Abstract
Research Highlights: Understanding of the spatial variation of root exudation on a regional scale can help understand the response of plant physiological activities to environmental changes. Background and Objectives: Although root exudation has become an important topic in belowground ecology, its relationship with root traits and environmental factors is poorly understood. Our objective was to explore how root traits and environmental factors influence root exudation. Materials and Methods: We used a multi-factorial design consisting of three tree species spanning across sites located at three latitudes to assess root exudation dynamics, which was measured using a syringe-basis incubation system. Results: The strongest and clearest effect observed in our study was a decrease in root exudation rates of Korean pine (Pinus koraiensis Sieb. et Zucc.) and larch (Larix gmelinii (Rupr.) Kuze.) at sites located in higher latitudes. Root exudation rates were positively related to mean annual temperature, mean annual precipitation, and negatively related to soil total organic carbon. Conclusions: Root exudation in coniferous species decreased at sites located in higher latitudes. Despite differences in root exudation rate among sites located at different latitudes and species with suitable variation in root morphological traits and environmental factors, we could not identify consistent influencing factors on root exudation rates.
1. Introduction
In addition to transferring carbon (C) and nutrients to the soil through the turnover of fine roots, plants also release labile carbon compounds from roots into the rhizosphere, a process referred to as root exudation [1]. Root exudates can comprise 5%–21% of assimilated carbon that trees allocate to roots [2] and consist of an array of simple molecules, such as soluble sugars, amino acids, organic acids, as well as complex polymers, such as mucilage [3,4]. Due to the magnitude and ubiquity of root exudation in forests, it is considered a key process responsible for shaping the rhizosphere environment [5]. For example, root exudation is a key mediator in plant–microbe–soil interactions and functions, thereby influencing soil organic matter (SOM) decomposition [6], nutrient cycling [7], microbial community assemblages [8], and soil enzyme activity [9]. Moreover, root exudation can explain differences in rhizosphere C cycling rates observed among different tree species [10]. For example, root exudation can stimulate heterotrophic respiration in the rhizosphere soil, which can account for 20% of total soil respiration [11,12]. However, despite the magnitude of carbon allocated to root exudation on an annual basis and its importance on ecosystem process, relatively little is known regarding the relative importance of root and environmental factors on root exudation.
The quantity of root exudation [13], as well as its composition [14], can exhibit considerable variation among species. Variation in root exudation among species may partially be explained by relative differences in the supply and distribution capacity of photosynthates that provide the basic substrate for exudates. Supply is determined by functional traits that result in higher rates of photosynthesis and productivity, such as leaf nitrogen (N) content [15,16,17]. Distribution is determined by root morphological traits that define the interface between plant and soil, such as root surface area [18], branching patterns [2,19], root tip density [20], or overall root system size [21]. Fine root orders 1–3 are considered absorbing roots and exhibit higher physiological activity than transport roots (<2 mm diameter) [22,23]. These low order absorptive roots mediate the exchange of material between the soil and plant [24,25]; thus, differences in morphological traits of absorptive roots among species or within species across different environments may be related to root exudation rates. Moreover, experiments have shown that the root exudation rate of deciduous tree species is higher than evergreen tree species, suggesting that leaf habit may strongly influence root exudation [13,26].
Soil characteristics [27] and environmental conditions [28,29,30] may exert a strong relationship with root exudation through direct effects on carbon assimilation or through indirect effects on decomposition rates that regulate the cycling and availability of nutrients. Studies aimed at assessing controls on root exudation mainly focus on environmental factors, such as temperature [31], soil nutrients [32], and pH [33]. Soil warming increased root exudation rates of Picea asperata Mast. [28] and Robinia pseudoacacia L. [31]. Exudation may respond to nutrient deficiency; however, past studies have found inconsistent results [34,35]. Similarly, root exudation may respond to soil pH, but previous studies have observed both increases [33] and decreases [36] with increasing soil pH, suggesting that pH alone does not control exudation, but may interact with other factors.
Although some experiments investigated the chemical composition, the interaction between exudates and microorganisms, and the influencing factors of root exudates [3,37,38], due to the influence of growth cycle and collection methods, previous studies on root exudates mainly focused on plants with short growth cycles [31,39], and few studies have been conducted on forest root exudates [23,40], especially those of mature forest trees in field. Moreover, most of these studies focus on the root exudates of one or more tree species at one site [23,38], there are few studies on the variations of root exudates on a regional scale and the relationship between root exudates and environmental factors.
Here, we explore fundamental questions about the influencing factors on the lateral three-order root exudation using three co-occurring tree species representing two different leaf habits (evergreen vs. deciduous) spanning across a latitudinal gradient consisting of three distinct sites, which represent a 6.23 ℃ range in mean annual temperature (MAT). We hypothesized that temperature differences associated with latitude would exert the strongest effect on root exudation, and that exudation would decrease with increasing latitude if the process is strongly related to temperature. We hypothesized that the two deciduous species would exhibit the largest root exudation relative to the other species because of its leaf habit.
2. Materials and Methods
2.1. Site Description and Study Species
This study was conducted in monoculture forest plantations of three tree species (Korean pine (Pinus koraiensis Sieb. et Zucc.), larch (Larix gmelinii (Rupr.) Kuze.), and white birch (Betula platyphylla Suk.)) at three sites in Northeast China: Caohekou (CHK) in Liaoning Province, Lushuihe (LSH) in Jilin Province, and Liangshui (LS) Forest Research Station of the Northeast Forestry University in Heilongjiang Province (Table 1). These forests span across temperate and cold temperate zones. This region is characterized by a cold, continental monsoon climate. The winter is cold and dry, and the summer is hot and humid. Across the latitudinal gradient, mean annual temperature (MAT) ranges from 5.92 to −0.31 ℃ and mean annual precipitation (MAP), which is concentrated in the summer, ranges from 835.89 to 588 mm (Table 1). From low to high latitude, MAT and the growing season length gradually decreases.

Table 1.
Geographic coordinates, mean annual temperature (MAT), and mean annual precipitation (MAP) at three sites in Northeast China.
The study species consist of the three common tree species in this region and include one evergreen conifer (Korean pine), one deciduous conifer (larch), and one deciduous broadleaf (white birch) species. Although plantations differed in age, all sampled trees were mature (Table 2).

Table 2.
Stand characteristics of three plantations in three sites.
2.2. Root Exudates Collection and Fine Root Sampling
Within each experiment site, we established three 10 m × 10 m replicate plots of each species with a 15 m buffer between plots. Within each replicate plot, we collected root exudates from three individual trees with similar diameter and height. Root exudates were collected in May, June, August, September, and October of 2018, using a modified non-soil syringe system developed specifically for root exudate collection in the field [41].
Fine roots of the three smallest orders (diameter <2 mm) that remained attached to the target trees were carefully excavated from the topsoil (0–10 cm). Fine roots were traced back to a parent tree or coarse root with characteristics unique to target trees. We washed intact fine roots with purified water from a squirt bottle to remove soil particles and other possible contaminants. Intact fine roots were carefully placed into a 30 mL glass syringe, filled with glass beads (c. 1 mm diameter) and sealed with a modified rubber septum (had a small slit cut to accommodate the protruding root, c. 2 mm). A small volume of C-free nutrient solution (0.1 mM KH2PO4, 0.2 mM K2SO4, 0.3 mM CaCl2, 0.2 mM MgSO4, and 0.5 mM NH4NO) [41] was added to the syringes to maintain humid conditions. Syringes were covered with aluminum foil, returned to the excavated area, and reburied in soil with leaf litter placed on top. We also collected a control sample (i.e., treated the same as exudate samples, but did not include a root) in each plot to adjust exudate values for possible contamination.
After a 48 h equilibration period, a fresh nutrient solution was flushed three times with a manual vacuum pump through each syringe to remove soluble C. After the third flush, an additional 25 mL of nutrient solution was added [13], and the syringe was re-buried as before. After 24 h, solutions containing exudates were collected from syringes with a manual vacuum pump, and the collected volumes were measured. Samples were filtered immediately through sterile 0.22 μm syringe filters within 2–5 h of collection, stored in a –20 °C freezer for further analysis. We avoided sampling on rainy days to protect systems from contamination.
Fine roots were removed from syringes, severed from the tree, and transported to the laboratory to determine specific root length (SRL), specific root surface area (SRA), and specific root tips (number of root tips per mass, SRT). Fine roots were photographed and analyzed for morphological traits using an Expression 10000XL 1.0 scanner (Epson Telford Ltd., Telford, UK) by WinRHIZO (Pro2004b) software (Regent Instruments Company, Quebec, Canada). After imaging, fine roots were oven−dried at 65 ℃ for 48 h and weighed to the nearest 0.1 mg. Filtered exudates solutions were analyzed for non-purgeable C on a TOC/TN analyzer (Multi N/C 2100s, Analytic Jena, Jena, Germany). The root exudation rate was calculated by dividing the total amount of organic C in the solution by the residence time and root area.
2.3. Soil Sampling and Laboratory Analyses
Three soil cores were sampled randomly in the 0−10 cm of the soil from each plot using a 5 cm diameter stainless steel core. Soil cores were collected during exudation incubations. Three replicate soil cores from the same plot were mixed and visible root and plant residues were removed. Soils were transported to the laboratory on ice, air-dried, sieved to pass through 2 mm mesh, and analyzed to determine total organic carbon (TOC), total nitrogen (TN), total phosphorus (TP), and soil pH. Soil TOC and TN were determined by a C/N analyzer (Multi N/C 2100s; Analytic Jena, Jena, Germany), whereas TP was determined by ammonium molybdate colorimetric analysis after H2SO4−H2O2 digestion [42]. Soil pH was measured using pH meter (PHS-3C, Scientific Instruments Co., Ltd., Shanghai, China) (soil: water = 1: 2.5).
2.4. Statistical Analyses
Response variables were averaged within-plot measurements (n = 3 replicate plots per site × species combination) and the plot was treated as the experimental unit. ANOVA was used to test for main effects of, and interactions between site (n = 3) and species (n = 3) on root exudation rate, root morphological traits (SRL, SRA, and SRT), and soil characteristics (TOC, TN, TP, and pH). Person correlation analysis was used to explore relationships between SRL, SRT, and environmental factors (soil characteristics, MAT, and MAP) and root exudation rates. Prior to statistical analyses, data were tested for ANOVA assumptions and transformed whenever necessary. The statistical tests were performed with R 3.6.1 [43], using α = < 0.05 for ANOVA.
3. Results
3.1. Variation of Root Exudation Rate Among Sites and Species
Root exudation rates ranged from 2.88 to 6.85 mg C m−2 h−1 across sites and species included in our study. There is an effect of species, site, and their interaction on root exudation rates (i.e., S× TS interaction; p = 0.002, Table 3). Regardless of species, root exudation rates of two coniferous species Korean pine and larch generally decreased with increasing latitude. The exudation rate of white birch was highest at LSH site, but there was no obvious trend that could be related to site location across the latitudinal gradient (Figure 1). Root exudation rates differed among species at sites located at the lowest latitudes (CHK, LSH; Figure 1), with larch exhibiting lower root exudation rates than Korean pine at CHK and white birch at LSH.

Table 3.
Results of ANOVA showing the P values for responses of root exudation rate, specific root length (SRL), area (SRA), and tips (SRT), soil total organic carbon (TOC), total nitrogen (TN), total phosphorus (TP), and pH to site (S) and tree species (TS). p values < 0.05 are bold.
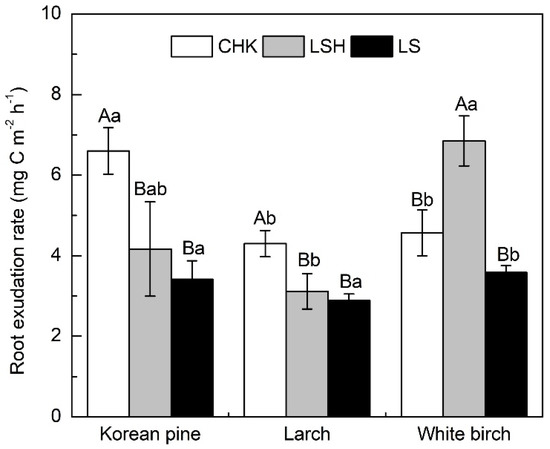
Figure 1.
Root exudation rates of Korean pine, larch, and white birch changes across sites and tree species in Northeast China. Error bars ± SE (n = 6). Different capital letters indicate significant differences (p < 0.05) within a site. Different lowercase letters indicate significant differences (p < 0.05) among within a species.
3.2. Root Morphological Traits
Specific root length (SRL) ranged from 9.78 to 32.19m g−1, specific root area (SRA) ranged from 184.87 to 385.4 cm2 g−1, and specific root tip (SRT) ranged from 5.15 to 24.16 tips mg−1 across species and sites. Among root morphological traits, only SRL was influenced by site (p = 0.017, Table 3); however, all traits differed among species (all p < 0.001, Table 3). All root morphological traits of white birch were higher at LS than at LSH (Figure 2a–c). Species differences in root morphological traits were almost always observed, but the differences of root morphological traits among tree species was not consistent across sites (Figure 2). Root morphological traits were generally lowest in Korean pine and highest in white birch (Figure 2).
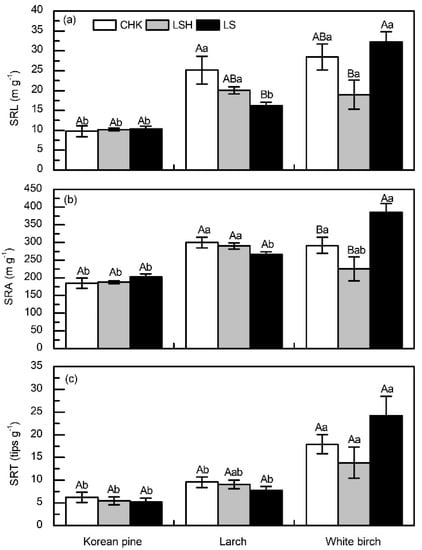
Figure 2.
Root morphological traits of Korean pine, larch, and white birch at three sites in Northeast China. (a) specific root length (SRL), (b) specific root surface area (SRA), and (c) specific root tips (SRT). Vertical bars are means ± SE, with a sample size of n = 6. Different capital letters indicate significant differences (p < 0.05) within a site. Different lowercase letters indicate significant differences (p < 0.05) within a species.
3.3. Soil Characteristics
Total organic carbon (TOC) and soil pH differed among sites and species (Figure 3); however, these main effects were rarely independent of each other (i.e., S × TS; p < 0.001, Table 3). Although TOC increased with latitude, regardless of species, the degree of difference among latitudes depended on species. TOC increased from 32.6 to 41.3 to 81.4 mg g−1, across the three sites. Likewise, TOC differed among species, but the rank order and degree of difference depended on latitude. Species differences in TOC were most apparent at highest and lowest site (LS and CHK; Figure 3a). Soil pH differed among sites and species, but species differences depended on site and site differences depended on species (i.e., S × TS interaction; p < 0.001, Table 3). Soil pH was highest at the intermediate latitudes (LSH), but this pattern was different for white birch (Figure 3d). Soil pH differed among tree species, the soil pH of Korean pine plantations was always higher than that of larch at three sites (Figure 3d).
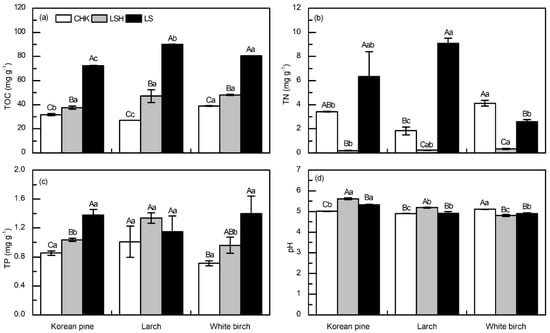
Figure 3.
Soil chemical traits of Korean pine, larch, and white birch plantations at three sites in Northeast China. (a) total organic carbon (TOC), (b) total nitrogen (TN), (c) total phosphorus (TP), and (d) pH. Vertical bars are means ± SE, with a sample size of n = 6. Different capital letters indicate significant differences (p < 0.05) within a site. Different lowercase letters indicate significant differences (p < 0.05) within a species.
Regardless of species, total N always differed among sites, but the rank order and degree of difference changed across species (i.e., S × TS interaction; p = 0.035, Table 3). For example, total N was always lowest at the middle site (LSH), regardless of species (Figure 3c). The highest site (LS) generally exhibited the highest total N, but again, this depended largely on species (Figure 3b). Total N differed among species, and these patterns remained independent of site (Figure 3b).
3.4. Relationship Between Root Exudation Rates and Root Traits and Environmental Factors
Root exudation rates were correlated with environmental factors. When all species were considered together, root exudation was negatively correlated TOC and positively related to MAT and MAP (p < 0.05, Table 4). Root exudation rates were most strongly correlated to MAT and MAP (both R = 0.443). Because root exudation rates were calculated by SRA, SRA was not considered in the correlation.

Table 4.
The matrix of correlation coefficients (Person) and p value between root exudation rates and root morphological traits and environmental factors. Correlations with a P values below 0.05 are in bold.
Within individual species, root exudation rates were related to root morphological traits and environmental factors. Korean pine root exudation rates were negatively correlated with TOC and TP (R = −0.471, p = 0.049 and R = −0.55, p = 0.018, respectively, Table 4), and were positively correlated with MAT and MAP (both R = 0.525, p = 0.025, Table 4). Larch root exudation rates were negatively correlated to TOC (R = 0.673, p = 0.002, Table 4) and positively correlated to MAT and MAP (both R = 0.637, p = 0.004, Table 4). Root exudation rates of white birch were negatively correlated with SRL and TN (R = −0.61, p = 0.007, R = −0.635, p = 0.005, and R = −0.508, p = 0.031, respectively, Table 4).
4. Discussion
Our results indicate that root exudation was related to a combination of factors, but failed to identify factors that consistently influenced the magnitude of root exudation. The strongest and clearest effect observed in our study was a decrease in root exudation of Korean pine and larch across the latitudinal gradient. There were no clear patterns in differences among species across site, suggesting that root traits and environmental factors may interact in complicated ways. Despite a large amount of variation in root morphological traits and soil chemical characteristics among species and sites, we were unable to identify consistent controls on root exudation.
4.1. Latitudinal and Tree Species Effects on Root Exudation Rates
In our study, root exudation rates of Korean pine and larch have inverse site trends (Figure 1). Site is not an environmental factor that can directly affect root exudates, but rather an indirect variable that comprises multiple factors. Mean annual temperature (MAT) and MAP showed inverse site trends in our research (Table 1), which is consistent with the generally accepted idea that the climate from southern to northern China gradually becomes colder and drier [44]. Total organic carbon (TOC) increased as site latitude increased, suggesting TOC may correlate with environmental factors associated with latitude. Huang et al. (2020) [45] reported that soil phosphorus was positively correlated with latitude. In our results, TP of Korean pine and white birch generally increased at sites of higher latitude, suggesting that TP may be negatively related to root exudation rates. The predicted response of root exudation across sites of increasing latitude was assumed to result from temperature variation, and we observed a response of MAT and MAP (Table 4), indicating that temperature and precipitation contributes to the formation of the site trend of root exudation rate. Many experiments have reported elevated temperature can increase belowground allocation of C in forests. Yin et al. (2013) [28] reported that root exudation rates increased under experimental warming and attributed this response to increased root activity with increasing temperature. Plant roots increase C input to soil when exposed to elevated temperature to improve soil microbial metabolic enzyme activity and N conversion processes, so as to meet the soil nutrient required for plant growth [46]. Wu et al. (2011) [47] found that increased precipitation promoted plant growth and ecosystem C fluxes. Shalik et al. (2018) [48] found that the growth of Pinus roxburghii Sarg. was positively corelated with precipitation. In the study of Ramırez-Valiente et al. (2020) [49] on oaks, the plant growth rates were related to precipitation in the driest month. Based on these previous observations, a decline in MAT and MAP along the site gradient is likely to cause a decrease in plant physiological activity and growth, thereby influencing root exudation. However, under realistic conditions, changes in root exudation are usually the result of multiple factors. Since changes in precipitation are usually closely related to factors such as temperature, there may be an interaction between temperature and precipitation that affects root exudation.
Although all stands in this study were mature, C allocation to fine roots may be affected by standing age because of C and N limitation in old stand [50,51]. The belowground C allocation are thought to be one of the primary aspects controlling root exudations [2]; thus, change in C allocation may cause changes in the root exudations [52]. In this study, the stand age in LS site was higher than other sites, and the root exudation rates in LS were lowest (Figure 1). Therefore, the root exudation rates in LS may be limited by the low C allocation to the root. The exudation rate of white birch (broadleaf) differs from that of Korean pine and larch (conifers) among sites, indicating that root exudation of conifers and broadleaf species may have differ in response to environment factors. Moreover, the influencing factors of root exudation rates of the three tree species in our study differed and the relative ranking of root exudation among species differed across site. Our observations suggest that functional traits, like leaf habit or phyla, may explain variation in exudation; however, our work only includes one example of each leaf habit and only one angiosperm, and there are currently few studies on root exudation of trees exhibiting different leaf habits, requiring further research to test this hypothesis.
4.2. The Relationship Between Root Exudation and Root Morphological Traits and Environmental Factors
Root exudation rates were correlated to environmental factors when assessed across all species, and were most positively correlated to MAT and MAP (Table 4). In addition to MAT and MAP, root exudates of individual tree species were also correlated to soil characteristics. Korean pine and larch root exudation rates were correlated to MAT and MAP. The positive correlation between root exudations and MAT and MAP might be because of MAT and MAP are the most obvious factors of change with latitude. In addition to MAT and MAP, root exudation rates of Korean pine and larch are also negatively related to soil characteristics (TOC and TP) (Table 4). Root exudation contributes to soil organic C directly and indirectly by stimulating microbial decomposition [23]. However, we found a negative correlation between root exudation and TOC. The reason may be that most of root exudation contribute to rhizosphere soil C rather than bulk soil [23]. In this study, only organic C in bulk soil were measured. Moreover, the restriction of phosphorus on plant growth is very common in forest ecosystems. In fact, enzymes in root exudates can directly decompose the organic phosphorus bound in soil organic matter for plant use, so that soil shows obvious phosphorus depletion [53]. Hence, the negative correlation between root exudates and phosphorus may be caused by the growth requirements of trees. In contrast to two conifers tree species, root exudation rates of deciduous species white birch were negatively corelated to SRL and TN. Root exudates are released from the fine root surface, and the surface area is often positively correlated with root length. Therefore, the longer the root length, the lower the exudation rates per unit surface area. The negative correlation between root exudations and TN may be related to soil nitrogen availability. Studies have shown that exudations are negatively related to N availability [38,54]. When soil N availability is limited, trees would exude more root exudates into rhizosphere to stimulated the growth and activity of microorganisms, access nitrogen bound up in SOM [46]. In addition to the factors in this study, other factors also affect the root exudation rate, such as soil microorganisms.
4.3. Other Factors That May Explain Root Exudation
Our results were based on an observational approach that opportunistically leveraged a series of co-occurring tree plantations arranged across a latitudinal gradient. As such, we could not control all of the factors that might influence root exudation rates. Indeed, other factors, including atmospheric N deposition and elevated CO2 concentration [55] have been shown to influence root exudation. For example, N deposition can enhance root exudation rates [34]. Both CO2 concentration and N supply stimulated tree root exudation rates [55]. In addition, this study only paid attention to the rate of root organic C exudation, but did not study the spatial and interspecies changes of root exudates composition. Qiao et al. (2014) [56] found that warming had significant impact on the relative contents of major compounds and enhanced the total phenolic acid compounds in root exudates. Zwetsloot et al. (2018) [37] found that root exudate compounds were different among tree species. These results indicated that environmental changes and tree species will affect the composition of root exudates, study the composition of root exudates at different sites and tree species is highly recommended.
In temperate and boreal forests, almost all plant roots have fungi attached to the surface to form ectomycorrhizas and produce a large number of epitaxial hyphae. Like roots, fungi also release exudations, forming ectomycorrhizas with tree roots [57]. Studies have shown that more than 70% of the oxalic acid secreted by plants into the soil comes from epitaxial hyphae [58]. Johansson et al., 2009 [59] found the root exudation rates of Pinus sylvestris L. seedlings infected by ectomycorrhiza was significantly higher than that of non-infected seedlings. These results suggested that ectomycorrhizal fungi secrete large amounts of C into the soil. Unfortunately, due to the limitation of research methods, this study lacks quantitative and qualitative results between root exudates and mycorrhizal exudates. How root system C and ectomycorrhiza fungi C regulate soil biogeochemical cycling process needs further research.
5. Conclusions
Our results demonstrated that root exudation rates were related to mean annual temperature, mean annual precipitation, and soil organic C; however, they fail to provide consistent and definitive information about the primary influencing factors. Inconsistencies in the relative ranking of root exudation among species across sites suggest that multiple factors interact to control root exudation. Future research should conduct more manipulative studies to tease apart specific control factors of root exudation. In addition, Ohta et al. (2019) [60] reported that the exudation rates were different among trees of different provenances. Some studies found Korean pine [61] and Larch [62] have geographical variation. Thus, future research should focus on the study of the root exudation rates among tree provenances.
Author Contributions
Conceptualization, L.Y., X.W., and D.P.A.; data curation, L.Y.; formal analysis, L.Y.; funding acquisition, X.W. and X.C.; investigation, Z.J.; methodology, L.Y.; project administration, X.W.; software, Y.G.; supervision, X.W. and Z.M.; validation, Z.M., X.C., and D.P.A.; visualization, L.Y. and X.W.; writing—original draft, L.Y.; writing—review and editing, X.W. and D.P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31670476), National Key R&D Program (2018YFC0507003) of China, the Fundamental Research Funds for the Central Universities (2572019BA15 and 2572019CP09), Applied technology research and Development Program of Heilongjiang Province (GA20B401), and Heilongjiang Touyan Innovation Team Program Technology Development Team for High-efficient Silviculture of Forest Resources.
Acknowledgments
The authors thank for Weiping Gu, Xingpeng Li, and Guoquan Ding for their assistance to field and laboratory work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hamilton, E.W.; Frank, D.A.; Hinchey, P.M.; Murray, T.R. Defoliation induces root exudation and triggers positive rhizospheric feedbacks in a temperate grassland. Soil Biol. Biochem. 2008, 40, 2865–2873. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Venturi, V.; Keel, C. Signaling in the Rhizosphere. Trends Plant Sci. 2016, 21, 187–198. [Google Scholar] [CrossRef]
- Mccully, M.E. Roots in soil: Unearthing the complexities of roots and their rhizospheres. Annu. Rev. Plant Biol. 1999, 50, 695–718. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Cheng, W. Photosynthesis controls of rhizosphere respiration and organic matter decomposition. Soil Biol. Biochem. 2001, 33, 1915–1925. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, M.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Effects of phenylcarboxylic acids on mitosis, endoreduplication and expression of cell cycle-related genes in roots of cucumber (Cucumis sativus L.). J. Chem. Ecol. 2009, 35, 679–688. [Google Scholar] [CrossRef]
- Haichar, F.Z.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef]
- Shi, S.; Richardson, A.E.; O’callaghan, M.; Deangelis, K.M.; Jones, E.E.; Stewart, A.; Firestone, M.K.; Condron, L.M. Effects of selected root exudate components on soil bacterial communities. FEMS Microbiol. Ecol. 2011, 77, 600–610. [Google Scholar] [CrossRef]
- Bengtson, P.; Barker, J.; Grayston, S.J. Evidence of a strong coupling between root exudation, C and N availability, and stimulated SOM decomposition caused by rhizosphere priming effects. Ecol. Evol. 2012, 2, 1843–1852. [Google Scholar] [CrossRef]
- Kelting, D.L.; Burger, J.A.; Edwards, G.S. Estimating root respiration, microbial respiration in the rhizosphere, and root-free soil respiration in forest soils. Soil Biol. Biochem. 1998, 30, 961–968. [Google Scholar] [CrossRef]
- Van Hees, P.A.W.; Jones, D.L.; Finlay, R.; Godbold, D.L.; Lundström, U.S. The carbon we do not see—The impact of low molecular weight compounds on carbon dynamics and respiration in forest soils: A review. Soil Biol. Biochem. 2005, 37, 1–13. [Google Scholar] [CrossRef]
- Sun, L.; Kominami, Y.; Yoshimura, K.; Kitayama, K. Root-exudate flux variations among four co-existing canopy species in a temperate forest, Japan. Ecol. Res. 2017, 32, 331–339. [Google Scholar] [CrossRef]
- Grayston, S.J. Rhizosphere carbon flow in trees, in comparison with annual plant, the importance of root exudation and its impact on microbial activity and nutrient availability. Appl. Soil Ecol. 1996, 5, 29–56. [Google Scholar] [CrossRef]
- Coleman, M.; Tolsted, D.; Nichols, T.; Johnson, W.; Wene, E.; Houghtaling, T. Post-establishment fertilization of Minnesota hybrid poplar plantations. Biomass Bioenergy 2006, 30, 740–749. [Google Scholar] [CrossRef]
- Shan, J.; Morris, L.A.; Hendrick, R.L. The effects of management on soil and plant carbon sequestration in slash pine plantations. J. Appl. Ecol. 2001, 38, 932–941. [Google Scholar] [CrossRef]
- Will, R.E.; Markewitz, D.; Hendrick, R.L.; Meason, D.F.; Crocker, T.R.; Borders, B.E. Nitrogen and phosphorus dynamics for 13-year-old loblolly pine stands receiving complete competition control and annual N fertilizer. For. Ecol. Manag. 2006, 227, 155–168. [Google Scholar] [CrossRef]
- Personeni, E.; Nguyen, C.; Marchal, P.; Pages, L. Experimental evaluation of an efflux-influx model of C exudation by individual apical root segments. J. Exp. Bot. 2007, 58, 2091–2099. [Google Scholar] [CrossRef]
- Lamont, B.B. Structure, ecology and physiology of root clusters—A review. Plant Soil 2003, 248, 1–19. [Google Scholar] [CrossRef]
- Mcdougall, B.M.; Rovira, A. Sites of exudation of 14C-labelled compounds from wheat roots. New Phytol. 1970, 69, 999–1003. [Google Scholar] [CrossRef]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef]
- Mccormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ataka, M.; Kominami, Y.; Yoshimura, K. Relationship between fine-root exudation and respiration of two Quercus species in a Japanese temperate forest. Tree Physiol. 2017, 37, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Pregitzer, K.S.; Deforest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine root architecture of nine North American trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Guo, D.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, J.; Ding, J.; Zou, T.; Zhang, Z.; Liu, Q.; Yin, H. Differences in root exudate inputs and rhizosphere effects on soil N transformation between deciduous and evergreen trees. In Plant and Soil; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Neumann, G.; Bott, S.; Ohler, M.A.; Mock, H.P.; Lippmann, R.; Grosch, R.; Smalla, K. Root exudation and root development of lettuce (Lactuca sativa L. cv. Tizian) as affected by different soils. Front. Microbiol. 2014, 5, 1–6. [Google Scholar] [CrossRef]
- Yin, H.; Xiao, J.; Li, Y.; Chen, Z.; Cheng, X.; Zhao, C.; Liu, Q. Warming effects on root morphological and physiological traits: The potential consequences on soil C dynamics as altered root exudation. Agric. For. Meteorol. 2013, 180, 287–296. [Google Scholar] [CrossRef]
- Majdi, H.; Öhrvik, J. Interactive effects of soil warming and fertilization on root production, mortality, and longevity in a Norway spruce stand in Northern Sweden. Glob. Chang. Biol. 2004, 10, 182–188. [Google Scholar] [CrossRef]
- Bai, W.; Wan, S.; Niu, S.; Liu, W.; Chen, Q.; Wang, Q.; Zhang, W.; Han, X.; Li, L. Increased temperature and precipitation interact to affect root production, mortality, and turnover in a temperate steppe: Implications for ecosystem C cycling. Glob. Chang. Biol. 2010, 16, 1306–1316. [Google Scholar] [CrossRef]
- Uselman, S.M.; Qualls, R.G.; Thomas, R.B. Effects of increased atmospheric CO2, temperature, and soil N availability on root exudation of dissolved organic carbon by a N-fixing tree (Robinia pseudoacacia L.). Plant Soil 2000, 222, 191–202. [Google Scholar] [CrossRef]
- Stovall, J.P.; Seiler, J.R.; Fox, T.R. Respiratory C fluxes and root exudation differ in two full-sib clones of Pinus taeda (L.) under contrasting fertilizer regimes in a greenhouse. Plant Soil 2012, 363, 257–271. [Google Scholar] [CrossRef]
- Meharg, A.; Killham, K. The effect of soil pH on rhizosphere carbon flow of Lolium perenne. Plant Soil 1990, 123, 1–7. [Google Scholar] [CrossRef]
- Ataka, M.; Sun, L.; Nakaji, T.; Katayama, A.; Hiura, T. Five-year nitrogen addition affects fine root exudation and its correlation with root respiration in a dominant species, Quercus crispula, of a cool temperate forest, Japan. Tree Physiol. 2020, 40, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Kusumoto, D.; Sekimoto, H.; Sugimoto, Y.; Takeuchi, Y.; Yoneyama, K. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta 2007, 227, 125–132. [Google Scholar] [CrossRef]
- Meier, I.C.; Tuckmantel, T.; Heitkotter, J.; Muller, K.; Preusser, S.; Wrobel, T.J.; Kandeler, E.; Marschner, B.; Leuschner, C. Root exudation of mature beech forests across a nutrient availability gradient: The role of root morphology and fungal activity. New Phytol. 2020, 226, 583–594. [Google Scholar] [CrossRef]
- Zwetsloot, M.J.; Kessler, A.; Bauerle, T.L. Phenolic root exudate and tissue compounds vary widely among temperate forest tree species and have contrasting effects on soil microbial respiration. New Phytol. 2018, 218, 530–541. [Google Scholar] [CrossRef]
- Xiong, D.; Huang, J.; Yang, Z.; Cai, Y.; Yang, Y. The effects of warming and nitrogen addition on fine root exudation rates in a young Chinese-fir stand. For. Ecol. Manag. 2020, 458, 117793. [Google Scholar] [CrossRef]
- Wasaki, J.; Rothe, A.; Kania, A.; Neumann, G.; Romheld, V.; Shinano, T.; Osaki, M.; Kandeler, E. Root exudation, phosphorus acquisition, and microbial diversity in the rhizosphere of white lupine as affected by phosphorus supply and atmospheric carbon dioxide concentration. J. Environ. Qual. 2005, 34, 2157–2166. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiao, M.; Li, D.; Yin, H.; Liu, Q. Do warming-induced changes in quantity and stoichiometry of root exudation promote soil N transformations via stimulation of soil nitrifiers, denitrifiers and ammonifiers? Eur. J. Soil Biol. 2016, 74, 60–68. [Google Scholar] [CrossRef]
- Phillips, R.P.; Erlitz, Y.; Bier, R.; Bernhardt, E.S. New approach for capturing soluble root exudates in forest soils. Funct. Ecol. 2008, 22, 990–999. [Google Scholar] [CrossRef]
- Shidan, B. Analysis Soil and Agricultural Chemistry, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 74–76. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Han, W.X.; Fang, J.Y.; Reich, P.B.; Ian Woodward, F.; Wang, Z.H. Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol. Lett. 2011, 14, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, C.; Teng, M.; Zhou, Z.; Wang, P. Net Primary Productivity of Pinus massoniana Dependence on Climate, Soil and Forest Characteristics. Forests 2020, 11, 404. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Bader, N.E.; Johnson, D.W.; Cheng, W. Does accelerated soil organic matter decomposition in the presence of plants increase plant N availability? Soil Biol. Biochem. 2009, 41, 1080–1087. [Google Scholar] [CrossRef]
- Wu, Z.; Dijkstra, P.; Koch, G.W.; PeÑuelas, J.; Hungate, B.A. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob. Chang. Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef]
- Sigdel, S.R.; Dawadi, B.; Camarero, J.J.; Liang, E.; Leavitt, S.W. Moisture-Limited Tree Growth for a Subtropical Himalayan Conifer Forest in Western Nepal. Forests 2018, 9, 340. [Google Scholar] [CrossRef]
- Ramirez-Valiente, J.A.; Lopez, R.; Hipp, A.L.; Aranda, I. Correlated evolution of morphology, gas exchange, growth rates and hydraulics as a response to precipitation and temperature regimes in oaks (Quercus). New Phytol. 2020, 227, 794–809. [Google Scholar] [CrossRef] [PubMed]
- Tateno, R.; Fukushima, K.; Fujimaki, R.; Shimamura, T.; Ohgi, M.; Arai, H.; Ohte, N.; Tokuchi, N.; Yoshioka, T. Biomass allocation and nitrogen limitation in a Cryptomeria japonicain plantation chronosequence. J. For. Res. 2017, 14, 276–285. [Google Scholar]
- Hishi, T.; Tateno, R.; Fukushima, K.; Fujimaki, R.; Itoh, M.; Tokuchi, N.; Näsholm, T. Changes in the anatomy, morphology and mycorrhizal infection of fine root systems of Cryptomeria japonicain relation to stand ageing. Tree Physiol. 2016, 37, 61–70. [Google Scholar]
- Ahonen-Jonnarth, U.; Hees, P.A.W.V.; Lundström, U.S.; Finlay, R.D. Organic acids produced by mycorrhizal Pinus sylvestris exposed to elevated aluminium and heavy metal concentrations. New Phytol. 2010, 146, 557–567. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Houlton, B.Z.; Smith, W.K.; Marklein, A.R.; Reed, S.C.; Parton, W.; Del Grosso, S.J.; Running, S.W. Patterns of new versus recycled primary production in the terrestrial biosphere. Proc. Natl. Acad. Sci. USA 2013, 110, 12733–12737. [Google Scholar] [CrossRef]
- Aitkenhead-Peterson, J.A.; Kalbitz, K. Short-term response on the quantity and quality of rhizo-deposited carbon from Norway spruce exposed to low and high N inputs. J. Plant Nutr. Soil Sci. 2005, 168, 687–693. [Google Scholar] [CrossRef]
- Phillips, R.P.; Bernhardt, E.S.; Schlesinger, W.H. Elevated CO2 increases root exudation from loblolly pine (Pinus taeda) seedlings as an N-mediated response. Tree Physiol. 2009, 29, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Xiao, J.; Yin, H.; Pu, X.; Yue, B.; Liu, Q. Analysis of the phenolic compounds in root exudates produced by a subalpine coniferous species as responses to experimental warming and nitrogen fertilisation. Chem. Ecol. 2014, 30, 555–565. [Google Scholar] [CrossRef]
- Vanhees, P.; Jones, D.; Jentschke, G.; Godbold, D. Organic acid concentrations in soil solution: Effects of young coniferous trees and ectomycorrhizal fungi. Soil Biol. Biochem. 2005, 37, 771–776. [Google Scholar] [CrossRef]
- Arvieu, J.C.; Leprince, F.; Plassard, C. Release of oxalate and protons by ectomycorrhizal fungi in response to P-deficiency and calcium carbonate in nutrient solution. Ann. For. Sci. 2003, 60, 815–821. [Google Scholar] [CrossRef]
- Johansson, E.M.; Fransson, P.M.A.; Finlay, R.D.; Van Hees, P.A.W. Quantitative analysis of soluble exudates produced by ectomycorrhizal roots as a response to ambient and elevated CO2. Soil Biol. Biochem. 2009, 41, 1111–1116. [Google Scholar] [CrossRef]
- Ohta, T.; Niwa, S.; Hiura, T. Geographical variation in Japanese cedar shapes soil nutrient dynamics and invertebrate community. Plant Soil 2019, 437, 355–373. [Google Scholar] [CrossRef]
- Zhang, H.Q.; An, L.J.; Zu, Y.G. Geographical variation of morphology characters for natural populations of Pinus koraiensis. Acta Ecol. Sin. 1999, 19, 932–938. [Google Scholar]
- Zhang, L.; Zhang, H.G.; Li, X.F. Analysis of genetic diversity in Larix gmelinii (Pinaceae) with RAPD and ISSR markers. Genet. Mol. Res. 2013, 12, 196–207. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).