Tree Growth and Water-Use Efficiency Do Not React in the Short Term to Artificially Increased Nitrogen Deposition
Abstract
1. Introduction
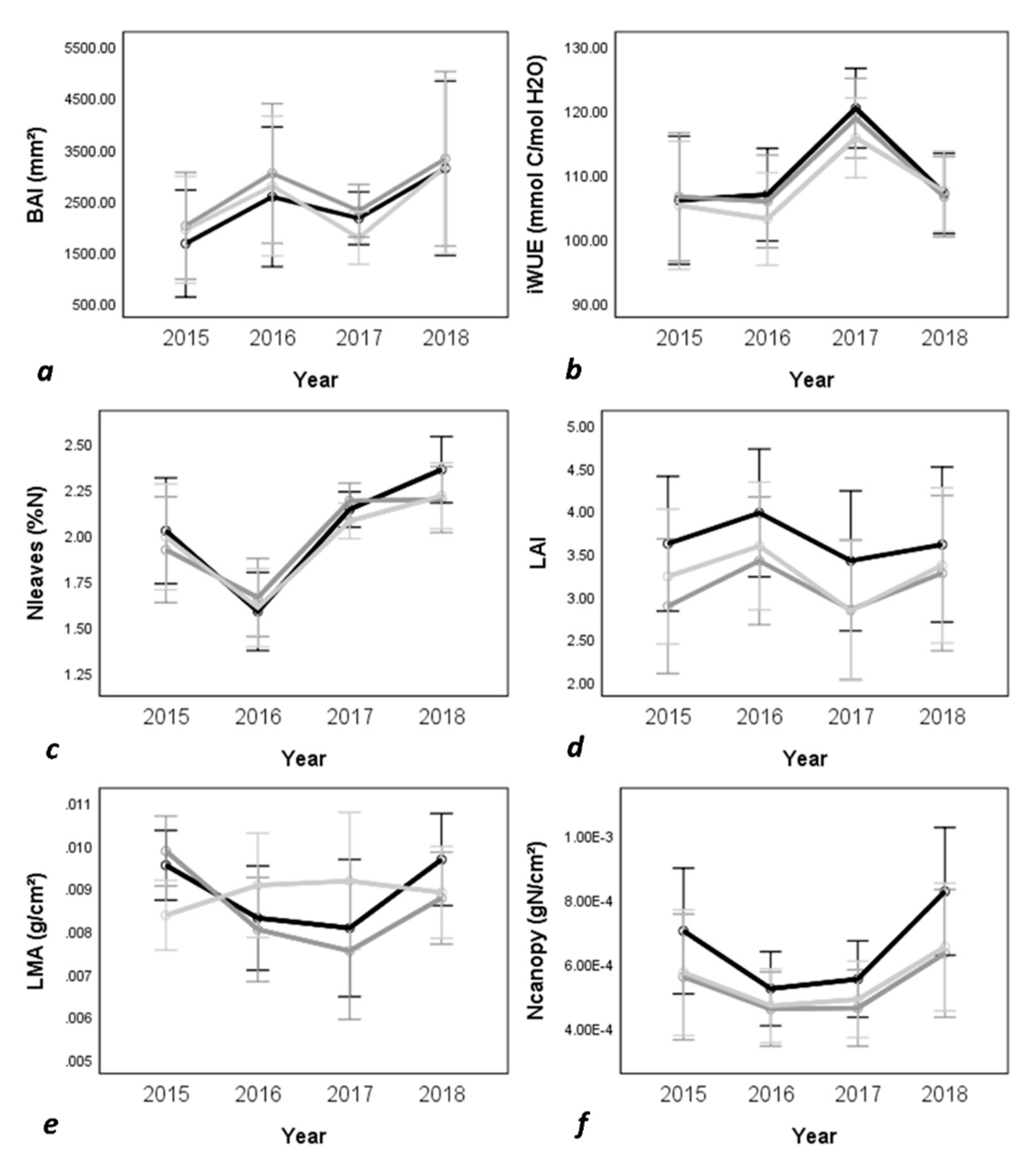
2. Results
3. Discussion
4. Material and Methods
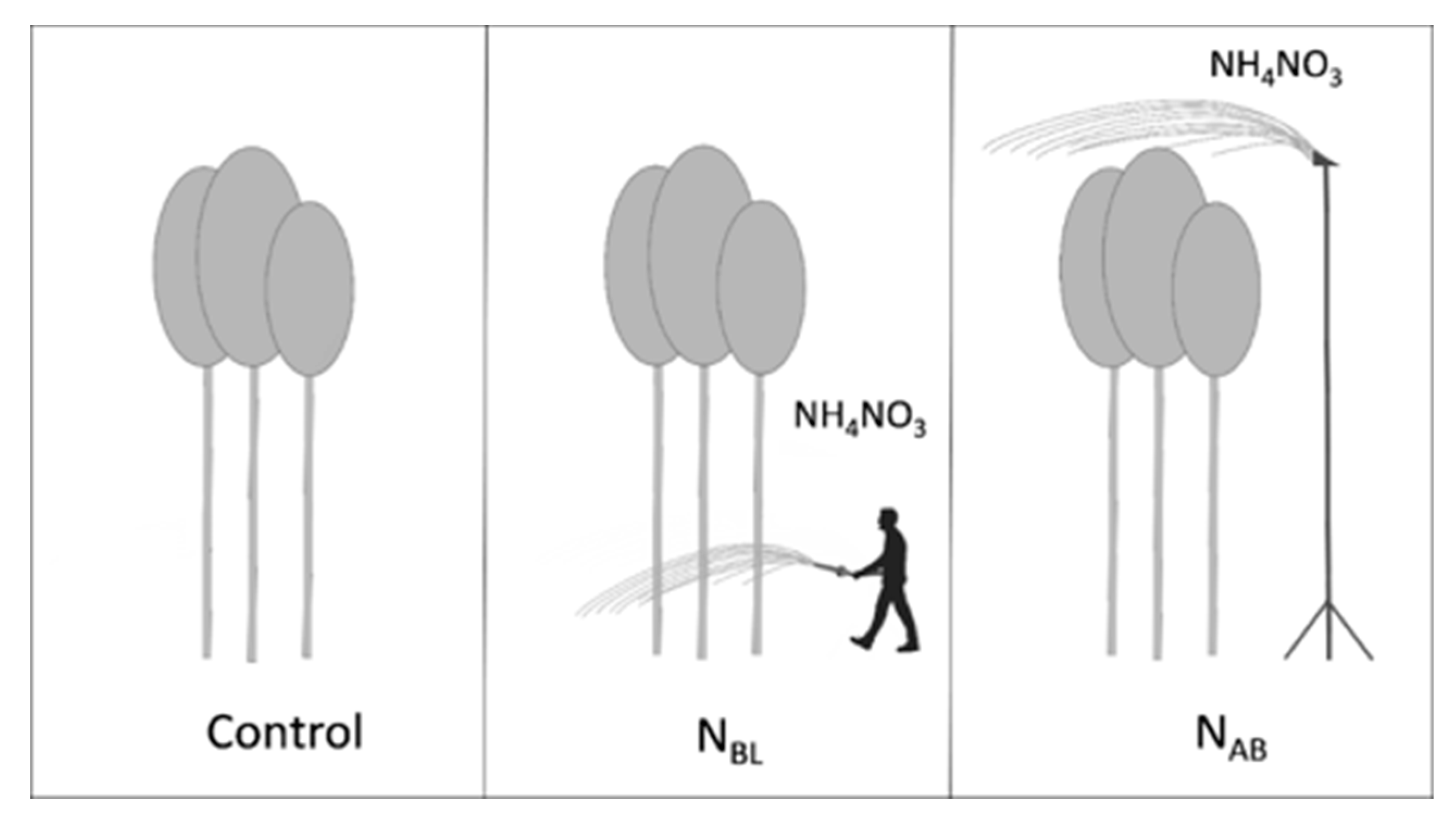
4.1. Study Site and Experimental Design
4.2. Tree Growth
4.3. iWUE and Leaves Parameters Data Collection
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hyvönen, R.; Agren, G.I.; Linder, S.; Persson, T.; Cotrufo, M.F.; Ekblad, A.; Freeman, M.; Grelle, A.; Janssens, I.A.; Jarvis, P.G.; et al. The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: A literature review. New Phytol. 2007, 173, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Core Writing Team; Pachauri, R.K.; Meyer, L.A. (Eds.) Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Gagen, M.; Finsinger, W.; Wagner-Cremer, F.; Mccarroll, D.; Loader, N.J.; Robertson, I.; Jalkanen, R.; Young, G.; Kirchhefer, A. Evidence of changing intrinsic water-use efficiency under rising atmospheric CO2 concentrations in Boreal Fennoscandia from subfossil leaves and tree ring δ13C ratios. Glob. Chang. Biol. 2011, 17, 1064–1072. [Google Scholar] [CrossRef]
- Leonardi, S.; Gentilesca, T.; Guerrieri, R.; Ripullone, F.; Magnani, F.; Mencuccini, M.; Noije, T.V.; Borghetti, M. Assessing the effects of nitrogen deposition and climate on carbon isotope discrimination and intrinsic water-use efficiency of angiosperm and conifer trees under rising CO 2 conditions. Glob. Chang. Biol. 2012, 18, 2925–2944. [Google Scholar] [CrossRef] [PubMed]
- Giammarchi, F.; Cherubini, P.; Pretzsch, H.; Tonon, G. The increase of atmospheric CO2affects growth potential and intrinsic water-use efficiency of Norway spruce forests: Insights from a multi-stable isotope analysis in tree rings of two Alpine chronosequences. Trees-Struct. Funct. 2017, 31, 503–515. [Google Scholar] [CrossRef]
- Andreu-Hayles, L.; Planells, O.; Gutiérrez, E.; Muntan, E.; Helle, G.; Anchukaitis, K.J.; Schleser, G.H. Long tree-ring chronologies reveal 20th century increases in water-use efficiency but no enhancement of tree growth at five Iberian pine forests. Glob. Chang. Biol. 2011, 17, 2095–2112. [Google Scholar] [CrossRef]
- Peñuelas, J.; Canadell, J.G.; Ogaya, R. Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob. Ecol. Biogeogr. 2011, 20, 597–608. [Google Scholar] [CrossRef]
- Galloway, J.N. The global nitrogen cycle: Past, present and future. Sci. China Ser. C Life Sci. Chin. Acad. Sci. 2005, 48, 669–677. [Google Scholar]
- Magnani, F.; Mencuccini, M.; Borghetti, M.; Berbigier, P.; Berninger, F.; Delzon, S.; Grelle, A.; Hari, P.; Jarvis, P.G.; Kolari, P.; et al. The human footprint in the carbon cycle of temperate and boreal forests. Nature 2007, 447, 848–850. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R.; Schimel, D.S.; Fisher, H.; Howarth, R.W.; Hedin, L.O.; Perakis, S.S.; Latty, E.F.; Von Fischer, J.C.; Hlseroad, A.; et al. Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Glob. Biogeochem. Cycles 1999, 13, 623–645. [Google Scholar] [CrossRef]
- LeBauer, D.S.; Treseder, K.K. Nitrogen Limitation of Net Primary Productivity in Terrestrial Ecosystems Is Globally Distributed. Ecology 2008. [Google Scholar] [CrossRef]
- Aber, J.; McDowell, W.; Nadelhoffer, K.; Magill, A.; Berntson, G.; Kamakea, M.; McNulty, S.; Currie, W.; Rustad, L.; Fernandez, I. Nitrogen Saturation in Temperate Forest Ecosystems. Bioscience 1998, 48, 921–934. [Google Scholar] [CrossRef]
- Townsend, A.R.; Braswell, B.H.; Holland, E.A.; Penner, J.E. Spatial and Temporal Patterns in Terrestrial Carbon Storage Due to Deposition of Fossil Fuel Nitrogen. Ecol. Appl. 1996, 6, 806–814. [Google Scholar] [CrossRef]
- Sutton, M.A.; Simpson, D.; Levy, P.E.; Smith, R.I.; Reis, S.; van Oijen, M.; de Vries, W. Uncertainties in the relationship between atmospheric nitrogen deposition and forest carbon sequestration. Glob. Chang. Biol. 2008, 14, 2057–2063. [Google Scholar] [CrossRef]
- Erisman, J.W.; Van Grinsven, H.; Grizzetti, B.; Bouraoui, F.; Powlson, D.; Sutton, M.A.; Bleeker, A.; Reis, S. The European nitrogen problem in a global perspective Executive summary Major uncertainties/challenges. In The European Nitrogen Assessment; Sutton, M.A., Britton, C., Erisman, J.W., Billen, G., Bleeker, A., Greenfelt, P., van Grinsven, H., Grizzetti, B., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 9–31. [Google Scholar]
- Hyvönen, R.; Persson, T.; Andersson, S.; Olsson, B.; Ågren, G.I.; Linder, S. Impact of long-term nitrogen addition on carbon stocks in trees and soils in northern Europe. Biogeochemistry 2008, 89, 121–137. [Google Scholar] [CrossRef]
- Ewers, B.E.; Oren, R.; Sperry, J.S. Influence of nutrient versus water supply on hydraulic architecture and water balance in Pinus taeda. Plant Cell Environ. 2000, 23, 1055–1066. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S., III; Pons, T. Plant Physiological Ecology; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Ripullone, F.; Lauteri, M.; Grassi, G.; Amato, M.; Borghetti, M. Variation in nitrogen supply changes water-use efficiency of Pseudotsuga menziesii and Populus x euroamericana; a comparison of three approaches to determine water-use efficiency. Tree Physiol. 2004, 24, 671–679. [Google Scholar] [CrossRef]
- Guerrieri, R.; Mencuccini, M.; Sheppard, L.J.; Saurer, M.; Perks, M.P.; Levy, P.; Sutton, M.A.; Borghetti, M.; Grace, J. The legacy of enhanced N and S deposition as revealed by the combined analysis of δ13C, δ18O and δ15N in tree rings. Glob. Chang. Biol. 2011, 17, 1946–1962. [Google Scholar] [CrossRef]
- Betson, N.R.; Johannisson, C.; Löfvenius, M.O.; Grip, H.; Granström, A.; Högberg, P. Variation in the δ13C of foliage of Pinus sylvestris L. in relation to climate and additions of nitrogen: Analysis of a 32-year chronology. Glob. Chang. Biol. 2007, 13, 2317–2328. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, J.; Zhang, W.; Shen, W.; Zhu, S.; Cai, X.; Liu, Z.; Wang, F.; Rao, X.; Mo, J.; et al. CAN Canopy Addition of Nitrogen Better Illustrate the Effect of Atmospheric Nitrogen Deposition on Forest Ecosystem? Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Sievering, H.; Tomaszewski, T.; Torizzo, J. Canopy uptake of atmospheric N deposition at a conifer forest: Part I-canopy N budget, photosynthetic efficiency and net ecosystem exchange. Tellus Ser. B Chem. Phys. Meteorol. 2007, 59, 483–492. [Google Scholar] [CrossRef]
- Gaige, E.; Dail, D.B.; Hollinger, D.Y.; Davidson, E.A.; Fernandez, I.J.; Sievering, H.; White, A.; Halteman, W. Changes in canopy processes following whole-forest canopy nitrogen fertilization of a mature spruce-hemlock forest. Ecosystems 2007, 10, 1133–1147. [Google Scholar] [CrossRef]
- Guerrieri, R.; Vanguelova, E.I.; Michalski, G.; Heaton, T.H.E.; Mencuccini, M. Isotopic evidence for the occurrence of biological nitrification and nitrogen deposition processing in forest canopies. Glob. Chang. Biol. 2015, 21, 4613–4626. [Google Scholar] [CrossRef] [PubMed]
- Houle, D.; Marty, C.; Duchesne, L. Response of canopy nitrogen uptake to a rapid decrease in bulk nitrate deposition in two eastern Canadian boreal forests. Oecologia 2015, 177, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen Cycles: Past, Present, and Future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Cape, J.N.; Sheppard, L.J.; Crossley, A.; Van Dijk, N.; Tang, Y.S. Experimental field estimation of organic nitrogen formation in tree canopies. Environ. Pollut. 2010, 158, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, F.S.; Adams, M.B. Effects of Nitrogen on Temporal and Spatial Patterns of Nitrate in Streams and Soil Solution of a Central Hardwood Forest. ISRN Ecol. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Jennings, K.A.; Guerrieri, R.; Vadeboncoeur, M.A.; Asbjornsen, H. Response of Quercus velutina growth and water use efficiency to climate variability and nitrogen fertilization in a temperate deciduous forest in the northeastern USA. Tree Physiol. 2016, 36, 428–443. [Google Scholar] [CrossRef]
- Fleischer, K.; Rebel, K.T.; Van Der Molen, M.K.; Erisman, J.W.; Wassen, M.J.; Van Loon, E.E.; Montagnani, L.; Gough, C.M.; Herbst, M.; Janssens, I.A.; et al. The contribution of nitrogen deposition to the photosynthetic capacity of forests. Global Biogeochem. Cycles 2013, 27, 187–199. [Google Scholar] [CrossRef]
- Bobbink, A.R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Cinderby, S.; Davidson, E.; Dentener, F.; Emmett, B.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef]
- Elhani, S.; Guehl, J.M.; Nys, C.; Picard, J.F.; Dupouey, J.L. Impact of fertilization on tree-ring δ15N and δ13C in beech stands: A retrospective analysis. Tree Physiol. 2005, 25, 1437–1446. [Google Scholar] [CrossRef][Green Version]
- Huang, Z.; Liu, B.; Davis, M.; Sardans, J.; Peñuelas, J.; Billings, S. Long-term nitrogen deposition linked to reduced water use efficiency in forests with low phosphorus availability. New Phytol. 2016, 210, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Kattge, J.; Knorr, W.; Raddatz, T.; Wirth, C. Quantifying photosynthetic capacity and its relationship to leaf nitrogen content for global-scale terrestrial biosphere models. Glob. Chang. Biol. 2009, 15, 976–991. [Google Scholar] [CrossRef]
- Pardo, L.H.; Robin-Abbott, M.J.; Driscoll, C.T. Assessment of nitrogen deposition effects and empirical critical loads of nitrogen for ecoregions of the United States. Gen. Tech. Rep. NRS 2011, 80, 291. [Google Scholar]
- Cornejo-Oviedo, E.H.; Voelker, S.L.; Mainwaring, D.B.; Maguire, D.A.; Meinzer, F.C.; Brooks, J.R. Basal area growth, carbon isotope discrimination, and intrinsic water use efficiency after fertilization of Douglas-fir in the Oregon Coast Range. For. Ecol. Manag. 2017, 389, 285–295. [Google Scholar] [CrossRef]
- Magill, A.H.; Aber, J.D.; Berntson, G.M.; McDowell, W.H.; Nadelhoffer, K.J.; Melillo, J.M.; Steudler, P. Long-term nitrogen additions and nitrogen saturation in two temperate forests. Ecosystems 2000, 3, 238–253. [Google Scholar] [CrossRef]
- Pitcairn, C.E.R.; Leith, I.; Fowler, D.; Hargreaves, K.; Moghaddam, M.; Kennedy, V.; Granat, L. Foliar Nitrogen as an Indicator of Nitrogen Deposition and Critical Loads Exceedance on a European Scale. Water Air Soil Pollut. 2001, 130, 1037–1042. [Google Scholar] [CrossRef]
- Talhelm, A.F.; Pregitzer, K.S.; Burton, A.J. No evidence that chronic nitrogen additions increase photosynthesis in mature sugar maple forests. Ecol. Appl. 2011, 21, 2413–2424. [Google Scholar] [CrossRef]
- Aber, J.D.; Goodale, C.L.; Ollinger, S.V.; Smith, M.-L.; Magill, A.H.; Martin, M.E.; Hallett, R.A.; Stoddard, J.L. Is Nitrogen Deposition Altering the Nitrogen Status of Northeastern Forests? Bioscience 2003, 53, 375. [Google Scholar] [CrossRef]
- Bauer, G.A.; Bazzaz, F.A.; Minocha, R.; Long, S.; Magill, A.; Aber, J.; Berntson, G.M. Effects of chronic N additions on tissue chemistry, photosynthetic capacity, and carbon sequestration potential of a red pine (Pinus resinosa Ait.) stand in the NE United States. For. Ecol. Manag. 2004, 196, 173–186. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, P.; Zhu, L.; Zhao, X.; Ni, G.; Ouyang, L.; Schäfer, K.V.R.; Shen, W. Responses of sap flux and intrinsic water use efficiency to canopy and understory nitrogen addition in a temperate broadleaved deciduous forest. Sci. Total Environ. 2019, 648, 325–336. [Google Scholar] [CrossRef]
- Sheppard, L.J.; Coward, P.; Skiba, U.; Harvey, F.J.; Crossley, A.; Ingleby, K. Effects of five years of frequent N additions, with or without acidity, on the growth and below-ground dynamics of a young Sitka spruce stand growing on an acid peat: Implications for sustainability. Hydrol. Earth Syst. Sci. 2010, 8, 377–391. [Google Scholar] [CrossRef]
- Marchetti, F.; Tait, D.; Ambrosi, P.; Minerbi, S.; Agrario, I.; Michele, S.; Michele, S.; Tn, A. Atmospheric deposition at four forestry sites in the Alpine Region of Trentino-South Tyrol, Italy. J. Limnol. 2002, 61, 148–157. [Google Scholar] [CrossRef]
- Farquhar, G. Carbon Isotope Discrimination And Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Francey, R.J.; Allison, C.E.; Etheridge, D.M.; Trudinger, C.M.; Enting, I.G.; Leuenberger, M.; Langenfelds, R.L.; Michel, E.; Steele, L.P. A 1000-year high precision record of delta13C in atmospheric CO2. Tellus B 1999, 51, 170–193. [Google Scholar] [CrossRef]
| BAI | iWUE | LAI | LMA | Ncanopy | Nleaves | |
|---|---|---|---|---|---|---|
| BAI | 0.0333 | 0.2139 | −0.0269 | 0.2658 | 0.1880 | |
| 0.8472 | 0.2104 | 0.8674 | 0.1171 | 0.2722 | ||
| iWUE | 0.0333 | −0.3310 | −0.1426 | −0.2403 | 0.3178 | |
| 0.8472 | 0.0487 | 0.4068 | 0.1580 | 0.0589 | ||
| LAI | 0.2139 | −0.3310 | −0.2953 | 0.5895 | −0.0401 | |
| 0.2104 | 0.0487 | 0.0804 | 0.0002 | 0.8165 | ||
| LMA | −0.0269 | −0.1426 | −0.2953 | 0.3290 | 0.0746 | |
| 0.8674 | 0.4068 | 0.0804 | 0.2060 | 0.6654 | ||
| Ncanopy | 0.2658 | −0.2403 | 0.5895 | 0.3290 | 0.6114 | |
| 0.1171 | 0.1580 | 0.0002 | 0.2060 | 0.0001 | ||
| Nleaves | 0.1880 | 0.3178 | −0.0401 | 0.0746 | 0.6114 | |
| 0.2722 | 0.0589 | 0.8165 | 0.6654 | 0.0001 |
| Variable | F | p | |
|---|---|---|---|
| Treatment | BAI | 0.119 | 0.890 |
| iWUE | 0.228 | 0.803 | |
| Nleaves | 0.180 | 0.839 | |
| LAI | 1.936 | 0.225 | |
| LMA | 2.060 | 0.208 | |
| Ncanopy | 2.060 | 0.208 | |
| Year | BAI | 16.985 | 0.000 |
| iWUE | 21.986 | 0.000 | |
| Nleaves | 62.191 | 0.000 | |
| LAI | 2.867 | 0.065 | |
| LMA | 2.925 | 0.062 | |
| Ncanopy | 11.638 | 0.000 | |
| Year × Treatment | BAI | 0.358 | 0.896 |
| iWUE | 0.310 | 0.923 | |
| Nleaves | 1.097 | 0.401 | |
| LAI | 0.156 | 0.985 | |
| LMA | 2.324 | 0.077 | |
| Ncanopy | 0.406 | 0.865 |
| N Trees | G (Basal Area) | Stand Characteristics | ||||
|---|---|---|---|---|---|---|
| N/ha | % | m2/ha | % | |||
| Quercus petraea | 1125 | 95.62% | 26.969 | 95.31% | gm (m2/ha) | 0.02 |
| Tilia cordata | 20 | 1.67% | 0.260 | 0.92% | dmg (cm) | 17.50 |
| Castanea sativa | 2 | 0.21% | 0.005 | 0.02% | Hmg (m) | 13.37 |
| Ostrya carpinifolia | 12 | 1.04% | 0.147 | 0.52% | Hd (m) | 16.40 |
| Pinus sylvestris | 15 | 1.25% | 0.786 | 2.78% | V (m3/ha) | 215.47 |
| Acer spp. | 2 | 0.21% | 0.001 | 0.01% | ||
| Total | 1177 | 28.168 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giammarchi, F.; Panzacchi, P.; Ventura, M.; Tonon, G. Tree Growth and Water-Use Efficiency Do Not React in the Short Term to Artificially Increased Nitrogen Deposition. Forests 2020, 11, 47. https://doi.org/10.3390/f11010047
Giammarchi F, Panzacchi P, Ventura M, Tonon G. Tree Growth and Water-Use Efficiency Do Not React in the Short Term to Artificially Increased Nitrogen Deposition. Forests. 2020; 11(1):47. https://doi.org/10.3390/f11010047
Chicago/Turabian StyleGiammarchi, Francesco, Pietro Panzacchi, Maurizio Ventura, and Giustino Tonon. 2020. "Tree Growth and Water-Use Efficiency Do Not React in the Short Term to Artificially Increased Nitrogen Deposition" Forests 11, no. 1: 47. https://doi.org/10.3390/f11010047
APA StyleGiammarchi, F., Panzacchi, P., Ventura, M., & Tonon, G. (2020). Tree Growth and Water-Use Efficiency Do Not React in the Short Term to Artificially Increased Nitrogen Deposition. Forests, 11(1), 47. https://doi.org/10.3390/f11010047





