Abstract
Field production of seedlings used to create nursery stock liners involves transplanting and root pruning that can alter root system architecture. Seedlings of eight species of trees commonly used in urban landscapes were selected based on the configuration of their woody lateral roots; Preferred (maximum gap between roots ≤90 degrees), Acceptable (maximum gap 120–150 degrees), and Inferior (≥180 degree gap—no lateral roots on one side). The lateral root configuration (LRC) of the seedlings was compared to the LRC one year after replanting. The number of lateral or regenerated roots alone was generally adequate to form an acceptable root flare (≥3 roots) one year after seedlings were replanted. The maximum gap in lateral roots as a seedling was not consistent with the maximum gap one year after the seedlings were replanted in most species. It often became larger. Neither lateral roots nor regenerated roots alone could reliably produce a root structure with an acceptable maximum gap between roots. Lateral roots and roots regenerated from the pruned end of the main root, together produced enough flare roots one year after replanting with a small enough maximum gap in the radial distribution for good stability. This information may be an initial step in developing criteria for seedling grading systems that will improve root systems of nursery stock grown for planting in urban landscapes.
1. Introduction
Mature forest trees that have been able to out-compete their neighbors over time typically have many prominent visible flare roots. Structural roots present as young trees persist and develop into these mature flare roots. The woody roots established during the first few years constituted the main structural root system of Sitka spruce trees at 34 years of age [1]. Though this kind of very long-term study is not common, there is no reason to believe this species is unusual. The lateral roots present on one-year-old seedlings of eight different tree species persisted, and increased in size, for at least four more years [2].
Typically, a tree has 3–15 primary structural roots emanating from the root collar that form the root flare [2,3,4,5]. The thickening of flare roots is caused by swaying of the stem in the wind and other mechanical and physiological stimuli from the adjacent stem [6]. The number and arrangement of these roots may affect tree stability [5,7]. The flare roots have been associated with soils that offer poor anchorage [8] and can produce sinker roots that plunge vertically into the soil, providing supplemental anchorage [7]. Where the number is small, the stability of the tree becomes sensitive to their symmetry [2]. A stool with only three legs can be stable only if the legs are evenly spaced. With four, or more, legs spaced unevenly, a gap larger than the 120 degrees between evenly spaced legs of the stool with only three legs would also be unstable.
The number of lateral roots is also closely related to tree growth rate from a very early age. For conifers (Picea sp., Abies sp., and Pinus taeda L.) and hardwoods (Quercus sp., Liquidambar styraciflua L., and Juglans nigra L.) studied, one-year old forest nursery seedlings with a high number of lateral roots consistently had more rapid growth after planting [9,10,11,12,13,14,15]. The fate of these roots was not followed after replanting. Fine roots are known to be short-lived [16]. Many of these young lateral roots of seedlings are quite small and are lost in a short time, even when plants are undisturbed [2,17].
Nursery practices result in the loss of lateral roots beyond those that would be lost naturally. Seedlings are routinely transplanted in landscape nursery field production [18]. The lower portion of the main root and lateral roots emanating from that portion are pruned off as seedlings are prepared for replanting. Others on the remaining portion of the main root are lost through exposure and handling during transplanting. There were significantly fewer lateral roots on nursery production seedlings one year after transplanting than on undisturbed seedlings [2,17].
New roots are almost always regenerated from the cut end of the main root after replanting. Pruning the taproot in the nursery, results in the formation of multiple taproots at the wound site or just above it [19,20,21]. These roots persist for at least four years and form an adventitious root flare. The few surviving lateral roots above it can be insufficient to form an acceptable root flare alone [2,17].
The objective of this study was to assess the contribution of natural lateral and regenerated roots resulting from landscape nursery field production practices to early root flare development of trees. Our findings suggest transplanting and root pruning seedlings have a minimal have a minimal effect on subsequent development of flare roots
2. Materials and Methods
One-year-old seedlings of eight species of trees commonly used in urban landscapes were selected, based on the configuration (size and arrangement) of their woody lateral roots at the harvested seedling stage of field-grown nursery production. Species used were hedge maple (Acer campestre L.), red maple (Acer rubrum L.), sugar maple (Acer saccharum Marshall), European black alder (Alnus glutinosa (L.) Gaertn.), northern catalpa (Catalpa speciosa (Warder) Warder ex Engelm.), honeylocust (Gleditsia triacanthos L.), Kentucky coffeetree (Gymnocladus dioicus (L.) K. Koch), and Siberian elm (Ulmus pumila L.).
One-year-old seedlings of each species were selected from normal bare root stock at J. Frank Schmidt & Sons Nursery, Boring, Oregon. Ten seedlings with at least four lateral roots >2 mm diameter were selected for each of three lateral root configurations (LRC) based on the following criteria:
- Preferred LRC: Maximum gap between existing lateral roots of 90 degrees, positioned for good anchorage on all four sides.
- Acceptable LRC: A larger gap in radial distribution of existing lateral roots (120–150 degrees) with potentially minor instability on one side.
- Inferior LRC: No lateral roots on one side (≥ 180 degree gap) and likely poorly anchored on the side without roots.
The seedling root systems were held against a template to evaluate the maximum gap between roots (Figure 1). Kentucky coffeetree did not have enough seedlings that fit the acceptable LRC criteria to include all three LRCs for that species.
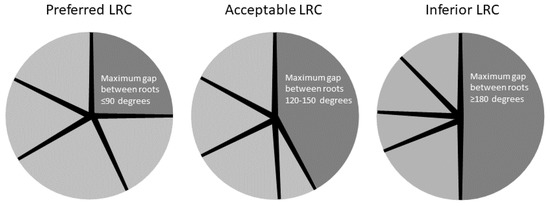
Figure 1.
Schematic illustration of how seedlings were selected based on a minimum of four lateral roots ≥2 mm, with a maximum gap between them for each of the three Lateral Root Configurations (LRC).
Individual seedlings were root pruned with hand pruners to prepare them for replanting, as is typical for seedlings in field-grown nursery propagation and production. The main root was pruned at approximately 10 cm below the natural soil level. Lateral roots larger than 2 mm diameter were pruned to approximately 2.5 cm from the main root.
When selecting the seedlings, the number and diameter of lateral roots could not be measured in the field without risking damage from exposure, and due to time limitations. Instead, photographs were taken of the seedlings in two directions, perpendicular to each other, and with a scale grid in the background. Diameters of the lateral roots were recorded from the photographs. Root diameter could not be measured with precision to less than a millimeter, so measurements were recorded as the closest whole millimeter unit.
The trees were planted in April as part of a larger new plant development field planting at Schmidt Nursery. Fertilizer was applied to the field at 16.8, 67.2, and 50.4 kg/h, for N, P, and K, respectively, before planting. The seedlings were replanted by hand at the original seedling root system depth in rows with 30 cm (12 inches) between plants in each row. The planting was maintained for one year according to normal nursery practices. During the growing season, urea (46-0-0) was applied at 112 kg/h (51.5 kg actual N) per application, in April, May, and June. Overhead irrigation was applied at 3.8 cm every two weeks from mid-June until late August. After one year, the young trees were dug with the equipment used to mechanically harvest trees in the nursery. The diameter of lateral roots, and regenerated roots from the cut end of the main root ≥1 mm was measured and recorded in whole millimeter units, the same as for the measurements from photographs. Radial position around the stem was recorded to the nearest 30 degree interval (positions of numbers on a clock face), and the maximum gap between roots on each plant was calculated.
Statistical analysis was performed using SigmaPlot for Windows, Verson 14.0 (Systat Software, Inc., San Jose, CA, USA). The number of roots was compared among LRCs for each species using One Way ANOVA, and the Holm-Sidak method of pairwise multiple comparison. The maximum gap between roots was compared among LRCs for each species using Kruskal–Wallis One Way Analysis of Variance on Ranks and Dunn’s Method of pairwise multiple comparison, or the Mann–Whitney Rank Sum Test when a species that had only two LRCs. The number of seedling lateral roots of different size classes was compared to the number of lateral roots one year later with t-tests. The maximum gap between regenerated roots alone was compared to the maximum gap between all roots, for each LRC treatment within each species, using the Mann–Whitney Rank Sum Test.
3. Results and Discussion
Woody roots established as young plants constitute the main structural root system of trees decades later [2]. In this study, seedlings of eight species of trees were selected for three groups based on the configuration of their woody lateral roots. One year after the seedlings were replanted, further development of lateral roots, and of roots regenerated from the cut end of the main root were assessed to provide insight into the relationship between seedling root configuration and structural root development in these species.
3.1. Lateral Roots
3.1.1. Number of Roots
As seedlings, six of the eight species showed a significant difference in the number of lateral roots among the three LRCs, but no LRC consistently had more lateral roots across species (Table 1). Fewer roots on inferior LRC seedlings than the other LRCs, would have been reasonable to expect since the roots were confined to a smaller space on one side of the plant, but this occurred only in red maple, catalpa, and Siberian elm. Kentucky coffeetree and honeylocust had significantly more lateral roots on seedlings with the inferior LRC. The distribution of lateral roots around the stem as a seedling is not a reliable indicator of the number of lateral roots on a seedling in most species.

Table 1.
Number and maximum gap between lateral roots on seedlings in a field-grown nursery production system prepared for replanting and one year later.
There was a significant difference in the number of lateral roots among the three LRCs one year after replanting in four of eight species. Kentucky coffeetree and honeylocust, which had more lateral roots on the inferior LRC as seedlings, had more lateral roots on the preferred LRC one year later. Sugar maple, which had more lateral roots on the preferred LRC as seedlings, maintained that difference. Hedge maple had no difference among LRCs as a seedling and had significantly fewer lateral roots in inferior seedling one year later. Red maple, northern catalpa, and Siberian elm, which had fewer roots on the inferior LRC as seedlings, had no difference among LRCs one year after replanting. There was no difference among LRCs in European black alder at either stage. (Table 1). The number of lateral roots in these larger plants did not follow the seedling root configuration.
As seedlings, the average number of lateral roots of all sizes varied among species and LRC groups from 9.7 to 33.2. All of the averages were within, or greater than, the range of lateral roots than reported is needed for an acceptable root flare (3–15), but not all of these roots would be expected to persist indefinitely. After an additional year of growth after replanting, there were significantly fewer roots in all LRCs of all species (Table 2). Though roots were lost, nearly all had more than the minimum of 3 roots needed.

Table 2.
The relationship between the mean number of lateral roots by size class as a seedling, and the mean number of lateral roots one year after replanting.
The roots that were lost may have been the smaller fine roots (<2 mm diameter) which are known to be shorter-lived [16], than larger, woody roots. When harvested one year after replanting the seedlings, the diameter of lateral roots for individual species was 3–6 times greater than as seedlings (data not shown). In red maple, sugar maple, and Siberian elm, there was no difference between the number of roots ≥2 mm diameters as seedlings and the number present one year after replanting in any LRC. (Table 2). There was a difference in only one of the three LRCs in five species. Hedge maple inferior LRC, and northern catalpa acceptable LRC seedlings had more lateral roots ≥2 mm diameter as seedlings than one year later, indicating that not all persisted. Kentucky coffeetree, and honeylocust preferred, and European black alder inferior seedlings had fewer ≥2 mm roots as seedlings than one year later, suggesting that some smaller roots persisted and grew larger. With a few minor inconsistencies, the data indicates that roots of 2 mm or greater, are the most likely persist and become part of the structural root system.
3.1.2. Maximum Gap between Roots
Though seedlings were visually evaluated and selected based on the maximum gap between woody lateral roots, one year after replanting the only difference in maximum gap among any of the LRCs was in hedge maple and honeylocust (Table 1). These species maintained the largest maximum gap in inferior LRC seedlings, and that appeared to expand to 300 degrees or greater based on the median value. The large median maximum gap value in the inferior LRC of these two species can be attributed to four of 10, and five of 8 plants, respectively, having only one or no lateral roots one year later (360 degree gap). In plants where lateral roots were present, the gap remained at approximately 180 degrees. The complete loss of all lateral roots in so many plants in only the inferior LRC in both of these species is unexplained.
The initial 90 degree, or less, maximum gap in the preferred LRC had expanded an additional 30 to 90 degrees by one year after planting (Table 1). This is inconsistent with the data showing persistence of laterals ≥2 mm (Table 2). Though individual roots could not be tracked, presumably, some roots ≥2 mm in crucial positions must have been lost for the gap to enlarge.
Laterals alone may not have the potential to consistently develop into an acceptable root flare, regardless of the LRC as a seedling. With the exception of the three inferior LRCs of maple seedlings, all other species LRC groups had at least four lateral roots one year after planting. Five or more lateral roots was common. This would be a sufficient number to form an acceptable root flare if well distributed around the main root. However, the maximum gap was greater than 120 degrees needed for good stability in 10 of 23 of the LRCs across all species. The maximum gap between lateral roots may be more of a limiting factor for acceptable root flare development than the number of lateral roots.
3.2. Regenerated Roots
3.2.1. Number of Roots
One year after the seedlings were replanted, all species regenerated multiple taproots from the cut end of the main root, and the number was not significantly different among LRCs in any species except European black alder (Table 1). A similar number of regenerated roots in all three LRCs would be a reasonable to expect, since none of the pruned ends of the main roots had any regenerated roots when they were replanted. This suggests that the number of lateral roots present on the main root does not affect root regeneration from the cut end.
The acceptable LRC European black alder seedlings had significantly fewer regenerated roots than the other two groups, but the reason for this lone difference could not be determined. Since the preferred and inferior LRCs had similar numbers of regenerated roots, and alder was the only species showing a difference, the lower number in the acceptable group without a reasonable explanation may simply be attributed Type I error (p = 0.011).
3.2.2. Maximum Gap between Roots
There was no significant difference in the maximum gap between regenerated roots among LRCs within any species. The maximum gap was an acceptable 90 degrees or less in only eight of the 25 LRCs across all species. The maximum gap was greater than 120 degrees needed for good stability in 13 of 25 LRCs across all species.
Regenerated roots alone may not have the potential develop into acceptable root flares, as well. Similar to lateral roots, the number of regenerated roots was sufficient to form an acceptable root flare in all but Kentucky coffeetree, but the uneven radial distribution left a gap too large for good stability (greater than 120 degrees) in approximately half of the adventitious root flares (Table 1).
3.3. All Roots
Though the data shows that lateral roots, or roots regenerated from the cut end of the main root may not be able to form an acceptable root flare alone. Young field-grown nursery trees could often have both lateral and regenerated roots forming the root flare. In this study, both types of roots together provided a sufficient number of roots (7.3–21.2), and the maximum gap is an acceptable 120 degrees, or less, in all but the sugar maple inferior LRC at slightly larger 150 degrees. The gap is the preferred 90 degrees or less in all but six of the 25 LRCs across all species.
The maximum gap among LRCs within species when all roots were considered together was different among LRCs only in honeylocust, where the inferior group gap was greater than the other two. Though significantly different, the inferior LRC gap was still an acceptable 120 degrees.
3.4. Seedlings without Lateral Roots
The data from this study suggest that persisting lateral roots develop from seedling roots ≥2 mm diameter. The seedlings used in this study were selected because they did have roots of this size. Seedlings without woody lateral roots on the portion of the main root remaining after pruning are not rejected as part of normal nursery procedures. The regenerated root data considered in this study suggest that regenerated roots alone are unlikely to reliably produce a root flare without large gaps between roots, if the seedling has no lateral roots when replanted. To further investigate whether regenerated roots alone are insufficient to form an acceptable root flare, a direct comparison of all roots (seedlings included did have at least four lateral roots ≥2 mm) and regenerated roots only (used to represent seedlings that have no lateral roots) of plants one year after replanting was performed.
There was no difference in maximum gap among LRCs in regenerated roots, or all roots, of any species, with the exception of all roots of honeylocust (Table 1). Though the inferior LRC in this species had a significantly greater maximum gap that the other two SLRCs, the inferior gap was still acceptable at 120 degrees. Based on this, there would be no reason to recommend grading seedlings based on maximum gap between woody roots, and root data from all three LRCs were combined for this comparison.
The maximum gap was significantly greater for regenerated roots only in all species except hedge maple and European black alder (Table 3). The lack of difference in these the two species is likely because they had the greatest number of regenerated roots (Table 1), and as a result has the smallest gap with only regenerated roots. The maximum gap of all roots is 90 degrees or less (preferred) in seven of eight species. Kentucky coffeetree is slightly larger at 120 degrees but still acceptable.

Table 3.
Comparison of the maximum gap between roots of regenerated roots alone, and both lateral and regenerated roots.
Though the regenerated root maximum gap is significantly larger than the maximum gap for all roots in most species, the maximum gap of regenerated roots only exceeds the acceptable 120 degrees in three of eight species (Table 3). The difference was only marginally greater in sugar maple at 135 degrees. This data suggest that regenerated root development may be a species characteristic. There may be a reason to discard seedlings without lateral roots after root pruning of these species with larger gaps in regenerated roots, such as red maple and Kentucky coffeetree. This could help to avoid producing plants with too large a gap between roots, but further study is needed before making such a recommendation.
4. Conclusions
Field nursery production systems include seedling transplanting and root pruning, and potential alteration of young tree root system architecture. The number of lateral roots remaining above the taproot pruning cut, or regenerated roots alone, were generally inadequate to form an acceptable root flare one year after seedlings were replanted, but both alone left a large gap between roots that could make the tree unstable (120 degrees or greater). Roots regenerated from the pruned end of the main root, and lateral roots present as a seedling, together produced enough flare roots with preferred or acceptable maximum gaps in the radial distribution of roots for good stability one year after replanting (Table 1).
Strict grading of seedlings based on the configuration of woody lateral roots does not seem to be a way to ensure that there will be lateral roots to form a root flare without large gaps between flare roots as the plant grows. Similarly, relying solely on the adventitious root flare formed by the roots regenerated from the cut end of the main root also resulted in a larger than acceptable maximum gap between roots in about half of the seedlings one year later. Both lateral roots and regenerated roots together are necessary to reliably form enough structural roots with a small enough maximum gap to form an acceptable root flare on root pruned nursery stock. Caution is warranted in moving seedlings without lateral roots after root pruning into later stages of production. The results of this study, along with future research have the potential to be used in improving seedling grading systems that will produce better root systems of nursery stock grown for planting in urban landscapes.
When root systems of young trees are left to develop undisturbed in nature, very few would meet the same standards as nursery produced root systems at a similar age [22]. Natural selection works over time to eliminate large numbers of inferior trees in the forest that are unable to compete with more vigorous ones. Nursery production systems appear to produce root systems that are of more consistent quality.
Author Contributions
Conceptualization G.W.W. and A.M.H.; methodology, G.W.W. and A.M.H.; formal analysis, G.W.W.; investigation G.W.W. and A.M.H.; resources, G.W.W. and A.M.H.; data curation, G.W.W. and A.M.H.; writing—original draft preparation, G.W.W.; writing—review and editing, G.W.W. and A.M.H.; visualization, G.W.W.; supervision, G.W.W.; and project administration, G.W.W. All authors have read and agreed to the published version of the manuscript.
Funding
Partial funding for this research was received through a grant from the J. Frank Schmidt Family Charitable Foundation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coutts, M.P. Development of the structural root system of sitka spruce. Forestry 1983, 56, 1–16. [Google Scholar] [CrossRef]
- Watson, G.; Hewitt, A. Changes in tree root architecture resulting from field nursery production practices. J. Environ. Hortic. 2020, 38, 22–28. [Google Scholar] [CrossRef]
- Perry, T.O. Tree roots: Facts and fallacies. Arnoldia 1989, 49, 1–21. [Google Scholar]
- Day, S.D.; Wiseman, P.E.; Dickinson, S.B.; Harris, J.R. Contemporary concepts of root system architecture of urban trees. Arboric. Urban. For. 2010, 36, 149–159. [Google Scholar]
- Smiley, E.T. Root pruning and stability of young willow oak. Arboric. Urban. For. 2008, 34, 123–128. [Google Scholar]
- Wilson, B.F. Structure and Growth of Woody Roots of Acer rubrum L.; Harvard University: Petersham, MA, USA, 1964; p. 14. [Google Scholar]
- Ghani, M.A.; Stokes, A.; Fourcaud, T. The effect of root architecture and root loss through trenching on the anchorage of tropical urban trees (Eugenia grandis Wight). Trees: Struc. Func. 2009, 23, 197–209. [Google Scholar] [CrossRef]
- Henwood, K. A structural model of forces in buttressed tropical rain forest trees. Biotropica 1973, 5, 83–93. [Google Scholar] [CrossRef]
- Kormanik, P.P. Lateral root morphology as an expression of sweetgum seedling quality. For. Sci. 1986, 32, 595–604. [Google Scholar] [CrossRef]
- Ruehle, J.L.; Kormanik, P.P. Lateral Root Morphology: A Potential Indicator of Seedling Quality in Northern Red Oak; USDA, Forest Service, Southeastern Forest Experiment Station: Asheville, NC, USA, 1986; p. 6. [Google Scholar]
- Kormanik, P.P. Frequency distribution of first-order lateral roots in forest tree seedlings: Silvicultural implications. In Proceedings of 5th Biennial Southern Silvicultural Research Conference; Miller, J.H., Ed.; USDA, Forest Service Southern Forest Experiment Station: New Orleans, LA, USA, 1989; pp. 101–105. [Google Scholar]
- Kormanik, P.P.; Ruehle, J.L.; Muse, H.D. Frequency Distribution of Lateral Roots of 1-0 Bare-Root White Oak Seedlings; USDA Forest Service Southeastern Forest Experiment Station: Asheville, NC, USA, 1989; p. 5. [Google Scholar]
- Schultz, R.C.; Thompson, J.R. Effect of density control and undercutting on root morphology of 1+0 bare root hardwood seedlings: Five-year field performance of rootgraded stock in the central USA. New For. 1997, 13, 301–314. [Google Scholar] [CrossRef]
- Kormanik, P.P.; Sung, S.S.; Kass, D.J.; Schlarbaum, S. Effect of Seedling Size and First-Order-Lateral Roots on Early Development of Northern Red Oak on Mesic Sites. In Proceedings of the 9th Biennial Southern Silvicultural Research Conference, Asheville, NC, USA, 17–25 February 1997; Waldrop, T.A., Ed.; USDA, Forest Service, Southeastern Forest Experiment Station: Asheville, NC, USA, 1998. [Google Scholar]
- Ponder, F., Jr. Survival and growth of planted hardwoods in harvested openings with first-order lateral root differences, root-dipping, and tree shelters. North. J. App. For. 2000, 17, 45–50. [Google Scholar] [CrossRef]
- McCormack, M.L.; Adams, T.S.; Smithwick, E.A.H.; Eissenstat, D.M. Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol. 2012, 195, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, A.; Watson, G. Bare root liner production can alter tree root architecture. J. Environ. Hortic. 2009, 27, 99–104. [Google Scholar] [CrossRef]
- Warren, K. Tree Root Characteristics and Growth from the Nursery Perspective. In The Landscape below Ground III; Watson, G., Costello, L., Scharenbroch, B., Gilman, E., Eds.; International Society of Arboriculture: Atlanta, GA, USA, 2009. [Google Scholar]
- Caliskan, S. Germination and seedling growth of holm oak (Quercus ilex L.): Effects of provenance, temperature, and radicle pruning. iForest-Biogeosci. For. 2013, 7, 103–109. [Google Scholar] [CrossRef]
- Tilki, F.; Unal Alptekin, C. Germination and seedling growth of Quercus vulcanica effects of stratification, desiccation, radicle pruning, and season of sowing. New For. 2006, 32, 243–251. [Google Scholar] [CrossRef]
- Insley, H.; Buckley, G.P. The influence of desiccation and root pruning on the survival and growth of broadleaved seedlings. J. Hortic. Sci. 1985, 60, 377–387. [Google Scholar] [CrossRef]
- Single, J. Good Roots Matter from Day One. In The Landscape below Ground III; Watson, G., Costello, L., Scharenbroch, B., Gilman, E., Eds.; International Society of Arboriculture: Atlanta, GA, USA, 2009. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).