW | −0.971 ** | Root average diameter | −0.279 | ||
| Total biomass | 0.962 ** | Branching number | 0.946 ** | ||
| Root biomass | 0.463 | Root tips | 0.946 ** | ||
| Stem biomass | 0.783 * | LF | 0.749 * | ||
| Leaf biomass | 0.922 ** | SAF | 0.848 ** | ||
| Gas exchange parameters | Pn | 0.695 * | Biomass allocation | Root/shoot ratio | −0.658 |
| Gs | 0.762 * | Nutrient absorption | Root N | 0.910 ** | |
| Ci | 0.141 | Stem N | 0.495 | ||
| Tr | 0.566 | Leaf N | 0.751 * | ||
| WUE | 0.809 ** | Root P | 0.336 | ||
| Phytohormone | IAA | 0.578 | Stem P | 0.626 | |
| GA3 | 0.747 * | Leaf P | −0.702 * | ||
| ZT | 0.432 | Root K | 0.423 | ||
| ABA | 0.386 | Stem K | −0.830 ** | ||
| Leaf K | −0.217 | ||||
| GRSP | EE-GRSP | 0.418 | Reactive oxygen species | Root H2O2 | −0.716 * |
| T-GRSP | −0.013 | Root O2− | −0.417 | ||
| Soil aggregates | <0.002 mm | 0.046 | Leaf H2O2 | −0.270 | |
| 0.002–0.01 mm | 0.099 | Leaf O2− | −0.714* | ||
| 0.01–0.25 mm | 0.132 | Photosynthetic pigments | Chlorophyll a | 0.800 ** | |
| 0.25–0.5 mm | −0.122 | Chlorophyll b | 0.757 * | ||
| 0.5–1 mm | 0.170 | Carotenoid | 0.910 ** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Meng, P.; Feng, H.; Wang, C. Effects of Arbuscular Mycorrhizal Fungi on Growth and Physiological Performance of Catalpa bungei C.A.Mey. under Drought Stress. Forests 2020, 11, 1117. https://doi.org/10.3390/f11101117
Chen W, Meng P, Feng H, Wang C. Effects of Arbuscular Mycorrhizal Fungi on Growth and Physiological Performance of Catalpa bungei C.A.Mey. under Drought Stress. Forests. 2020; 11(10):1117. https://doi.org/10.3390/f11101117
Chicago/Turabian StyleChen, Wei, Panpan Meng, Huan Feng, and Chunyan Wang. 2020. "Effects of Arbuscular Mycorrhizal Fungi on Growth and Physiological Performance of Catalpa bungei C.A.Mey. under Drought Stress" Forests 11, no. 10: 1117. https://doi.org/10.3390/f11101117
APA StyleChen, W., Meng, P., Feng, H., & Wang, C. (2020). Effects of Arbuscular Mycorrhizal Fungi on Growth and Physiological Performance of Catalpa bungei C.A.Mey. under Drought Stress. Forests, 11(10), 1117. https://doi.org/10.3390/f11101117








