Competition, Climate, and Size Effects on Radial Growth in an Old-Growth Hemlock Forest
Abstract
1. Introduction
Response to Competition and Climate in Northern Hardwood Forests
2. Materials and Methods
Statistical Modeling
3. Results
3.1. Climate Trends
3.2. Climate Effects on Radial Growth Analyzed with Linear and Likelihood Models
3.3. Size and Competition Effects
4. Discussion
4.1. Competition
4.2. Size Effects on Growth
4.3. Climate and Growth
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aakala, T.; Fraver, S.; D’Amato, A.W.; Palik, B.J. Influence of competition and age on tree growth in structurally complex old-growth forests in northern Minnesota. For. Ecol. Manag. 2013, 308, 128–135. [Google Scholar] [CrossRef]
- Fraver, S.; D’Amato, A.W.; Bradford, J.B.; Jonsson, B.G.; Jönsson, M.; Esseen, P.-A. Tree growth and competition in an old-growth Picea abies forest of boreal Sweden: Influence of tree spatial patterning. J. Veg. Sci. 2013, 25, 374–385. [Google Scholar] [CrossRef]
- Das, A.J. The effect of size and competition on tree growth rate in old-growth coniferous forests. Can. J. For. Res. 2012, 42, 1983–1995. [Google Scholar] [CrossRef]
- Ford, K.R.; Breckheimer, I.K.; Franklin, J.F.; Freund, J.A.; Kroiss, S.J.; Larson, A.J.; Theobald, E.J.; Hille Ris Lambers, J. Competition alters tree growth responses to climate at individual and stand scales. Can. J. For. Res. 2017, 47, 53–62. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Linares, J.C.; Camarero, J.J.; Madriga-Gonzalez, J.; Hevia, A.; Sanchez-Miranda, A.; Ballesteros-Canovas, J.A.; Alfaro-Sanchez, C.; Garcia-Cervignon, A.K.; Bigler, C.; et al. Disentangling the effects of competition and climate on individual tree growth: A retrospective and dynamic approach in Scots pine. For. Ecol. Manag. 2015, 358, 12–25. [Google Scholar] [CrossRef]
- Clark, J.S.; Bell, D.M.; Hersh, M.H.; Nichols, L. Climate change vulnerability of forest biodiversity: Climate and competition tracking of demographic rates. Glob. Chang. Biol. 2011, 17, 1834–1849. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Garcia-Valdes, R.; Ruiz-Benito, P.; Zavala, M.A. Disentangling the relative importance of climate, size, and competition on tree growth in Iberian forests: Implications for forest management under global change. Glob. Chang. Biol. 2011, 17, 2400–2414. [Google Scholar] [CrossRef]
- Cescatti, A.; Piutti, E. Silvicultural alternatives, competition regime and sensitivity to climate in a European beech forest. For. Ecol. Manag. 1998, 102, 213–223. [Google Scholar] [CrossRef]
- Misson, L.; Vincke, C.; Devillez, F. Frequency responses of radial growth series after different thinning intensities in Norway spruce (Picea abies (L.) Karst.) stands. For. Ecol. Manag. 2003, 177, 51–63. [Google Scholar] [CrossRef]
- Bottero, A.; D’Amato, A.W.; Palik, B.J.; Bradford, J.B.; Fraver, S.; Battaglia, M.A.; Asherin, L.A. Density-dependent vulnerability of forest ecosystems to drought. J. Appl. Ecol. 2016, 54, 1605–1614. [Google Scholar] [CrossRef]
- Gleason, K.E.; Bradford, J.; Bottero, A.; D’Amato, A.W.; Fraver, S.; Palik, B.; Battaglia, M.A.; Iverson, L.R.; Kenefic, L.; Kern, C.C. Competition amplifies drought stress in forests across broad climatic and compositional gradients. Ecosphere 2017, 8, E01849. [Google Scholar] [CrossRef]
- Baker, F.S. A revised tolerance table. J. For. 1949, 47, 179–181. [Google Scholar] [CrossRef]
- Duchesne, L.; Prévost, M. Canopy disturbance and intertree competition: Implications for tree growth and recruitment in two yellow birch–conifer stands in Quebec, Canada. J. For. Res. 2013, 18, 168–178. [Google Scholar] [CrossRef]
- Canham, C.; Murphy, L. The demography of tree species response to climate: Sapling and canopy tree growth. Ecosphere 2016, 7. [Google Scholar] [CrossRef]
- Goldberg, D.E.; Fleetwood, L. Competitive effect and response in four annual plants. J. Ecol. 1987, 75, 1131–1143. [Google Scholar] [CrossRef]
- Tardif, J.; Brisson, J.; Bergeron, Y. Dendroclimatic analysis of Acer saccharum, Fagus grandifolia, and Tsuga canadensis from an old-growth forest, southwestern Quebec. Can. J. For. Res. 2001, 31, 1491–1501. [Google Scholar] [CrossRef]
- Bourque, C.P.-A.; Cox, R.M.; Allen, D.J.; Arp, P.A.; Meng, F.-R. Spatial extent of winter thaw events in eastern North America: Historical weather records in relation to yellow birch decline. Glob. Chang. Biol. 2005, 11, 1477–1492. [Google Scholar] [CrossRef]
- Lane, C.J.; Reed, D.D.; Mroz, G.D.; Liechty, H.O. Width of sugar maple (Acer saccharum) tree rings as affected by climate. Can. J. For. Res. 1993, 23, 2370–2375. [Google Scholar] [CrossRef]
- Oswald, E.M.; Pontius, J.; Rayback, S.A.; Schaberg, P.G.; Wilmot, S.H.; Dupigny-Giroux, L.-A. The complex relationship between climate and sugar maple health: Climate change implications in Vermont for a key northern hardwood species. For. Ecol. Manag. 2018, 422, 303–312. [Google Scholar] [CrossRef]
- Huntington, T.G.; Richardson, A.D.; McGuire, K.J.; Hayhoe, K. Climate and hydrological changes in the northeastern United States: Recent trends and implications for forested and aquatic ecosystems. Can. J. For. Res. 2009, 39, 199–212. [Google Scholar] [CrossRef]
- Tang, G.; Beckage, B. Projecting the distribution of forests in New England in response to climate change. Divers. Distrib. 2010, 16, 144–158. [Google Scholar] [CrossRef]
- Hayhoe, K.; Wake, C.P.; Andersen, B.T.; Liang, X.-Z.; Maurer, E.P.; Zhu, J.; Bradbury, J.A.; DeGaetano, A.T.; Stoner, A.M.; Wuebbles, D.J. Regional climate change projections for the Northeast USA. Mitig. Adapt. Strateg. Glob. Chang. 2008, 13, 425–436. [Google Scholar] [CrossRef]
- Frumhoff, P.C.; McCarthy, J.; Melillo, J.M.; Moser, S.C.; Wuebbles, D.J. Confronting Climate Change in the U.S. Northeast: Science, Impacts, Solutions; Union of Concerned Scientists: Cambridge, MA, USA, 2007. [Google Scholar]
- Iverson, L.R.; Prasad, A.M.; Matthews, S.N.; Peters, M. Estimating potential habitat for 134 US tree species under six climate scenarios. For. Ecol. Manag. 2008, 254, 390–406. [Google Scholar] [CrossRef]
- Russell, N.R. Natural forests of the Edmund Niles Huyck Preserve, New York. Proc. Iowa Acad. Sci. 1995, 62, 231–244. [Google Scholar]
- Odum, E.P. The vegetation of the Edmund Niles Huyck Preserve, New York. Am. Midl. Nat. 1943, 29, 72–88. [Google Scholar] [CrossRef]
- Brown, J.H. Soil Survey of Albany County, New York; USDA Soil Conservation Service: Washington, DC, USA, 1992. [Google Scholar]
- Bigelow, S.W.; Canham, C.D. Community organization of tree species along soil gradients in a north-eastern USA forest. J. Ecol. 2002, 90, 188–200. [Google Scholar] [CrossRef]
- Runkle, J.R. Twenty-four years of change in an old Tsuga canadensis woods affected by beech bark disease. J. Torrey Bot. Soc. 2005, 132, 483–491. [Google Scholar] [CrossRef]
- Runkle, J.R. Eight years change in an old Tsuga canadensis woods affected by beech bark disease. Bull. Torrey Bot. Club 1990, 117, 409–419. [Google Scholar] [CrossRef]
- Garcia, O. A generic approach to spatial individual-based modelling and simulation of plant communities. Math. Comput. For. Nat. Resour. Sci. 2014, 6, 36–47. [Google Scholar]
- Bunn, A.G. Statistical and visual crossdating in R using the dplR library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- Oyler, J.W.; Ballantyne, A.; Jencso, K.; Sweet, M.; Running, S.W. Creating a topoclimatic daily air temperature dataset for the conterminous United States using homogenized station data and remotely sensed land skin temperature. Int. J. Climatol. 2015, 35, 2258–2279. [Google Scholar] [CrossRef]
- Livneh, B.; Rosenberg, A.; Lin, C.; Nijssen, B.; Mishra, V.; Andreadis, K.M.; Maurer, E.P.; Lettenmaier, D.P. A long-term hydrologically based dataset of land surface fluxes and states for the conterminous United States: Update and extension. J. Clim. 2013, 26, 9384–9392. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Grömping, U. Relative importance for linear regression in R: The package relaimpo. J. Stat. Softw. 2006, 17, 1–27. [Google Scholar] [CrossRef]
- Canham, C.D.; Papaik, M.J.; Uriarte, M.; McWilliams, W.H.; Jenkins, J.C.; Twery, M.J. Neighborhood analyses of canopy tree competition along environmental gradients in New England forests. Ecol. Appl. 2006, 16, 540–554. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Simard, S.W.; Sachs, D.L.; Vyse, A.; Blevins, L.L. Paper birch competitive effects vary with conifer tree species and stand age in interior British Columbia forests: Implications for reforestation policy and practice. For. Ecol. Manag. 2004, 198, 55–74. [Google Scholar] [CrossRef]
- Boivin, F.; Paquette, A.; Papaik, M.J.; Thiffault, N.; Messier, C. Do position and species identity of neighbours matter in 8–15-year-old post harvest mesic stands in the boreal mixedwood? For. Ecol. Manag. 2010, 260, 1124–1131. [Google Scholar] [CrossRef]
- Ducey, M.J. Evergreenness and wood density predict height–diameter scaling in trees of the northeastern United States. For. Ecol. Manag. 2012, 279, 21–26. [Google Scholar] [CrossRef]
- Tripler, C.E.; Canham, C.D.; Inouye, R.S.; Schnurr, J.L. Soil nitrogen availability, plant luxury consumption, and herbivory by white-tailed deer. Oecologia 2002, 133, 517–524. [Google Scholar] [CrossRef]
- Catovsky, S.; Kobe, R.K.; Bazzaz, F.A. Nitrogen-induced changes in seedling regeneration and dynamics of mixed conifer-broad-leaved forests. Ecol. Appl. 2002, 12, 1611–1625. [Google Scholar] [CrossRef]
- Bigelow, S.W.; Canham, C.D. Nutrient limitation of juvenile trees in a northern hardwood forest: Calcium and nitrate are preeminent. For. Ecol. Manag. 2007, 243, 310–319. [Google Scholar] [CrossRef]
- Huston, M.; Smith, T. Plant succession: Life history and competition. Am. Nat. 1987, 130, 168–198. [Google Scholar] [CrossRef]
- Goerlich, D.L.; Nyland, R.D. Natural Regeneration of Eastern Hemlock: A Review; GTR-NE-267; USDA-Forest Service, Northeastern Forest Experiment Station: Newtown Square, PA, USA, 2000; pp. 14–23. [Google Scholar]
- Long, R.P.; Horsley, S.B.; Hallett, R.A.; Bailey, S.W. Sugar maple growth in relation to nutrition and stress in the northeastern United States. Ecol. Appl. 2009, 19, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Canham, C.D. Growth and canopy architecture of shade-tolerant trees: Response to canopy gaps. Ecology 1988, 69, 786–795. [Google Scholar] [CrossRef]
- Woods, K.D. Dynamics in late-successional hemlock–hardwood forests over three decades. Ecology 2000, 81, 110–126. [Google Scholar] [CrossRef]
- Teets, A.; Fraver, S.; Weiskittel, A.R.; Hollinger, D.Y. Quantifying climate–growth relationships at the stand level in a mature mixed-species conifer forest. Glob. Chang. Biol. 2018, 24, 3587–3602. [Google Scholar] [CrossRef] [PubMed]
- D’Arrigo, R.D.; Schuster, W.S.F. Climate-growth relationships of eastern hemlock and Chestnut Oak from Black Rock Forest in the Highlands of Southeastern New York. Tree-Ring Res. 2001, 57, 183–190. [Google Scholar]
- Paradis, A.; Elkington, J.; Hayhoe, K.; Buonaccorsi, J. Role of winter temperature and climate change on the survival and future range expansion of the hemlock woolly adelgid (Adelges tsugae) in eastern North America. Mitig. Adapt. Strateg. Glob. Chang. 2008, 13, 541–554. [Google Scholar] [CrossRef]
- Zhu, X.B.; Cox, R.M.; Bourque, C.P.-A.; Arp, P.A. Thaw effects on cold-hardiness parameters in yellow birch. Can. J. Bot. 2002, 2002, 390–398. [Google Scholar] [CrossRef]
- Comerford, D.P.; Schaberg, P.G.; Templer, P.H.; Socci, A.M.; Campbell, J.L.; Wallin, K.F. Influence of experimental snow removal on root and canopy physiology of sugar maple trees in a northern hardwood forest. Oecologia 2013, 171, 261–269. [Google Scholar] [CrossRef]
- Reinmann, A.B.; Susser, J.R.; Demaria, E.M.C.; Templer, P.H. Declines in northern forest tree growth following snowpack decline and soil freezing. Glob. Chang. Biol. 2019, 25, 420–430. [Google Scholar] [CrossRef]
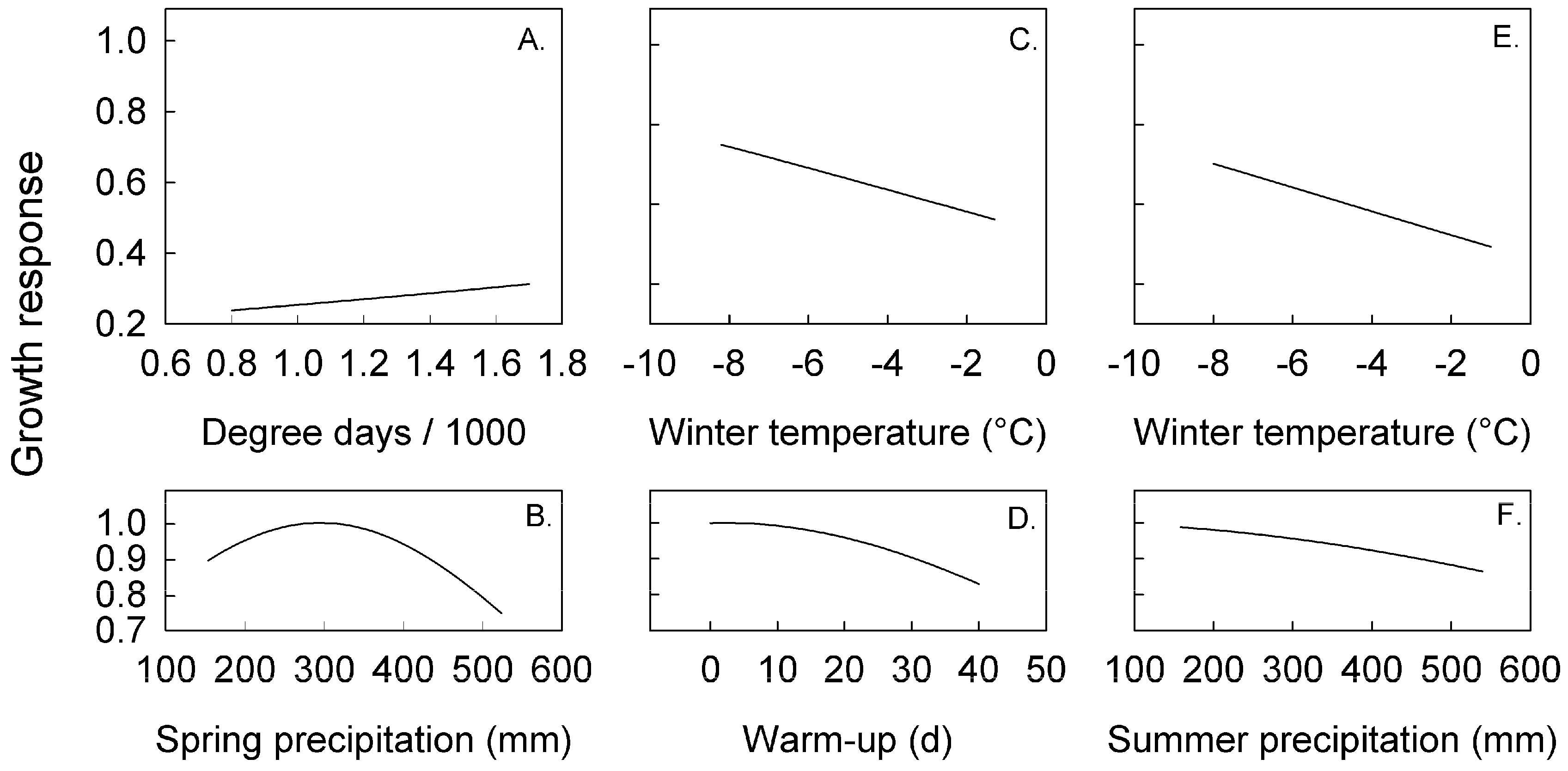
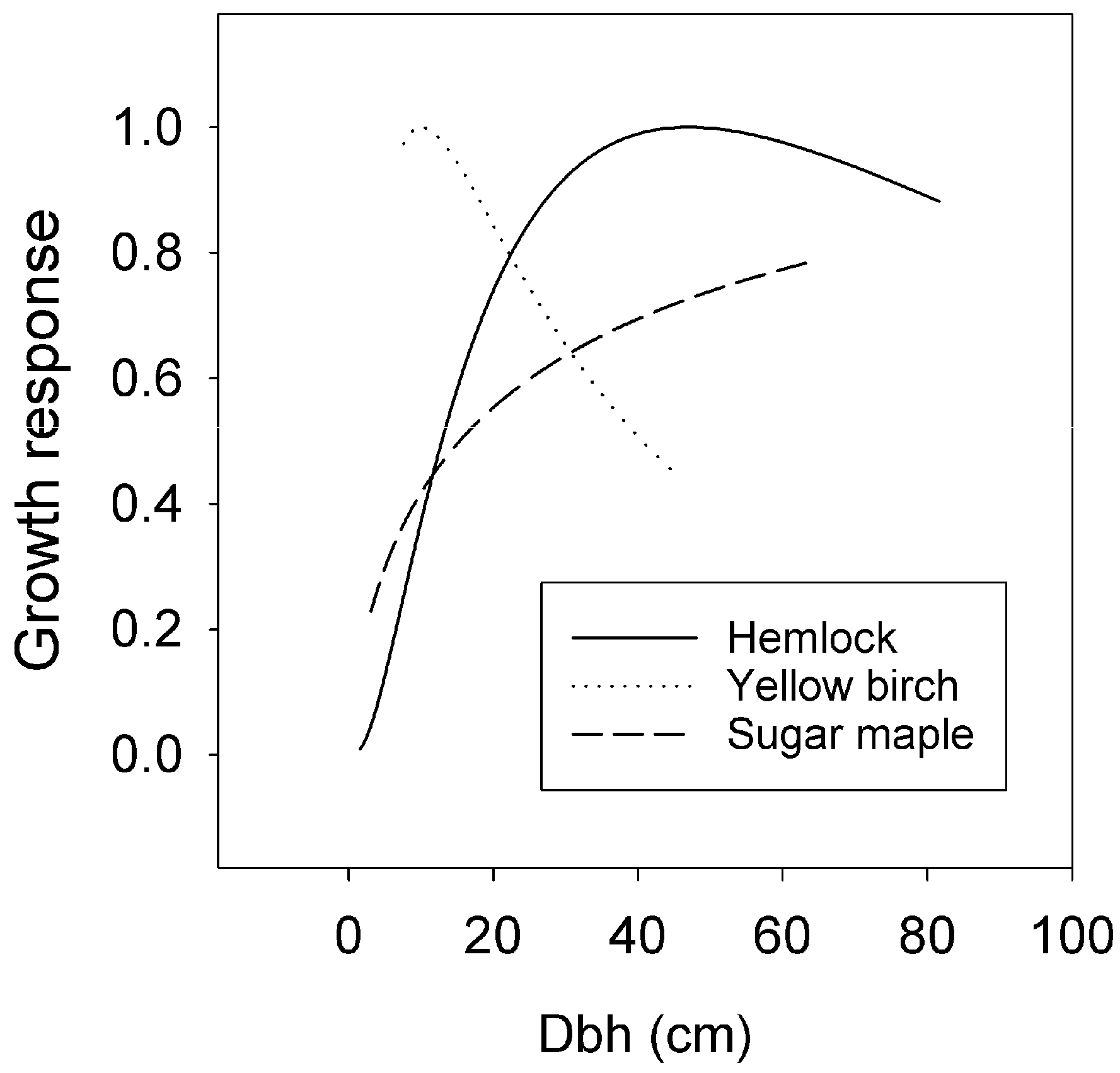
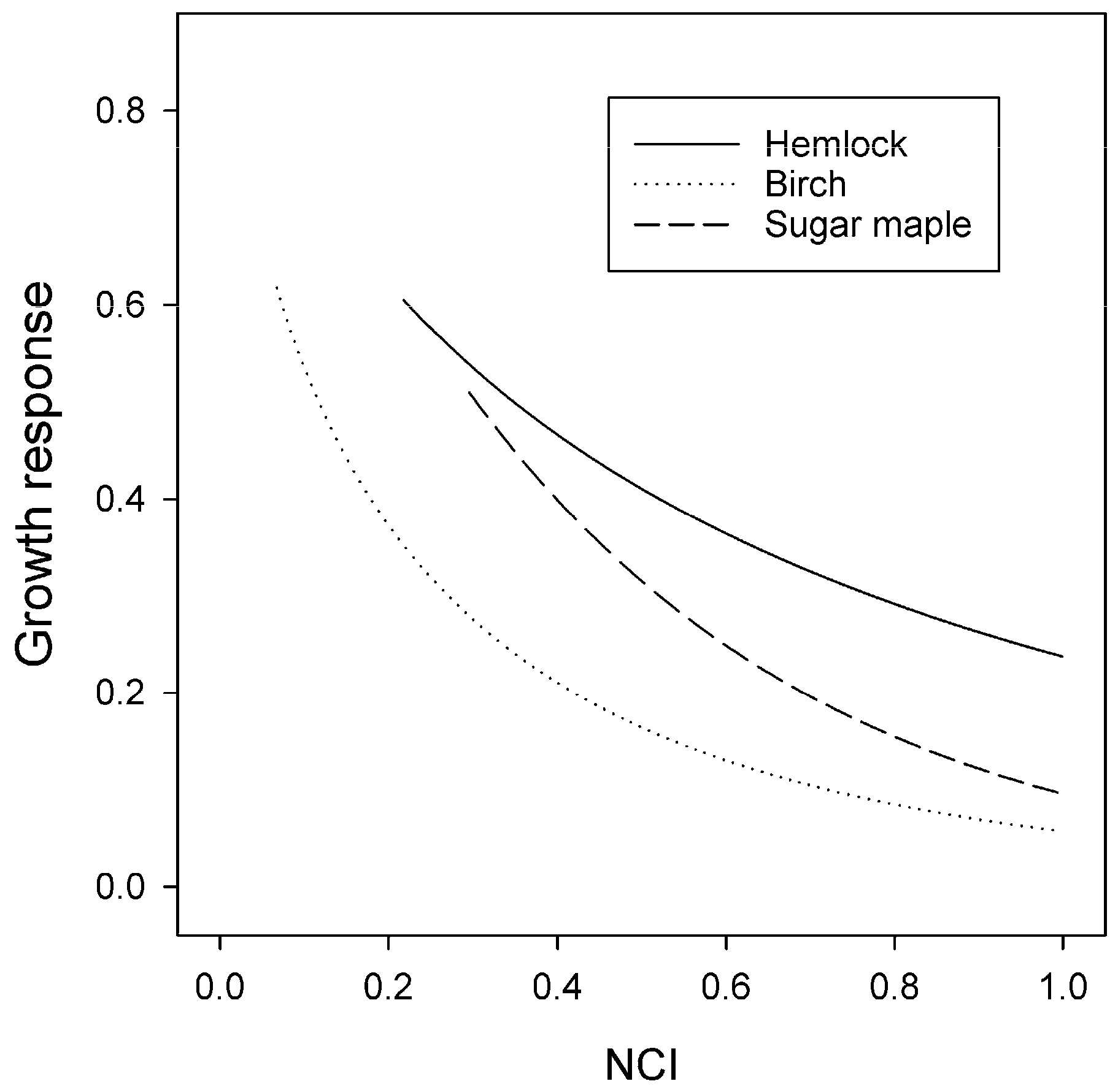
| Climate Index | Mean (SD) | Unit | Hemlock * | Birch | Sugar Maple |
|---|---|---|---|---|---|
| Winter temperature | −5.2 (1.7) | °C | 0.052 (+/−) | 0.058 (−) | 0.061 (−) |
| Snow | 171 (65) | inches | 0.030 (−) | 0.006 (+/−) | 0.005 (+/−) |
| Warm-up | 22.5 (6.1) | days | 0.024 (+/−) | 0.124 (−) | 0.010 (−) |
| Max. 3-day temperature | 30.4 (1.3) | °C | 0.020 (+/−) | 0.026 (−) | 0.060 (−) |
| Temperature extremes | 1.8 (2.5) | days | 0.025 (+) | 0.007 (−) | 0.043 (−) |
| Degree-days | 1224 (206) | °C days | 0.133 (+) | 0.035 (−/+) | 0.090 (−) |
| Thaws | 8.6 (5.4) | days | 0.048 (+/−) | 0.047 (−) | 0.024 (−) |
| Hardening | 7.0 (3.9) | days | 0.027 (−) | 0.003 (+) | 0.035 (+) |
| Spring precipitation | 301 (86) | mm | 0.052 (−) | 0.004 (−) | 0.003 (+/−) |
| Summer precipitation | 309 (85) | mm | 0.006 (+) | 0.006 (+/−) | 0.138 (−) |
| Spring temperature | 11.9 (2) | °C | 0.055 (+/−) | 0.062 (+) | 0.015 (+/−) |
| Summer temperature | 21.2 (2) | °C | 0.014 (+/−) | 0.027 (−) | 0.033 (−) |
| Sum (=r2) | 0.48 | 0.40 | 0.52 |
| Hemlock | Yellow Birch | Sugar Maple | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model * | K† | AICc‡ | Δi§ | R2|| | AICc | Δi | R2 | AICc | Δi | R2 |
| pg | 2 | 4535.6 | 955.4 | 0.00 | 2858.3 | 393.1 | 0.00 | 3294.9 | 514.9 | 0.00 |
| pg × se | 4 | 3841.0 | 254.4 | 0.24 | 2856.7 | 391.5 | 0.00 | 3130.3 | 350.4 | 0.07 |
| pg × ce | 6 | 4481.1 | 900.4 | 0.02 | 2842.8 | 377.6 | 0.01 | 3268.5 | 488.5 | 0.02 |
| pg × ne | 8 | 4280.2 | 632.9 | 0.10 | 2524.4 | 59.2 | 0.19 | 2985.8 | 205.9 | 0.17 |
| pg × se·ce | 8 | 3771.1 | 190.5 | 0.26 | 2841.0 | 375.8 | 0.01 | 3062.9 | 283.0 | 0.10 |
| pg × se·ne | 10 | 3691.4 | 104.8 | 0.29 | 2478.7 | 13.5 | 0.22 | 2802.6 | 22.7 | 0.24 |
| pg × ce·ne | 12 | 4126.5 | 545.9 | 0.16 | 2500.8 | 35.6 | 0.21 | 2934.1 | 154.1 | 0.20 |
| pg × se × ce × ne | 14 | 3580.6 | 0 | 0.32 | 2448.7 | 0 | 0.24 | 2779.9 | 0 | 0.25 |
| Hemlock | Yellow Birch | Sugar Maple | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model * | K† | AICc‡ | Δi§ | R2|| | AICc | Δi | R2 | AICc | Δi | R2 |
| pg | 2 | 4535.6 | 955.4 | 0.00 | 2858.3 | 393.1 | 0.00 | 3294.9 | 514.9 | 0.00 |
| pg × se | 4 | 3841.0 | 254.4 | 0.24 | 2856.7 | 391.5 | 0.00 | 3130.3 | 350.4 | 0.07 |
| pg × ce | 6 | 4481.1 | 900.4 | 0.02 | 2842.8 | 377.6 | 0.01 | 3268.5 | 488.5 | 0.02 |
| pg × ne | 8 | 4280.2 | 632.9 | 0.10 | 2524.4 | 59.2 | 0.19 | 2985.8 | 205.9 | 0.17 |
| pg × se·ce | 8 | 3771.1 | 190.5 | 0.26 | 2841.0 | 375.8 | 0.01 | 3062.9 | 283.0 | 0.10 |
| pg × se·ne | 10 | 3691.4 | 104.8 | 0.29 | 2478.7 | 13.5 | 0.22 | 2802.6 | 22.7 | 0.24 |
| pg × ce·ne | 12 | 4126.5 | 545.9 | 0.16 | 2500.8 | 35.6 | 0.21 | 2934.1 | 154.1 | 0.20 |
| pg × se × ce × ne | 14 | 3580.6 | 0 | 0.32 | 2448.7 | 0 | 0.24 | 2779.9 | 0 | 0.25 |
| Factor | Hemlock | Yellow Birch | Sugar Maple |
|---|---|---|---|
| Climate | 0.02 | 0.02 | 0.02 |
| Size | 0.21 | 0.01 | 0.07 |
| Competition | 0.09 | 0.21 | 0.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bigelow, S.W.; Runkle, J.R.; Oswald, E.M. Competition, Climate, and Size Effects on Radial Growth in an Old-Growth Hemlock Forest. Forests 2020, 11, 52. https://doi.org/10.3390/f11010052
Bigelow SW, Runkle JR, Oswald EM. Competition, Climate, and Size Effects on Radial Growth in an Old-Growth Hemlock Forest. Forests. 2020; 11(1):52. https://doi.org/10.3390/f11010052
Chicago/Turabian StyleBigelow, Seth W., James R. Runkle, and Evan M. Oswald. 2020. "Competition, Climate, and Size Effects on Radial Growth in an Old-Growth Hemlock Forest" Forests 11, no. 1: 52. https://doi.org/10.3390/f11010052
APA StyleBigelow, S. W., Runkle, J. R., & Oswald, E. M. (2020). Competition, Climate, and Size Effects on Radial Growth in an Old-Growth Hemlock Forest. Forests, 11(1), 52. https://doi.org/10.3390/f11010052





