Abstract
Light is a major environmental factor limiting the growth and survival of plants. The heterogeneity of the light environment after gap formation in forest influences the leaf chlorophyll contents, net photosynthetic rate (Pn), and chlorophyll fluorescence, thus influencing the growth and regeneration of Castanopsis kawakamii seedlings. The aim of this study was to explore the effects of weak light on the photosynthetic physiology of C. kawakamii seedlings in forest gaps and non-gaps. The results showed that (1) the contents of chlorophyll a (Chl-a), chlorophyll b (Chl-b), and total chlorophyll (Chl-T) in forest gaps were lower than in non-gaps. Seedlings tended to increase chlorophyll content to absorb light energy to adapt to low light intensity in non-gap environments. (2) The Pn values of C. kawakamii seedlings in forest gaps were significantly higher than in non-gaps, and forest gaps could improve the seedlings’ photosynthetic capacity. (3) The C. kawakamii seedlings in forest gaps were more sensitive to weak light and control group treatment, especially the tall seedlings, indicating that seedlings require more light to satisfy their growth needs in the winter. The seedlings in non-gaps demonstrated better adaptability to low light intensity. The light intensity was not adequate in weak light conditions and limited seedling growth. We suggest that partial forest selection cutting could improve light intensity in non-gaps, thus promoting seedling growth and regeneration of C. kawakamii more effectively in this forest.
1. Introduction
The variation in the forest canopy structure directly changes the understory light availability and spatial distribution. This variation influences the growth and survival of many forest plant species, and affects their renewal and community succession [1]. Long-term light intensity is significantly lower than the light saturation points for plants. Thus, weak light stress is produced, which may affect plant growth [2,3]. These changes play an important role in plant adaptation to variations in light conditions. The most direct impact of weak light on plants is changes in chlorophyll content, which affects the photosynthetic rate [4]. Weak light decreases the chlorophyll content in plants, which decreases leaf thickness, leaf mass per area (LMA), and chlorophyll content (SPAD value) of Daphniphyllum macropodum plants [5]. It also decreases the chlorophyll content of cucumber leaves [6] and two maize near-isogenic lines (NILs) [3]. However, some results reported that chlorophyll a (Chl-a) and total chlorophyll content for two varieties of maize leaves increased after shading treatment [7]. The chlorophyll content, especially chlorophyll b (Chl-b) content, in tomato seedlings increased in low light [8]. Therefore, weak light is a key factor in the photochemical efficiency of plants and has different influences on leaf chlorophyll contents. These significant differences in leaf characteristics and photochemical efficiency can be seen as adaptation to weak light.
Shading is a common treatment for exploring how weak light stress influences seedling photosynthesis. Under weak light, the photosynthetic rate (Pn) and chlorophyll fluorescence characteristics, like maximal photochemical quantum efficiency of photosystem II (PSII; Fv/Fm), decreased for different plant species, including in maize leaves [3,7], tomato seedlings [8], and cucumber leaves [9]. Weak light sometimes influences plant growth along with low temperature. Under this condition, the Fv/Fm of cut chrysanthemum decreased significantly in low temperature and weak light conditions [10], and the actual photochemical efficiency and maximal photochemical efficiency of photosystem II in Cucummis sativus seedlings declined, leading to decreased photosynthetic rate and stomatal conductance (Gs) [11]. Therefore, plant photosynthesis and chlorophyll fluorescence characteristics also decrease under weak light conditions, thus reducing plant growth and biomass allocation [12]. However, studies on the response of plant photosynthesis to weak light conditions have mainly focused on crop plants. Little is known about the response of endangered species to weak light condition. Therefore, endangered plants should be studied under different light conditions, which could help us protect natural resources.
Castanopsis kawakamii Hayata is a tertiary endangered relict plant that is only distributed in the subtropical area of China [13]. The C. kawakamii Nature Reserve, located in the Fujian province of Southern China is 700 hectares of natural forest dominated by the C. kawakamii population, which contains the largest area of this population [13]. However, this forest has been in recession and the over mature population of C. kawakamii experiencing regeneration issues, which has caused severe fragmentation in the forest canopy layer and increased the number of forest gaps [13,14,15]. Studies conducted here have included the population age structure, community structure, gap characteristics, population competition, and species diversity in this area, providing a scientific basis for revealing the reasons the species is endangered in this forest [13]. However, forest management techniques for this species are still needed to promote its seedling establishment and regeneration in this forest.
The heterogeneity of the light environment after gap formation was shown to influence the regeneration of C. kawakamii seedling [14,15,16,17,18]. Canopy gap play a crucial role in forest dynamics. They largely determine light transmission to lower canopy strata, thereby influencing species composition and regeneration [19]. Light intensity in the forest gaps gradually decreased from the gap center, canopy gap, and extended gap to the non-gaps in four seasons from 2008 to 2010 [14]. The maximum light intensity was recorded in the gap center, whereas the minimum was found in non-gaps [14,18]. In forest gaps, the growth of C. kawakamii seedlings increased gradually, with the exception of the early stage (one to four years) of seedling growth. In non-gaps, seedlings have been shown to grow well in the early seedling stage (one to four years), but a downward trend for growth has been identified in the later seedling stages (5–10 years) [20].
Here, we hypothesized that the responses of leaf chlorophyll content, photosynthesis rate, and chlorophyll fluorescence characteristics to the variations of light intensity in forest gaps and non-gaps were different, thus influencing the seedling growth strategy for adaptation to the environment. Therefore, we addressed the following questions: (1) Do the chlorophyll content and photosynthesis rate of C. kawakamii seedlings vary in forest gaps and non-gaps? (2) Do the seedlings’ chlorophyll fluorescence characteristics in forest gaps and non-gaps vary with weak and natural light conditions? (3) Does the seedlings’ adaptability vary in forest gaps and non-gaps? Thus, our findings regarding seedling adaptability in forest gaps and non-gaps could provide valuable information for the regeneration of C. kawakamii.
2. Material and Methods
2.1. Study Site and Species
The experimental site was located in C. kawakamii Nature Reserve, Fujian province, China (latitude: 26°07′–26°12′ N, longitude: 117°24′–117°29′ E). This area has a subtropical monsoon climate, with an elevation ranging from 180 to 604 m. The average annual temperature, precipitation, and relative humidity are 19.5 °C (the extreme minimum temperature is −5.5 °C and the maximum temperature is 40 °C), 1500 mm (March to August accounts for about 75% of the year), and 79%, respectively. The soil type in this forest mainly consists of ferric acrisols with abundant humus. The forest type is a subtropical evergreen broad-leaved forest. The average canopy height is 15 m, the average diameter at breast height (dbh) of the tree layer is about 30 cm, and the average population age is about 120 to 150 years [13]. This forest is mainly composed of C. kawakamii, C. carlesii, C. eyrei, Pinus massoniana, and Schima superba [14,17].
The experiment was conducted in C. kawakamii Nature Reserve from December 2012 to December 2014. In this study, we selected 3–5 C. kawakamii seedling individuals with different heights in similar forest gaps and selected relatively similar non-gaps as the control. However, finding different height classes of seedlings in one or two similar gaps in this natural forest was difficult. Therefore, we chose 4 forest gaps and 4 non-gaps to investigate the C. kawakamii seedlings’ growth and regeneration dynamics. The forest gaps were generally oval-shaped, and the average area was 66.5 ± 11.3 m2 [20]. We labeled the representative C. kawakamii seedlings with similar growth rates, as well as the disease-free seedlings with different heights, to measure the relative indices in 2012 and 2013. The same size plots were built in non-gaps about 10 m away from the backlight direction of the major axis of the oval shape in each forest gap. We selected 15 individual C. kawakamii seedlings in forest gaps as well as 15 individuals in the non-gaps. The seedling heights ranged from 16.3 to 131 cm in forest gaps (71.29 ± 38.85 cm) and non-gaps (63.75 ± 27.95 cm).
First, we collected leaves to measure the leaf chlorophyll content, and then investigated the leaf diurnal photosynthetic characteristics in winter from 2013 to 2014. We analyzed the variation in the seedlings’ chlorophyll fluorescence characteristics in forest gaps and non-gaps in the winter of 2014.
2.2. Experimental Design
2.2.1. Leaf Chlorophyll Contents
Healthy and complete leaves from branches, in the current year, were collected to conduct this research. Leaf chlorophyll was extracted from five or six fully expanded leaves with 80% acetone, following Xue’s method [21]. The absorbance value (optical density, OD) of the clarified chlorophyll extracts were measured using a UV-visible spectrophotometer (UV-2550, Shimadzu Corporation, Kyoto, Japan) at 645 and 663 nm, and the chlorophyll a (Chl-a), chlorophyll b (Chl-b), total chlorophyll (Chl-T), and chlorophyll a/b ratio (Chl-a/b) were calculated. Leaf chlorophyll contents were determined with three replicates on average.
2.2.2. Leaf Net Photosynthesis Rate
Diurnal photosynthetic characteristics for different C. kawakamii seedling heights were measured in forest gaps and non-gaps. The selected leaves of C. kawakamii seedling were enclosed in a 2 × 2 cm transparent leaf chamber and the net photosynthetic rates (Pn, μmol·m−2·s−1) were measured every two hours between 08:00 and 16:00 for three sunny days under natural light conditions in the winter. This was done using a GFS 3000 portable photosynthetic system (Heinz Walz Co., Effeltrich, Germany) [22]. A fully expanded leaf in the middle of each plant was of the same height plant, in each direction, were measured. Each leaf was allowed to adapt to its natural nearly vertical orientation when the angles of incidence of the leaf to light intensity were largest [23]. Then, the mean value of the three leaf measurements for each seedling was used as the net photosynthetic rate. The diurnal net photosynthetic rate indicated variations in Pn at 8:00, 10:00, 12:00, 14:00, and 16:00.
2.2.3. Leaf Chlorophyll Fluorescence
According to our previous study [20], light intensity in four seasons decreased from the gap center and canopy gap, and expanded gap to non-gap, with the highest recorded in the gap center and lowest in the non-gap. The average light intensity was highest in summer and lowest in winter [20]. We used the minimum and average of the light intensity in the winter as the weak light conditions and control groups in forest gaps and non-gaps, respectively. During winter, the maximum, minimum, and average light intensities were 1359.74, 119.32, and 746.73 lux in forest gaps, and 990.64, 92.77, and 577.65 lux in non-gaps, respectively [20]. Here, we set the light intensities to 120 and 95 lux, as the weak light conditions, and to 750 and 580 lux in the control groups (CK) in forest gaps and non-gaps, respectively. This was done to evaluate the variation of seedlings’ chlorophyll fluorescence characteristics under weak light conditions and for control groups. Chlorophyll fluorescence parameters were measured using an OS5P portable pulse modulation chlorophyll fluorescence analyzer (Opti-science, Hudson, NH., USA), including the PSII photochemical conversion efficiency (Fv/Fm), photochemical quenching (qP), non-photochemical quenching (NPQ), and the actual quantum yield of PSII photochemical (Y). Leaves were maintained in a dark environment for 15 min for dark adaptation before measuring the minimum (F0) and maximum fluorescence (Fm). The chlorophyll fluorescence parameters included Fv/Fm, qP, NPQ and Y, and were determined and calculated according to Maxwell and Johnson [24]. The mean value of each seedling and the leaf photosynthesis were measured for six leaves on average.
2.3. Data Analysis
We tested the normal distribution and homogeneity of variance for the data in different habitats (forest gaps and non-gaps) for different seedling heights including chlorophyll content, net photosynthetic rate (Pn), and chlorophyll fluorescence parameters before variance analysis. The data for Pn did not fit the normal distribution so we used the Box–Cox power transformation with λ set to 0.15. We used log transformation and sine transformation for qP and NPQ of chlorophyll fluorescence parameters, respectively. Two-way or three-way ANOVA was used to test the effects of habitats (forest gaps and non-gaps) and height on the seedling chlorophyll content, Pn, and chlorophyll fluorescence parameters. This was followed by a Tukey–Kramer multiple comparison test for which the p-value was below 0.05. Data are expressed as mean ± standard deviation (SD). All statistical analysis and graphs were prepared using Excel 2016 and R 3.5.1 software [25].
3. Results
3.1. Leaf Chlorophyll Contents of C. kawakamii Seedlings
The two-way ANOVA showed that the seedling height significantly influenced the Chl-b content and Chl-a/b, and the habitat significantly influenced the contents of Chl-a, Chl-b, and Chl-T (Table 1). We found no significant interaction between habitat and height on the leaf chlorophyll contents (Table 1). According to the multiple comparison test, the Chl-a, Chl-b, and Chl-T contents in forest gaps was significantly lower than in non-gaps (Table 2). In forest gaps, the Chl-b content decreased with increasing seedling height; the trend for the ratio of Chl-a/b was the opposite (Figure 1a,b). No obvious trend was observed for Chl-b content or Chl-a/b ratio in non-gaps with increasing seedling height (Figure 1a,b).

Table 1.
Two-way analysis of variance (ANOVA) for the chlorophyll contents of C. kawakamii seedlings.

Table 2.
The multiple comparison test between forest gaps (GAP) and non-gaps (NG) for the leaf chlorophyll contents, net photosynthetic rate (Pn), and chlorophyll fluorescence parameters of C. kawakamii seedlings.
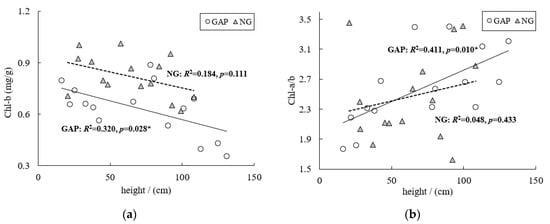
Figure 1.
The Chl-b content and Chl-a/b ratio of C. kawakamii seedlings in forest gaps and non-gaps.
3.2. Pn of C. kawakamii Seedlings
The two-way ANOVA showed that the between-group interactions (height, habitat, and interaction) significantly influenced the diurnal variations in Pn (Table 3). The within-group (time) and between-group (height × habitat) interactions also significantly influenced the diurnal variations in Pn (Table 3). The diurnal variations of Pn of C. kawakamii seedlings in forest gaps and non-gaps showed a single peak curve, with peak values at 12:00 (Figure 2a). According to the multiple comparison test, significant differences in the average diurnal variations in Pn (Figure 2a) were observed. The average diurnal variation in Pn in forest gaps was significantly higher than in non-gaps (Table 2). In non-gaps, the average diurnal variation in Pn for C. kawakamii seedlings increased with increasing seedling height, whereas it was relatively stable and high in forest gaps (Figure 2b).

Table 3.
Two-way ANOVA for the Pn of C. kawakamii seedlings.
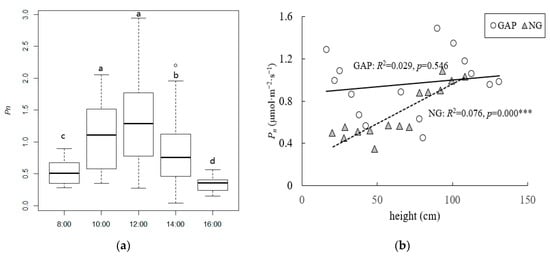
Figure 2.
The average diurnal variations in Pn (μmol·m−2·s−1) for C. kawakamii seedlings in forest gaps and non-gaps. (a) Mean values of Pn for all the seedlings at different times in forest gaps and non-gaps. This was followed by the multiple comparison test. Different letters indicate significant difference when p < 0.05. (b) Pn values for different heights of seedlings in forest gaps and non-gaps. Circles—seedlings in forest gaps; triangles—seedlings in non-gaps, *** p < 0.001.
3.3. Chlorophyll Fluorescence Parameters of C. kawakamii Seedlings
The three-way ANOVA showed that the treatment significantly influenced the chlorophyll fluorescence parameters of Fv/Fm, qP, NPQ, and Y for C. kawakamii seedlings (Table 4). The height, habitat, and their interaction significantly influenced qP; the height and the interaction between habitat and treatment significantly influenced NPQ; the habitat and the interaction between treatment and height significantly influenced Y (Table 4).

Table 4.
The three-way ANOVA for the chlorophyll fluorescence of C. kawakamii seedlings.
According to the multiple comparison test, the chlorophyll fluorescence parameters of qP and Y values in forest gaps were significantly lower than in non-gaps (Table 2). Comparing the weak light treatment and control group, we found that the chlorophyll fluorescence parameters of qP, NPQ, and Y under weak light were significantly lower than in the control group, with the opposite result for Fv/Fm (Table 5). The NPQ value under weak light was lower than in the control group in forest gaps, whereas the difference in non-gaps was not obvious (Figure 3a). In forest gaps, NPQ and qP decreased with increasing seedling height in both weak light and CK treatment, whereas the trend was not obvious in non-gaps (Figure 3b,c). Y decreased with increasing seedling height in the control group in forest gaps, and no obvious trend was observed under the weak light condition (Figure 3d). The qP and Y of seedlings in the weak light condition in forest gaps were strained and remained low, whereas in the CK treatment qP and Y continued to decline with increasing seedling height, indicating that taller seedlings require more light to satisfy their growth needs in winter. We observed little difference between weak light and CK treatment, which indicated that seedlings in non-gaps are better adapted to the weak light conditions.

Table 5.
Multiple comparison test of the chlorophyll fluorescence parameters of C. kawakamii seedlings.
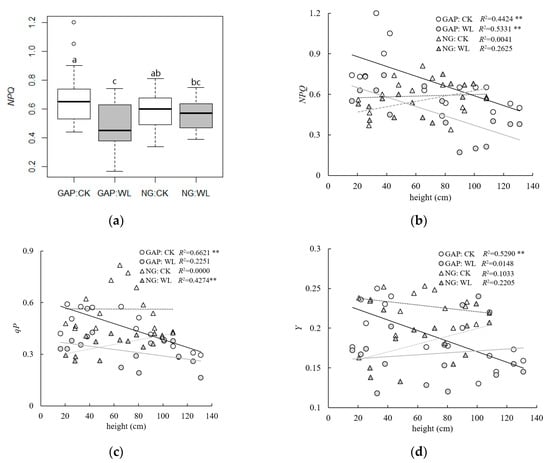
Figure 3.
The chlorophyll fluorescence parameters of C. kawakamii seedlings in forest gaps and non-gaps. (a) The results of the multiple comparison test of the interaction between habitat (GAP and NG) and treatment (CK and WL), where different letters indicate significant difference when p < 0.05. (b) NPQ, (c) qP, and (d) Y values for different seedling heights under different treatments in forest gaps and non-gaps. Unfilled circles—seedlings under the CK treatment in forest gaps; solid circles—seedlings under the WL treatment in forest gaps; unfilled triangles—seedlings under the CK treatment in non-gaps; solid triangles—seedlings under the WL treatment in non-gaps; black solid line—fitted curves of NPQ, qP, and Y values for CK treatment in forest gaps (GAP-CK); grey solid line—WL treatment in forest gaps (GAP-WL); black dotted line—fitted curves of NPQ, qP, and Y values for CK treatment in non-gaps (NG-CK); grey dotted line—WL treatment in non-gaps (NG-WL). ** p < 0.01).
4. Discussion
4.1. Leaf Chlorophyll Contents of C. kawakamii Seedlings
Light is a major environmental factor limiting the growth and survival of many forest species, and its distribution may affect stand-level regeneration patterns [1]. Chlorophyll content is an important indicator of plant adaption to and use of the living environment [26]. Under high light conditions chlorophyll is not only easily synthesized but can also be destroyed easily, whereas weak light reduces the photo-oxidative damage in pigments [27,28]. With decreasing light intensity from forest gaps to non-gaps, the Chl-a, Chl-b, and Chl-T contents in forest gaps were significantly lower than in non-gaps (Table 2). The photosynthetically active radiation (PAR) was relatively low under weak light, with low photosynthesis efficiency. Therefore, plants tended to increase the chlorophyll content to absorb light energy to adapt to environmental conditions, a finding that is in accordance with those of previous studies [29]. The Chl-b content decreased with increasing seedling height, whereas the ratio of Chl-a/b was the opposite in forest gaps (Figure 1a,b). This occurred because the short seedlings in forest gaps likely use violet light that dominates in diffused light, thereby increasing their Chl-b content to absorb and transform the light energy, thus leading to a decline in the Chl-a/b ratio. Tall seedlings can receive more direct light from the canopy layer and decrease the transformation of Chl-a to Chl-b, leading to an increase in the Chl-a/b ratio. The short and tall seedlings of C. kawakamii adapted to the light in accordance with the characteristics of shade tolerant species [13]. Afterward, more light was needed for growth, especially for tall seedlings, saplings, and mature plants [13].
4.2. Net Photosynthetic Rate of C. kawakamii Seedlings in Forest Gaps and Non-Gaps
In natural conditions, two main types of diurnal variation curves of the net photosynthetic rate (Pn) were observed: one single peak curve with a peak Pn appearing close to noon and a bimodal curve with two maximum Pn that appeared before and after noon [30]. We found that the diurnal variation in Pn of C. kawakamii seedlings in forest gaps and non-gaps showed a single peak curve in the daytime (Figure 2a). The light intensity received by seedlings was not adequate for meeting the growth demand and diminished the noon phenomenon to take full advantage of illumination. Forest gaps ensured better PAR penetration, which is advantageous for photosynthesis and improves light use efficiency to seedlings [22]. The forest gaps received higher light intensity [20]; thus, the Pn values of C. kawakamii seedlings in forest gaps were significantly higher than those in non-gaps (Table 2). In non-gaps, the average diurnal variations in Pn for C. kawakamii seedlings increased with increasing seedling height, whereas it was relatively stable and high in forest gaps (Figure 2b). The formation of forest gaps could improve meteorological heterogeneity in this species’ habitat, allowing seedlings to increase their photosynthetic rate [22], thereby promoting their growth [20].
4.3. Chlorophyll Fluorescence Parameters of C. kawakamii Seedlings
The Fv/Fm can be characterized as photo inhibition that is widely used for identifying the capability of plant resistance to stress. The Fv/Fm value is approximately 0.83 for mature healthy leaves of taller plants [31]. The Fv/Fm values of C. kawakamii seedlings were all below 0.83 (Table 5), indicating that weak light decreased the PSII photochemical conversion efficiency, inhibited photosynthesis of the primary reaction, and then affected the photosynthesis process and led to light inhibition.
The values of qP for C. kawakamii seedlings in forest gaps were significantly lower than in non-gaps (Table 2). The values qP of C. kawakamii seedlings under weak light conditions were significantly lower than in control groups (Table 5). Increasing qP values indicate higher electron transfer activity in PSII [30]. In forest gaps, the qP value decreased with increasing seedling height, and this change was not obvious in non-gaps (Figure 3c), indicating that tall seedlings were more sensitive to weak light. The evident decline in qP values in forest gaps was due to the reduction in the re-oxidation capacity of the PSII primary electron acceptor (QA) and the decline in electron transport activity of PSII under weak light conditions. This caused a reduction in carbon assimilation, and transfer of the electrons carried by reduced QA to later electron mediators was difficult. Finally, the photosynthetic electron transfer process was blocked, which decreased the photosynthesis rate of seedlings [32]. The seedlings in forest gaps were more sensitive to weak light, while the seedlings were better adapted to weak light due to long-term growth in a shady environment.
The coefficient of non-photochemical quenching (NPQ) reflects the heat dissipation capacity of plants to a certain extent. It also represents the proportion of light energy allocation, which is a self-protection mechanism of photosynthesis [32]. The NPQ values under the weak light condition were significantly lower than in control groups, especially for the seedlings in forest gaps (Table 5, Figure 3a). This indicated that the heat dissipation capacity declined and constrained the growth of C. kawakamii seedlings in forest gaps under weak light conditions. The NPQ values of C. kawakamii seedlings decreased with increasing seedling height (Figure 3b), indicating that the reaction of tall seedlings was more sensitive to weak light, especially in forest gaps. Similar to the qP values, we observed no obvious trend in NPQ values in non-gaps (Figure 3b,c), which revealed that seedlings in forest gaps were more sensitive to weak light.
The PSII actual photochemical quantum yield (Y) reflects the actual energy capture efficiency and the proportion of energy used during the photochemical reaction to the energy absorbed by the plant when the PSII reaction center is partly closed [31]. The Y values for C. kawakamii seedlings in forest gaps were lower than in non-gaps (Table 2). The low values of Y for C. kawakamii seedlings indicated that weak light conditions decreased the actual photochemical conversion efficiency. This then prevented the formation of plant assimilation (ATP), thus negatively affecting carbon fixation and assimilation to plants [30,33]. The Y values under the weak light condition were lower than in control groups (Table 5). This was in accordance with weak light stress reducing the chlorophyll fluorescence characteristics [2,3]. The Y values decreased with increasing seedling height in the control group of forest gaps (Figure 3d), indicating that the selected light intensity may not have been enough for tall seedlings, which decreased their actual photochemical conversion efficiency. Light inhibition was more obvious for the tall seedlings in the control group of forest gaps, whereas it was not obvious under weak light and CK treatment for seedlings in non-gaps.
5. Conclusions
The light intensity in forest gaps was significantly higher than in non-gaps [20]. C. kawakamii seedlings tended to increase their chlorophyll content to adapt to the environmental conditions and increase growth in non-gaps. Thus, the contents of Chl-a, Chl-b, and Chl-T in non-gaps were significantly higher than in forest gaps. The relatively low light intensity in non-gaps produced a low-efficiency photosynthesis rate. The Pn of C. kawakamii seedlings in forest gaps was significantly higher than in non-gaps, which promoted the growth and regeneration of C. kawakamii seedlings in forest gaps. In addition, the C. kawakamii seedlings in forest gaps were more sensitive to weak light conditions. The NPQ and qP values of C. kawakamii seedlings decreased with increasing seedling height in forest gaps, indicating that the reaction of tall seedlings was more sensitive to weak light than that of short seedlings. The PSII reaction center reduced the efficiency of the PSII photochemical conversion and the electron transfer rate. It then restrained the primary process of photosynthesis, resulting in a decreased photosynthetic capacity. Finally, it affected the normal growth of seedlings under weak light conditions. Seedlings in forest gaps require more light to satisfy their growth needs in the winter. Due to long-term growth in a shady environment, the seedlings’ NPQ and qP values in non-gaps were not obvious, which demonstrated better adaptability to low light intensity with high chlorophyll content and low-efficiency photosynthesis rate.
We suggest that partial forest selection cutting be used as a measure to improve light intensity and, thus, promote the rate of photosynthesis and regeneration for C. kawakamii seedlings in non-gaps. In this study, we aimed to determine the response of leaf photosynthesis and fluorescence characteristics to weak light, but the response to strong light requires further study. Forest gap characteristics (including gap size, shape, age, and canopy openness) and environmental factors play vital roles in the regeneration of plant species. Therefore, the main factors that influence the leaf photosynthesis and fluorescence characteristics of C. kawakamii seedlings need to be identified. By addressing these two issues, the regeneration of this endangered species.
Author Contributions
Conceptualization, Z.-s.H., J.-f.L., and W.H.; funding acquisition, Z.-s.H. and J.-f.L.; investigation, Z.-s.H., R.T., M.-r.J., M.-j.L., and C.X.; methodology, Z.-s.H., R.T., M.-r.J., and C.X.; writing—original draft, Z.-s.H., R.T., and J.-f.L.; writing—review and editing, Z.-s.H. and J.-f.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (NSFC), grant numbers 31700550 and 31770678; the Nature Science Fund of the Fujian Province Science and Technology of China, grant number 2019J01367; the Science and Technology Promotion of Project Forestry Bureau of the Fujian Province, grant number 2018TG14-2; and the Innovation and Technology Fund of Fujian Agriculture and Forestry University, grant number CXZX2018125.
Acknowledgments
We wish to express our thanks for the support received from the Castanopsis kawakamii Nature Reserve in Sanming City, Fujian province, and for allowing us to collect samples. The authors wish to thank Songjin Su, Ruifeng Ma, Kaijin Kuang, and Lixia Qi for field and experiment work. We also thank Dehuang Zhu from Sun Yat-sen University and Yuxin Chen from Xiamen University for helpful discussions and valuable suggestions during the revision of the manuscript. The authors also sincerely appreciate the helpful and constructive comments provided by the three reviewers of the draft manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nicotra, A.B.; Chazdon, R.L.; Iriarte, S.V.B. Spatial heterogeneity of light and woody seedling regeneration in tropical wet forests. Ecology 1999, 80, 1908–1926. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.M.; Wei, M.; Shi, Q.H.; Yang, F.J.; Wang, X.F. Mechanisms of tolerance differences in cucumber seedlings grafted on rootstocks with different tolerance to low temperature and weak light stresses. Turk. J. Bot. 2015, 39, 606–614. [Google Scholar] [CrossRef]
- Qian, C.J.; Zhang, W.; Zhong, X.M.; Li, F.H.; Shi, Z.S. Comparative studies on the photosynthetic characteristics of two maize (Zea mays L.) near-isogenic lines differing in their susceptibility to low light intensity. Emir. J. Food Agric. 2017, 29, 300–311. [Google Scholar] [CrossRef]
- Sui, X.L.; Mao, S.L.; Wang, L.H.; Zhang, B.X.; Zhang, Z.X. Effect of low light on the characteristics of photosynthesis and chlorophyll a fluorescence during leaf development of sweet pepper. J. Integr. Agric. 2012, 11, 1633–1643. [Google Scholar] [CrossRef]
- Park, S.G.; Matsumoto, M. A Study on the effects of light conditions on the longevity and characteristics of Daphniphyllum macropodum leaves. J. Fac. Agric. Kyushu Univ. 2018, 63, 15–19. [Google Scholar]
- Ding, X.T.; Jiang, Y.P.; Wang, H.; Jin, H.J.; Zhang, H.M.; Chen, C.H.; Yu, J.Z. Effects of cytokinin on photosynthetic gas exchange, chlorophyll fluorescence parameters, antioxidative system and carbohydrate accumulation in cucumber (Cucumis sativus L.) under low light. Acta Physiol. Plant. 2013, 35, 1427–1438. [Google Scholar] [CrossRef]
- Wang, J.; Huang, H.J.; Jia, S.; Zhong, X.M.; Li, F.H.; Zhang, K.Y.; Shi, Z.S. Photosynthesis and chlorophyll fluorescence reaction to different shade stresses of weak light sensitive maize. Pak. J. Bot. 2017, 49, 1681–1688. [Google Scholar]
- Wang, M.; Jiang, W.J.; Yu, H.J. Effects of exogenous epibrassinolide on photosynthetic characteristics in tomato (Lycopersicon esculentum Mill) seedlings under weak light stress. J. Agric. Food Chem. 2010, 58, 3642–3645. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Yuan, C.H.; Han, W.; Li, Y.X.; Xiao, F. Effects of low irradiation on photosynthesis and antioxidant enzyme activities in cucumber during ripening stage. Photosynthetica 2016, 54, 251–258. [Google Scholar] [CrossRef]
- Liang, F.; Zheng, C.S.; Sun, X.Z.; Wang, W.L. Effects of low temperature and weak light stress and its recovery on the photosynthesis and chlorophyll fluorescence parameters of cut flower chrysanthemum. Chin. J. Appl. Ecol. 2010, 21, 29–35. [Google Scholar] [CrossRef]
- Bi, H.G.; Liu, P.P.; Jiang, Z.S.; Ai, X.Z. Overexpression of the rubisco activase gene improves growth and low temperature and weak light tolerance in Cucumis sativus. Physiol. Plant. 2017, 161, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.J.; Shen, Z.G.; Liu, Y.; Wang, L.L.; Hannaway, D.; Lu, H.F. Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ. Exp. Bot. 2009, 65, 177–182. [Google Scholar] [CrossRef]
- Liu, J.F.; He, Z.S.; Hong, W.; Zheng, S.Q.; Wang, Z.J. Conservation ecology of endangered plant Castanopsis kawakamii. J. Beijing For. Univ. 2011, 33, 136–143. [Google Scholar] [CrossRef]
- He, Z.S.; Liu, J.F.; Wu, C.T.; Zheng, S.Q.; Hong, W.; Su, S.J.; Wu, C.Z. Effects of forest gaps on some microclimate variables in Castanopsis kawakamii natural forest. J. Mt. Sci. 2012, 9, 706–714. [Google Scholar] [CrossRef]
- Buajan, S.; Liu, J.F.; He, Z.S.; Feng, X.P.; Muhammad, A. Effects of gap size and locations on the regeneration of Castanopsis kawakamii in a subtropical natural forest, China. J. Trop. For. Sci. 2018, 30, 39–48. [Google Scholar] [CrossRef]
- He, Z.S.; Liu, J.F.; Zheng, S.Q.; Su, S.J.; Hong, W.; Wu, Z.Y.; Xu, D.W.; Wu, C.Z. Studies on the seeds dispersal and seedlings regeneration in gaps and understory of Castanopsis kawakamii natural forest. J. Trop. Subtrop. Bot. 2012, 20, 506–512. [Google Scholar]
- He, Z.S.; Wang, L.J.; Jiang, L.; Wang, Z.; Liu, J.F.; Xu, D.W.; Hong, W. Effect of microenvironment on species distribution patterns in the regeneration layer of forest gaps and non-gaps in a subtropical natural forest, China. Forests 2019, 10, 90. [Google Scholar] [CrossRef]
- Buajan, S.; Liu, J.F.; He, Z.S.; Feng, X.P.; Muhammad, A. The effect of light on micro-environment and specific leaf area within the gap, subtropical forest, China. Pak. J. Bot. 2017, 49, 273–282. [Google Scholar]
- Feldmann, E.; Drossler, L.; Hauck, M.; Kucbel, S.; Pichler, V.; Leuschner, C. Canopy gap dynamics and tree understory release in a virgin beech forest, Slovakian Carpathians. For. Ecol. Manag. 2018, 415, 38–46. [Google Scholar] [CrossRef]
- He, Z.S. Study on the Micro-Environment Characteristics and Seedlings Dynamic Regeneration in Castanopsis Kawakamii Natural Forest Gaps; Fujian Agriculture and Forestry University: Fuzhou, China, 2012. [Google Scholar]
- Xue, Y.L. Plant. Physiology Laboratory Manual; Shanghai Science and Technology Press: Shanghai, China, 1985. [Google Scholar]
- He, Z.S.; Liu, J.F.; Zheng, S.Q.; Hong, W.; Wu, C.Z.; Li, J. Diurnal variation of photosynthetic rates of Castanopsis kawakamii seedlings and their relationships with meteorological factors in forest gaps and non-gaps. Pak. J. Bot. 2018, 50, 1361–1368. [Google Scholar]
- Andrews, T.J.; Muller, G.J. Photosynthetic gas exchange of the mangrove, Rhizophora stylosa in its natural environment. Oecologia 1985, 65, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Liu, Y.Q.; Sun, X.Y.; Wang, Y.; Liu, Y. Effects of shades on the photosynthetic characteristics and chlorophyll fluorescence parameters of Urtica dioica. Acta Ecol. Sin. 2007, 27, 3457–3464. [Google Scholar]
- Liu, S.L.; Ma, M.D.; Pan, Y.Z.; Wei, L.L.; He, C.X.; Yang, K.M. Effects of light regime on growth and photosynthetic characteristics of Alnus formosana and A.cremastogyne seedlings. Chin. J. Appl. Ecol. 2013, 24, 351–358. [Google Scholar] [CrossRef]
- Sarijeva, G.; Knapp, M.; Lichtenthater, H.K. Differences in photosynthetic activity, chlorophyll and carotenoid levels, and in chlorophyll fluorescence parameters in green sun and shade leaves of Ginkgo and Fagus. J. Plant Physiol. 2007, 164, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Niu, D.K.; Zhao, Z.H. A Study on photosynthesis and physiological characteristics of Camellia oleifera Abel Clones. Acta Agric. Univ. Jiangxiensis 2007, 29, 219–224. [Google Scholar] [CrossRef]
- Duan, R.Y.; Wei, X.L.; Meng, X.S. Physiological and biochemical responses and growth effects of Ormosia henryi seedlings under different light conditions. J. Cent. South Univ. For. Technol. 2013, 33, 30–34. [Google Scholar] [CrossRef]
- Qi, H.Y.; Hua, L.J.; Zhao, L.; Tang, Y.F. Effect of low night temperature on chlorophyll fluorescence parameters in different genotypes tomato leaves. Acta Agric. Boreali-Sin. 2011, 26, 222–227. [Google Scholar]
- Jiang, Y.; Hao, H.K.; Huang, Z.L.; Shen, W.H.; He, Q.F.; Peng, Y.H.; Huang, X.R. Effects of different light intensity on characteristics of growth and chlorophyll fluorescence of Castanopsis hystrix seedlings. J. Cent. South Univ. For. Technol. 2013, 33, 61–65. [Google Scholar] [CrossRef]
- Wu, F.Y.; Yi, L.T.; Li, X.P.; Yin, X.M.; Liu, M.H.; Yu, S.Q. Effect of different light intensity on intensity chlorophyll content and chlorophyll fluorescence in Lithocarpus glaber. J. Northeast Agric. Univ. 2012, 43, 88–92. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).