Effects of Bird Traits on Seed Dispersal of Endangered Taxus chinensis (Pilger) Rehd. with Ex-Situ and In-Situ Conservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species and Sites
2.2. Seed Dispersal in In-Situ and Ex-Situ Sites
2.3. Effects of Bird Traits on Seed Dispersal in In-Situ and Ex-Situ Sites
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.B.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.L.; Petchey, O.L.; et al. Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Fanin, N.; Gundale, M.J.; Farrell, M.; Ciobanu, M.; Baldock, J.A.; Nilsson, M.C.; Kardol, P.; Wardle, D.A. Consistent effects of biodiversity loss on multifunctionality across contrasting ecosystems. Nature Ecol. Evol. 2018, 2, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.P.; Fox, C.W. Conservation Biology Evolution in Action; Oxford University Press: New York, NY, USA, 2008. [Google Scholar]
- Braverman, I. Conservation without nature: The trouble with in situ versus ex situ conservation. Geoforum 2014, 51, 47–57. [Google Scholar] [CrossRef]
- Primack, R.B. Essentials of Conservation Biology, 6th ed; Oxford University Press: New York, NY, USA, 2014. [Google Scholar]
- Guerrant, E.O.; Havens, K.; Vitt, P. Sampling for effective ex situ plant conservation. Int. J. Plant Sci. 2014, 175, 11–20. [Google Scholar] [CrossRef]
- Guerrant, E.O.; Havens, K.; Maunder, M. Ex Situ Plant Conservation: Supporting Species Survival in the Wild; Island Press: Washington, DC, USA, 2004. [Google Scholar]
- Oldfield, S.F. Botanic gardens and the conservation of tree species. Trends Plant Sci. 2009, 14, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bascompte, J.; Jordano, P. Plant-animal mutualistic networks: The architecture of biodiversity. Ann. Rev. Ecol. Evol. Syst. 2007, 38, 567–593. [Google Scholar] [CrossRef]
- Li, N.; An, S.Q.; Liu, Z.; Lu, C.H. Fruit consumption and seed dispersal by birds in native vs. ex-situ individuals of the endangered Chinese yew, Taxus chinensis. Ecol. Res. 2014, 29, 917–923. [Google Scholar]
- Gosper, C.R.; Stansbury, C.D.; Vivian-Smith, G. Seed dispersal of fleshy-fruited invasive plants by birds: Contributing factors and management options. Divers. Distrib. 2005, 11, 549–558. [Google Scholar] [CrossRef]
- Aslan, C.E. Implications of newly-formed seed-dispersal mutualisms between birds and introduced plants in northern California, USA. Biol. Invasions 2011, 13, 2829–2845. [Google Scholar] [CrossRef]
- Schleuning, M.; Fründ, J.; García, D. Predicting ecosystem functions from biodiversity and mutualistic networks: An extension of trait-based concepts to plant–animal interactions. Ecography 2015, 38, 380–392. [Google Scholar] [CrossRef]
- Bregman, T.P.; Lees, A.C.; MacGregor, H.E.A.; Darski, B.; de Moura, N.G.; Aleixo, A.; Barlow, J.; Tobias, J.A. Using avian functional traits to assess the impact of land-cover change on ecosystem processes linked to resilience in tropical forests. Proc. R. Soc. Lond. B 2016, 283, 1289. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.C.; Schaefer, H.M.; Böhning-Gaese, K.; Schleuning, M. Importance of animal and plant traits for fruit removal and seedling recruitment in a tropical forest. Oikos 2017, 126, 823–832. [Google Scholar] [CrossRef]
- Jordano, P.; Schupp, E.W. Determinants of seed disperser effectiveness: The quantity component and patterns of seed rain for Prunus mahaleb. Ecol. Monog. 2000, 70, 591–615. [Google Scholar] [CrossRef]
- Spiegel, O.; Nathan, R. Incorporating dispersal distance into the disperser effectiveness framework: Frugivorous birds provide complementary dispersal to plants in a patchy environment. Ecol. Lett. 2007, 10, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Moles, A.T. A mammoth mouthful? A test of the idea that larger animals disperse larger seeds. Glob. Ecol. Biogeog. 2015, 24, 1269–1280. [Google Scholar]
- Wotton, D.M.; Kelly, D. Do larger frugivores move seeds further? Body size, seed dispersal distance, and a case study of a large, sedentary pigeon. J. Biogeogr. 2012, 39, 1973–1983. [Google Scholar]
- Farwig, N.; Schabo, D.G.; Albrecht, J. Trait-associated loss of frugivores in fragmented forest does not affect seed removal rates. J. Ecol. 2017, 105, 20–28. [Google Scholar] [CrossRef]
- Zwolak, R. How intraspecific variation in seed-dispersing animals matters for plants. Biol. Rev. 2017. [Google Scholar] [CrossRef]
- Puerta-Piñero, C.; Pino, J.; Gómez, J.M. Direct and indirect landscape effects on Quercus ilex regeneration in heterogeneous environments. Oecologia 2012, 170, 1009–1020. [Google Scholar] [CrossRef]
- Côrtes, M.; Uriarte, M. Integrating frugivore behavior and animal movement: A review of the evidence and implication for scaling seed dispersal. Biol. Rev. 2013, 88, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Z.; Zhang, S.; Yan, C.; Li, X.H.; Lu, C.H. Importance of bird traits for seed dispersal patterns of co-fruiting trees in a patchy forest. Integr. Zool. 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; An, S.Q.; Fu, W.Y.; Lu, C.H. Ecological rescue of remnant fengshui trees in farmlands by avian frugivores. Plant Ecol. Diver. 2015, 8, 401–405. [Google Scholar] [CrossRef]
- Moermond, T.C.; Denslow, J.S. Neotropical avian frugivores: Patterns of behavior, morphology, and nutrition, with consequences for fruit selection. Ornithol. Monogr. 1985, 36, 865–897. [Google Scholar] [CrossRef]
- Dehling, D.M.; Fritz, S.A.; Töpfer, T.; Päckert, M.; Estler, P.; Böhning-Gaese, K.; Schleuning, M. Functional and phylogenetic diversity and assemblage structure of frugivorous birds along an elevational gradient in the tropical Andes. Ecography 2014, 37, 1047–1055. [Google Scholar] [CrossRef]
- MacKinnon, J.R.; Phillipps, K.; He, F.Q. A Field Guide to the Birds of China; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Breitbach, N.; Laube, I.; Steffan-Dewenter, I.; Bohning-Gaese, K. Bird diversity and seed dispersal along a human land-use gradient: High seed removal in structurally simple farmland. Oecologia 2010, 162, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.M. Vertebrate frugivores and their interaction with invertebrate fruit predators: Supporting evidence a Costa Rican dry forest. Oikos 1989, 54, 185–188. [Google Scholar] [CrossRef]
- Holbrook, K.M.; Smith, T.B.; Hardesty, B.D. Implications of long-distance movements of frugivorous rain forest hornbills. Ecography 2002, 25, 745–749. [Google Scholar] [CrossRef]
- Eshiamwata, G.E.; Berens, D.G.; Bleher, B.; Dean, W.R.J.; Bohning-Gaese, K. Bird assemblages in isolated Ficus trees in Kenyan farmland. J. Trop. Ecol. 2006, 22, 723–726. [Google Scholar] [CrossRef]
- Herrera, J.M.; Morales, J.M.; García, D. Differential effects of fruit availability and habitat cover for frugivore-mediated seed dispersal in a heterogeneous landscape. J. Ecol. 2011, 99, 1100–1107. [Google Scholar] [CrossRef]
- Mueller, T.; Lenz, J.; Caprano, T.; Fiedler, W.; Böhning-Gaese, K. Large frugivorous birds facilitate functional connectivity of fragmented landscapes. J. Appl. Ecol. 2014, 51, 684–692. [Google Scholar] [CrossRef]
- Howe, H.F.; Smallwood, J. Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 1982, 13, 201–228. [Google Scholar] [CrossRef]
- Charco, J.; Venturas, M.; Gil, L.; Nanos, N. Effective Seed Dispersal and Fecundity Variation in a Small and Marginal Population of Pinus pinaster Ait. Growing in a Harsh Environment: Implications for Conservation of Forest Genetic Resources. Forests 2017, 8, 312. [Google Scholar]
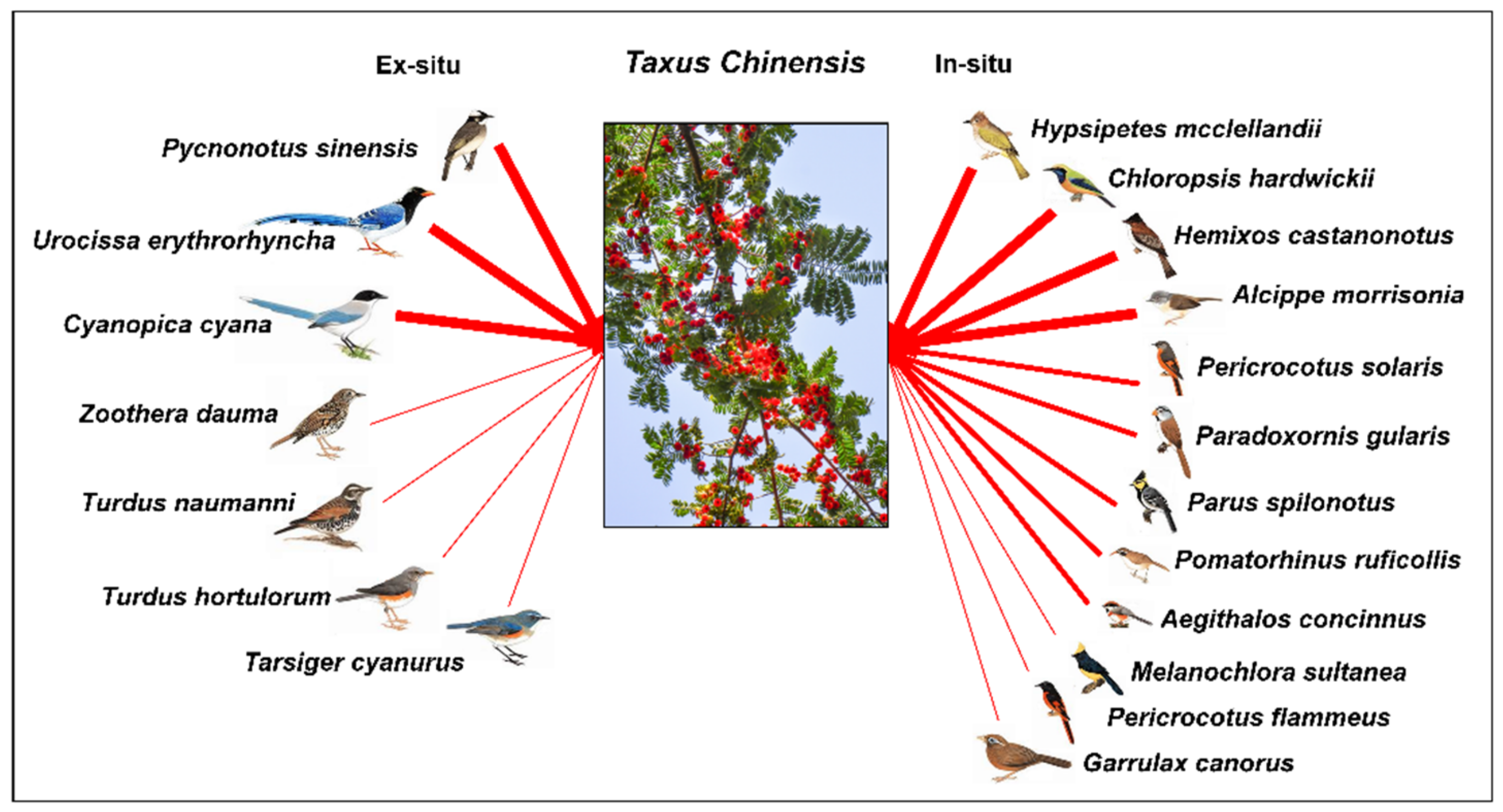
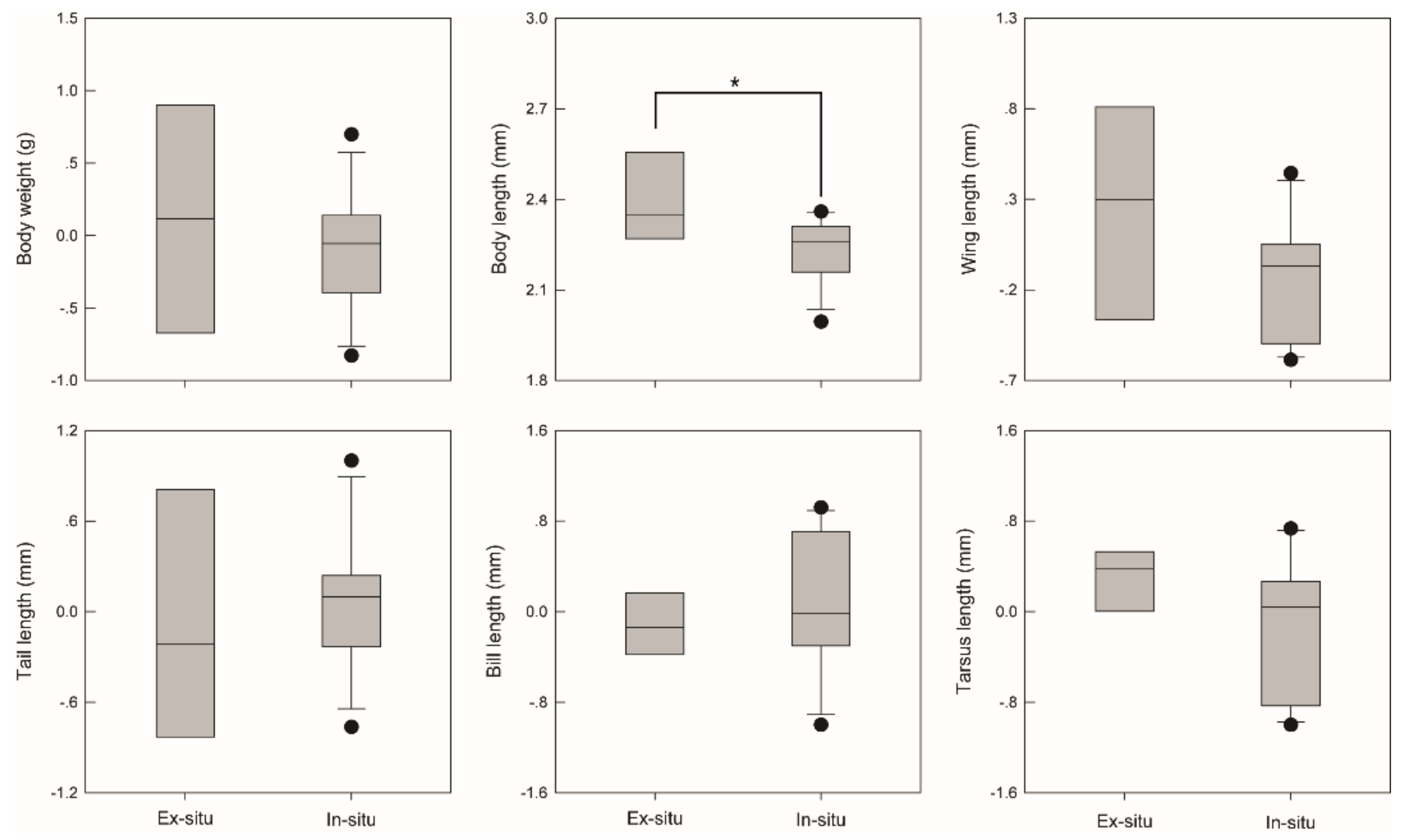
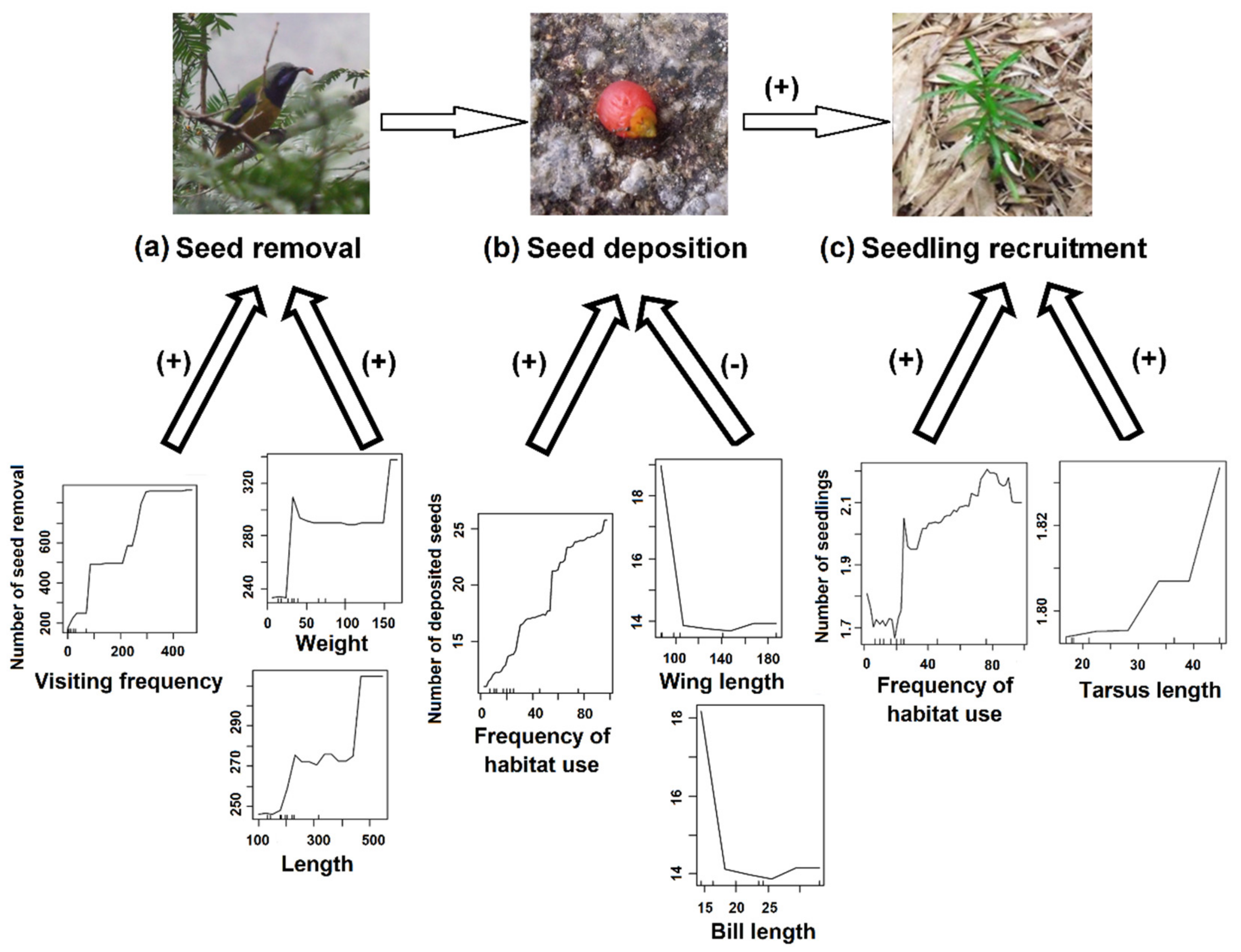
| Sites | Number of Bird Species | Mean Visitation Frequency | Mean Number of Seed Removal | Total Number of Seed Removal |
|---|---|---|---|---|
| In-situ forest | 12 | 11.00 ± 1.98 | 121.42 ± 28.55 | 4371 |
| Ex-situ forest | 7 | 87.81 ± 33.91 | 477.62 ± 176.34 | 10,030 |
| Parameter | Estimate | Standard Error | Z | p |
|---|---|---|---|---|
| Effects of bird traits on seed deposition | ||||
| Intercept | 4.820 | 0.950 | 5.073 | 3.92e−7 |
| Frequency of habitat use | 0.018 | 0.002 | 9.232 | <2e−16 *** |
| Wing length | −0.027 | 0.009 | −2.992 | 0.003 ** |
| Habitat use by Urocissa erythrorhyncha | 2.472 | 0.850 | 2.908 | 0.004 ** |
| Sites | −0.240 | 0.100 | −2.396 | 0.016 * |
| Random effects | ||||
| Groups | Variance | Std. Dev. | ||
| Years | 0.004 | 0.061 | ||
| Effects of bird traits on seedling recruitment | ||||
| Intercept | −2.305 | 2.257 | −1.021 | 0.307 |
| Number of deposited seeds | 0.024 | 0.012 | 2.124 | 0.034 * |
| Habitat use by Chloropsis hardwickii | 0.846 | 0.374 | 2.263 | 0.023 * |
| Sites | 0.033 | 0.271 | 0.125 | 0.901 |
| Random effects | ||||
| Groups | Variance | Std. Dev. | ||
| Years | 0 | 0 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Wang, Z.; Li, X.-H.; Yi, X.-F.; Yan, C.; Lu, C.-H.; Chen, S.-C. Effects of Bird Traits on Seed Dispersal of Endangered Taxus chinensis (Pilger) Rehd. with Ex-Situ and In-Situ Conservation. Forests 2019, 10, 790. https://doi.org/10.3390/f10090790
Li N, Wang Z, Li X-H, Yi X-F, Yan C, Lu C-H, Chen S-C. Effects of Bird Traits on Seed Dispersal of Endangered Taxus chinensis (Pilger) Rehd. with Ex-Situ and In-Situ Conservation. Forests. 2019; 10(9):790. https://doi.org/10.3390/f10090790
Chicago/Turabian StyleLi, Ning, Zheng Wang, Xin-Hai Li, Xian-Feng Yi, Chuan Yan, Chang-Hu Lu, and Si-Chong Chen. 2019. "Effects of Bird Traits on Seed Dispersal of Endangered Taxus chinensis (Pilger) Rehd. with Ex-Situ and In-Situ Conservation" Forests 10, no. 9: 790. https://doi.org/10.3390/f10090790
APA StyleLi, N., Wang, Z., Li, X.-H., Yi, X.-F., Yan, C., Lu, C.-H., & Chen, S.-C. (2019). Effects of Bird Traits on Seed Dispersal of Endangered Taxus chinensis (Pilger) Rehd. with Ex-Situ and In-Situ Conservation. Forests, 10(9), 790. https://doi.org/10.3390/f10090790







