Environmental Controls of Diurnal and Seasonal Variations in the Stem Radius of Platycladus orientalis in Northern China
Abstract
1. Introduction
2. Materials and Methods
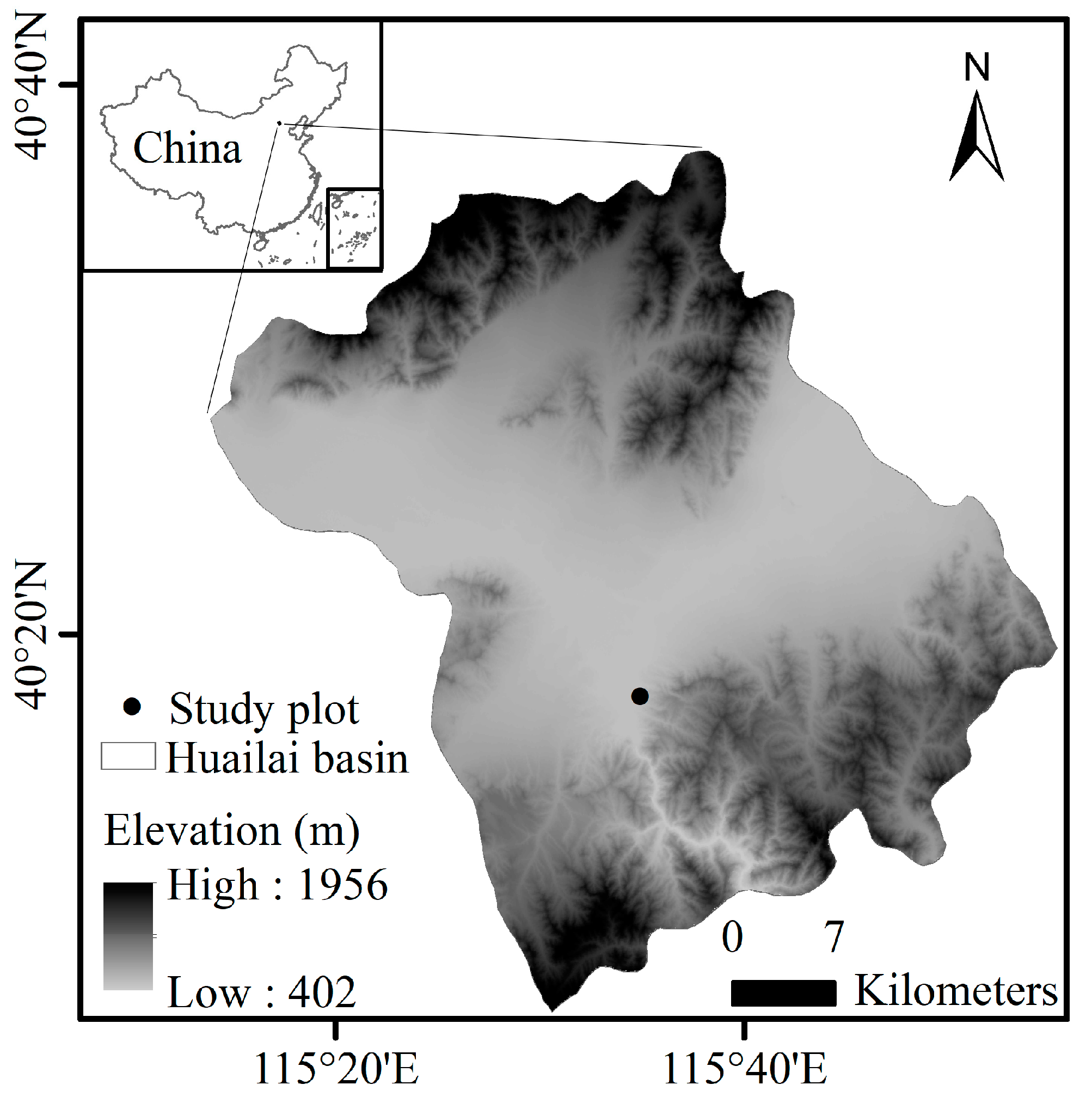
2.1. Study Area
2.2. Dendrometer Measurements
2.3. Meteorological Measurements
2.4. Data Analysis
3. Results
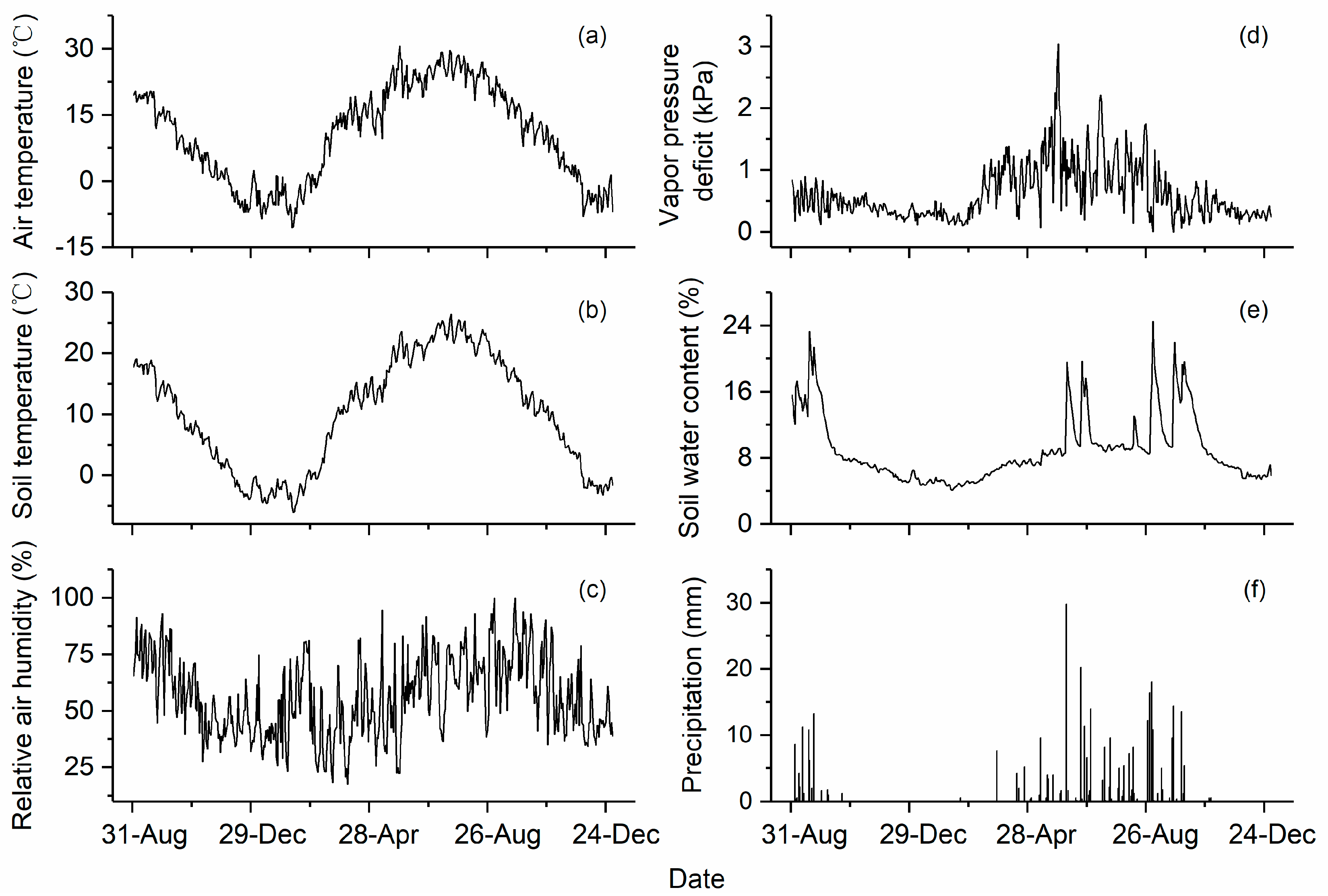
3.1. Environmental Conditions
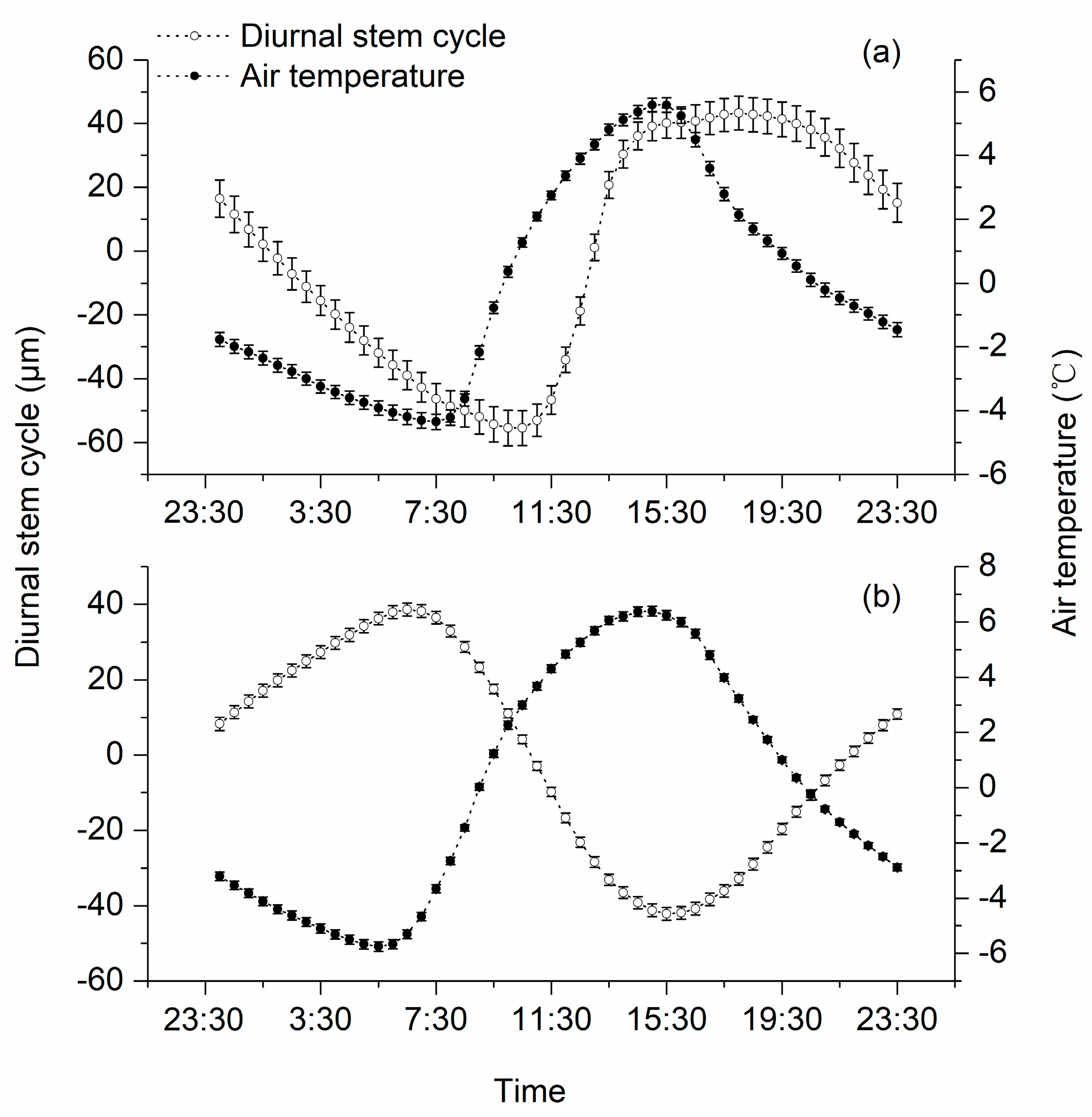
3.2. Diurnal Stem Cylces
3.3. Seasonal Stem Radial Variation
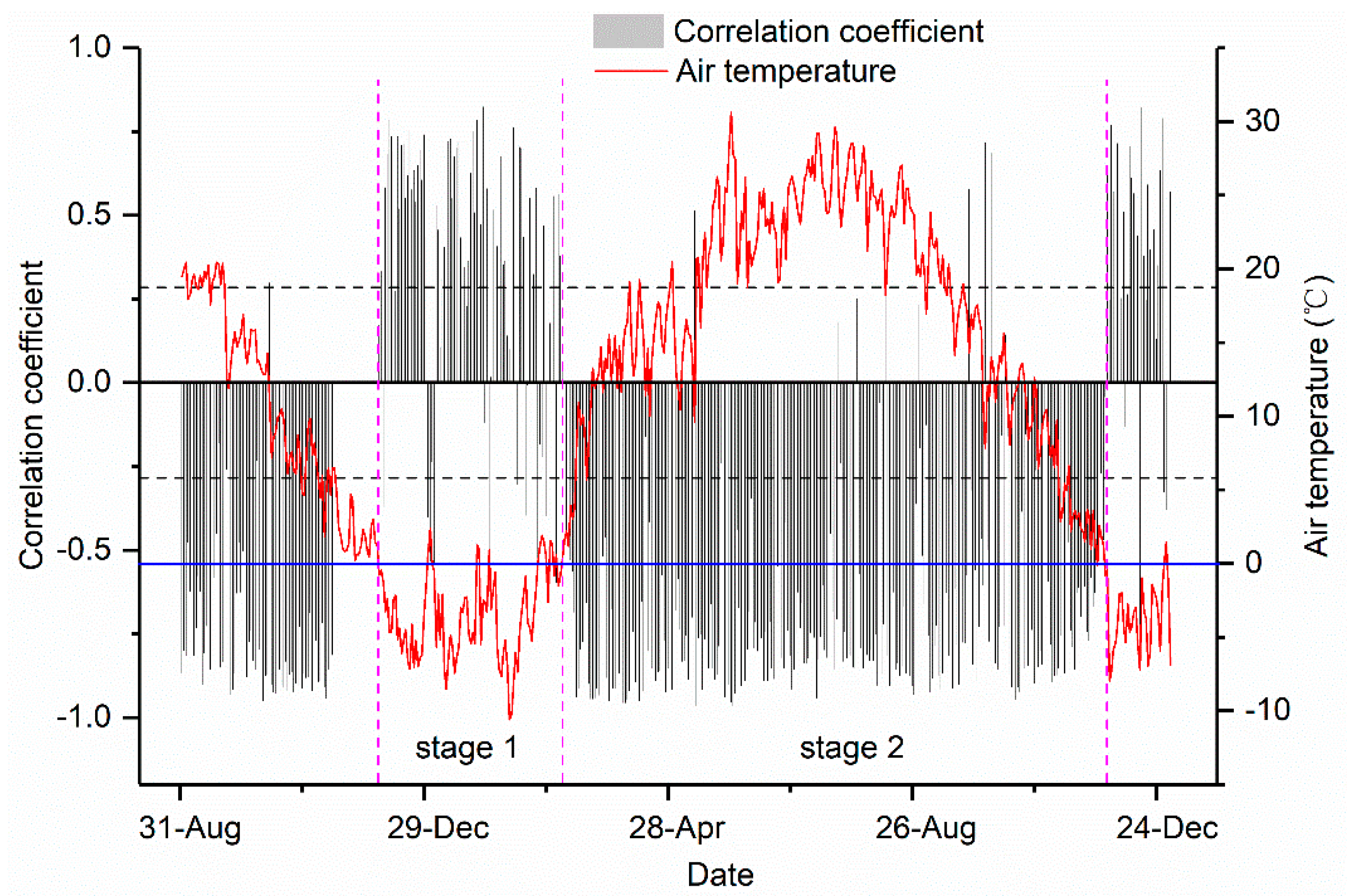
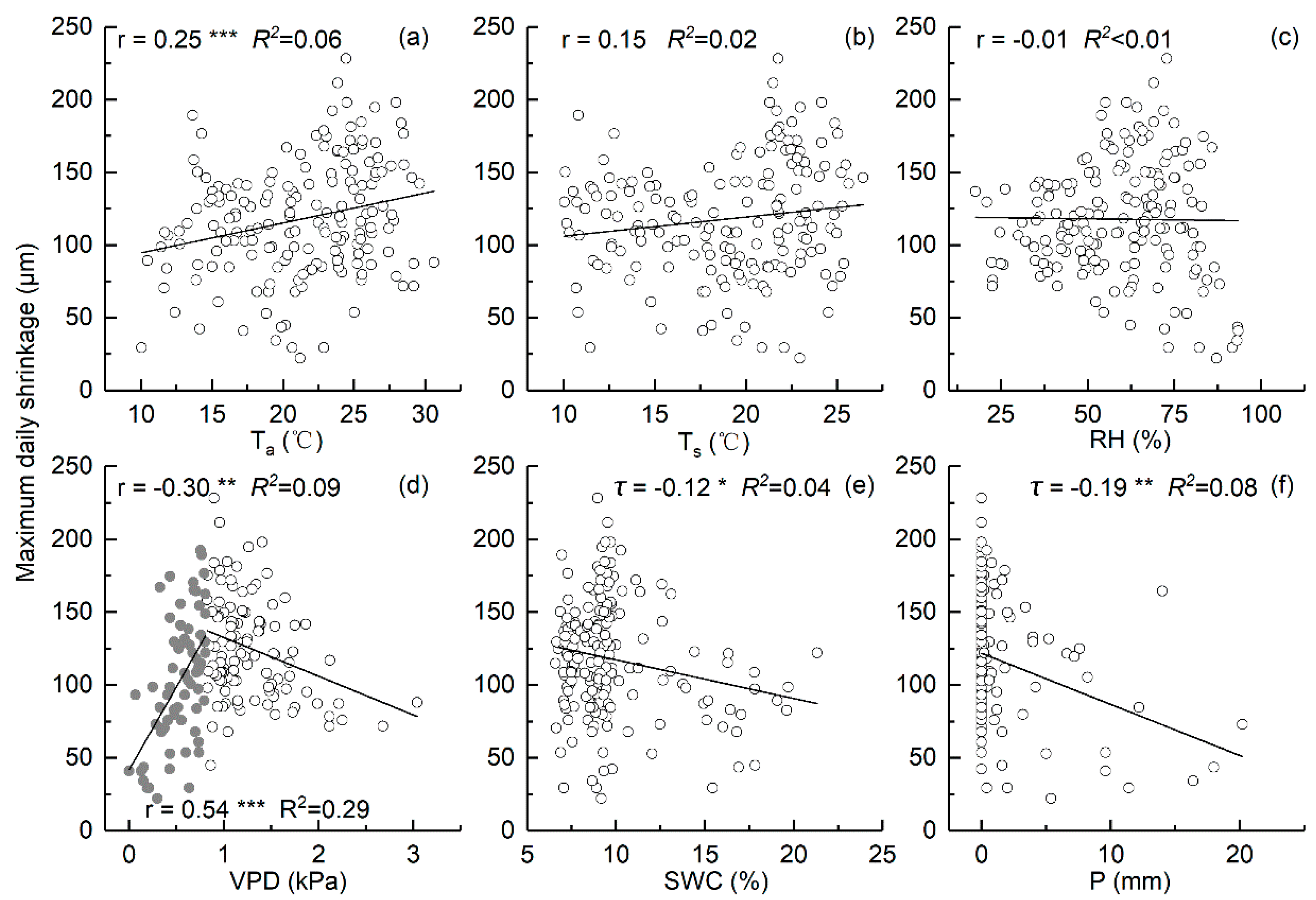
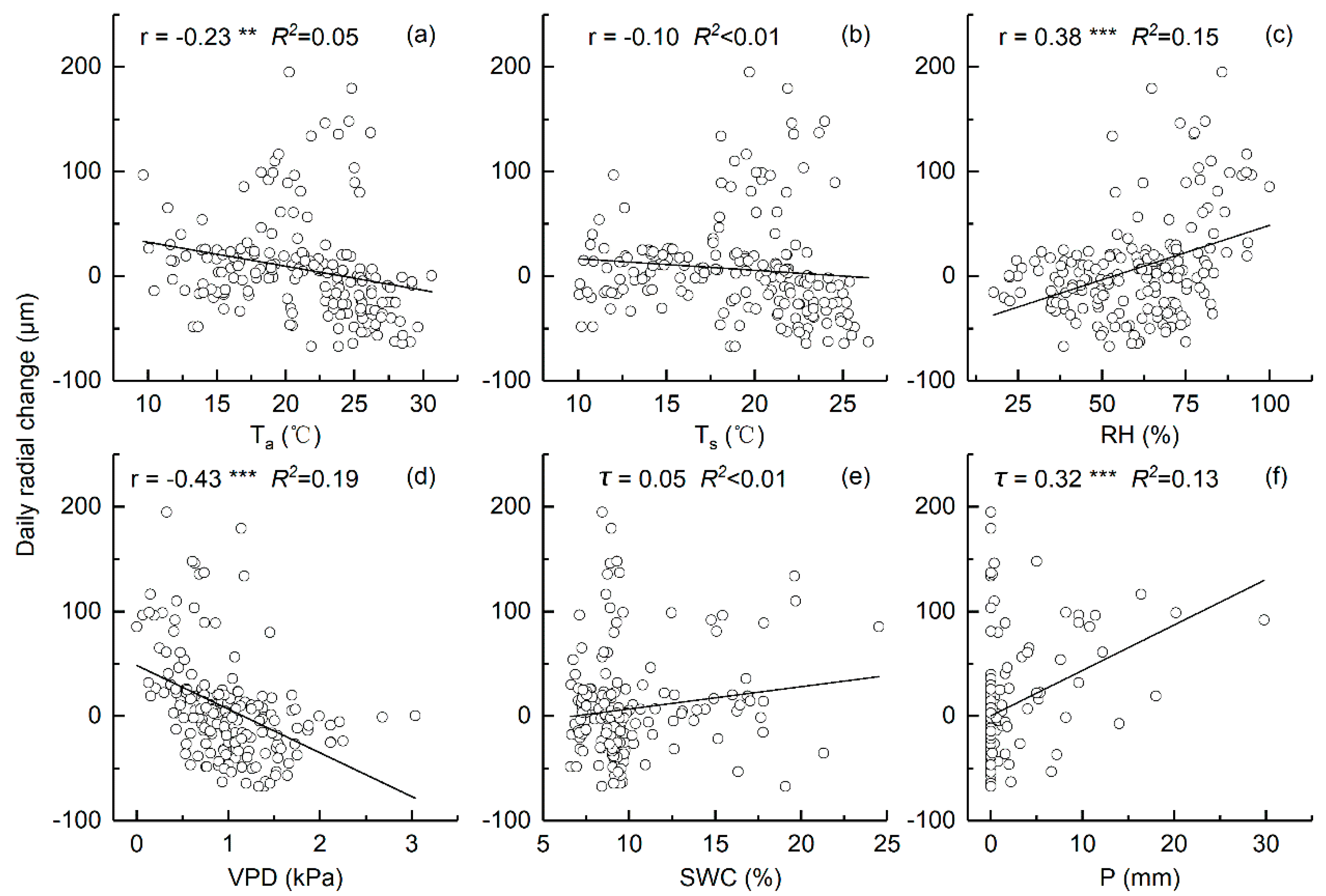
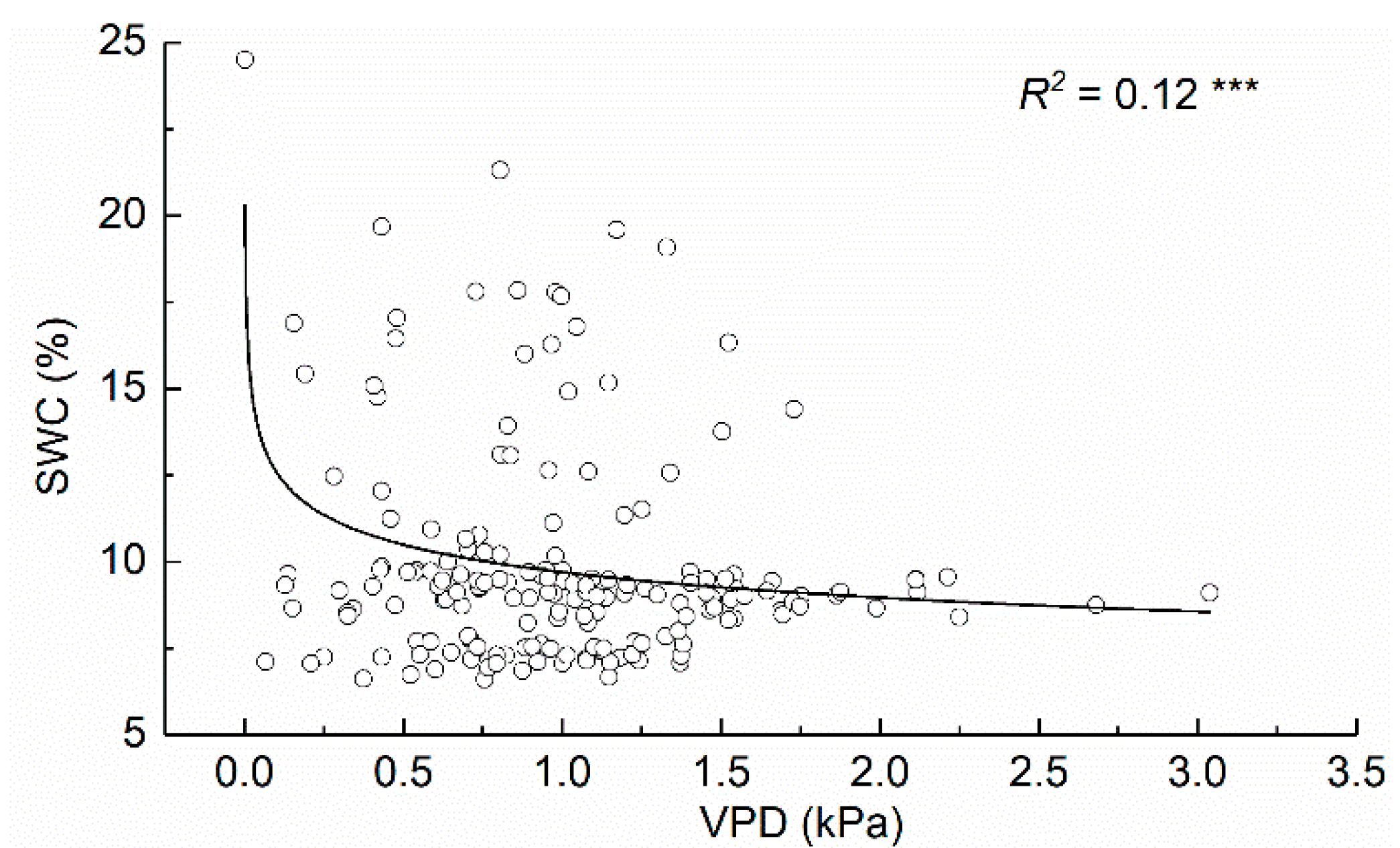
3.4. Relationships between the Daily Stem Radial Variation and Environmental Factors during Growing Season
4. Discussion
4.1. Patterns of Diurnal Stem Cycle
4.2. Seasonal Patterns of Stem Radius Variation
4.3. Environmental Factors That Influence Daily Stem Variation during Growing Season
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, R.B.; Yuan, Y.J.; Gou, X.H.; Zhang, T.W.; Zou, C.; Ji, C.R.; Fan, Z.A.; Qin, L.; Shang, H.M.; Li, X.J. Intra-annual radial growth of Schrenk spruce (Picea schrenkiana Fisch et Mey) and its response to climate on the northern slopes of the Tianshan mountains. Dendrochronologia 2016, 40, 36–42. [Google Scholar] [CrossRef]
- Fritts, H.C.; Shatz, D.J. Selecting and characterizing tree-ring chronologies for dendroclimatic analysis. Tree-Ring Bull. 1975, 35, 31–46. [Google Scholar]
- Gutierrez, E.; Campelo, F.; Julio Camarero, J.; Ribas, M.; Muntan, E.; Nabais, C.; Freitas, H. Climate controls act at different scales on the seasonal pattern of Quercus ilex L. stem radial increments in NE Spain. Trees 2011, 25, 637–646. [Google Scholar] [CrossRef]
- Perez, C.A.; Carmona, M.R.; Aravena, J.C.; Farina, J.M.; Armesto, J.J. Environmental controls and patterns of cumulative radial increment of evergreen tree species in montane, temperate rainforests of Chiloe Island, southern Chile. Austral Ecol. 2009, 34, 259–271. [Google Scholar] [CrossRef]
- Liu, Z.B.; Wang, Y.H.; Tian, A.; Yu, P.T.; Xiong, W.; Xu, L.H.; Wang, Y.R. Intra-annual variation of stem radius of Larix principis-rupprechtii and its response to environmental factors in Liupan mountains of Northwest China. Forests 2017, 8, 382. [Google Scholar] [CrossRef]
- Tian, Q.Y.; He, Z.B.; Xiao, S.C.; Peng, X.M.; Ding, A.J.; Lin, P.F. Response of stem radial growth of Qinghai spruce (Picea crassifolia) to environmental factors in the Qilian Mountains of China. Dendrochronologia 2017, 44, 76–83. [Google Scholar] [CrossRef]
- Liu, X.S.; Nie, Y.Q.; Wen, F. Seasonal dynamics of stem radial increment of Pinus taiwanensis Hayata and its response to environmental factors in the Lushan mountains, Southeastern China. Forests 2018, 9, 387. [Google Scholar] [CrossRef]
- Downes, G.; Beadle, C.; Worledge, D. Daily stem growth patterns in irrigated Eucalyptus globulus and E. nitens in relation to climate. Trees 1999, 14, 102–111. [Google Scholar] [CrossRef]
- Dong, M.Y.; Jiang, Y.; Zhang, W.T.; Yang, Y.G.; Yang, H.C. Effect of alpine treeline conditions on the response of the stem radial variation of Picea Meyeri Rebd. Et Wils to environmental factors. Pol. J. Ecol. 2011, 59, 729–739. [Google Scholar]
- Drew, D.M.; Downes, G.M. The use of precision dendrometers in research on daily stem size and wood property variation: A review. Dendrochronologia 2009, 27, 159–172. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, B.Q.; Dong, M.Y.; Huang, Y.M.; Wang, M.C.; Wang, B. Response of daily stem radial growth of Platycladus orientalis to environmental factors in a semi-arid area of North China. Trees 2015, 29, 87–96. [Google Scholar] [CrossRef]
- Sprengel, L.; Stangler, D.F.; Sheppard, J.; Morhart, C.; Spiecker, H. Comparative analysis of the effects of stem height and artificial pruning on seasonal radial growth dynamics of Wild Cherry (Prunus avium L.) and Sycamore (Acer pseudoplatanus L.) in a widely spaced system. Forests 2018, 9, 174. [Google Scholar] [CrossRef]
- Makinen, H.; Seo, J.-W.; Nojd, P.; Schmitt, U.; Jalkanen, R. Seasonal dynamics of wood formation: A comparison between pinning, microcoring and dendrometer measurements. Eur. J. For. Res. 2008, 127, 235–245. [Google Scholar] [CrossRef]
- Bräuning, A.; Volland-Voigt, F.; Burchardt, I.; Ganzhi, O.; Nauss, T.; Peters, T. Climatic control of radial growth of Cedrela montana in a humid mountain rainforest in Southern Ecuador. Erdkunde 2009, 63, 337–345. [Google Scholar] [CrossRef]
- Urrutia-Jalabert, R.; Rossi, S.; Deslauriers, A.; Malhi, Y.; Lara, A. Environmental correlates of stem radius change in the endangered Fitzroya cupressoides forests of southern Chile. Agric. For. Meteorol. 2015, 200, 209–221. [Google Scholar] [CrossRef]
- Cocozza, C.; Tognetti, R.; Giovannelli, A. High-resolution analytical approach to describe the sensitivity of tree-environment dependences through stem radial variation. Forests 2018, 9, 134. [Google Scholar] [CrossRef]
- Zweifel, R.; Hasler, R. Frost-induced reversible shrinkage of bark of mature subalpine conifers. Agric. For. Meteorol. 2000, 102, 213–222. [Google Scholar] [CrossRef]
- King, G.; Fonti, P.; Nievergelt, D.; Buntgen, U.; Frank, D. Climatic drivers of hourly to yearly tree radius variations along a 6 degrees C natural warming gradient. Agric. For. Meteorol. 2013, 168, 36–46. [Google Scholar] [CrossRef]
- Wang, W.B.; Zhang, F.; Yuan, L.M.; Wang, Q.T.; Zheng, K.; Zhao, C.Y. Environmental factors effect on stem radial variations of Picea crassifolia in Qilian Mountains, Northwestern China. Forests 2016, 7, 210. [Google Scholar] [CrossRef]
- Turcotte, A.; Morin, H.; Krause, C.; Deslauriers, A.; Thibeault-Martel, M. The timing of spring rehydration and its relation with the onset of wood formation in black spruce. Agric. For. Meteorol. 2009, 149, 1403–1409. [Google Scholar] [CrossRef]
- Vieira, J.; Rossi, S.; Campelo, F.; Freitas, H.; Nabais, C. Seasonal and daily cycles of stem radial variation of Pinus pinaster in a drought-prone environment. Agric. For. Meteorol. 2013, 180, 173–181. [Google Scholar] [CrossRef]
- Drew, D.M.; Richards, A.E.; Cook, G.D.; Downes, G.M.; Gill, W.; Baker, P.J. The number of days on which increment occurs is the primary determinant of annual ring width in Callitris intratropica. Trees 2013, 28, 31–40. [Google Scholar] [CrossRef]
- Tardif, J.; Flannigan, M.; Bergeron, Y. An analysis of the daily radial activity of 7 boreal tree species, northwestern Quebec. Environ. Monit. Assess. 2001, 67, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Devine, W.D.; Harrington, C.A. Factors affecting diurnal stem contraction in young Douglas-fir. Agric. For. Meteorol. 2011, 151, 414–419. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Q.L.; Liu, X.; Meng, M. The relationship between stem diameter shrinkage and tree bole moisture loss due to transpiration. Forests 2019, 10, 290. [Google Scholar] [CrossRef]
- Jimenez, M.N.; Navarro, F.B.; Sanchez-Miranda, A.; Ripoll, M.A. Using stem diameter variations to detect and quantify growth and relationships with climatic variables on a gradient of thinned Aleppo pines. For. Ecol. Manag. 2019, 442, 53–62. [Google Scholar] [CrossRef]
- Oberhuber, W.; Gruber, A.; Kofler, W.; Swidrak, I. Radial stem growth in response to microclimate and soil moisture in a drought-prone mixed coniferous forest at an inner Alpine site. Eur. J. For. Res. 2014, 133, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.G.; Wang, T.L.; Liu, S.S.; Jiao, S.Q.; Jia, K.H.; Zhou, S.S.; Jin, Y.Q.; Li, Y.; El-Kassaby, Y.A.; Mao, J.F. Predicting future seed sourcing of Platycladus orientalis (L.) for future climates using climate niche models. Forests 2017, 8, 471. [Google Scholar] [CrossRef]
- Huang, R.F.; Zhao, Y.K.; Lv, J.X.; Bao, F.C. Response of ring width and ring density of Platycladus orientalis to climate change in Beijing. Sci. Silv. Sin. 2006, 42, 78–82. (In Chinese) [Google Scholar]
- Lu, W.W.; Yu, X.X.; Jia, G.D.; Li, H.Z.; Liu, Z.Q. Responses of intrinsic water-use efficiency and tree growth to climate change in semi-arid areas of North China. Sci. Rep. 2018, 8, 308. [Google Scholar]
- Ziaco, E.; Biondi, F. Stem circadian phenology of four pine species in naturally contrasting climates from sky-island forests of the Western USA. Forests 2018, 9, 396. [Google Scholar] [CrossRef]
- Murray, F.W. On the computation of saturation vapor pressure. J. Appl. Meteorol. 1967, 6, 203–204. [Google Scholar] [CrossRef]
- Deslauriers, A.; Rossi, S.; Anfodillo, T. Dendrometer and intra-annual tree growth: What kind of information can be inferred? Dendrochronologia 2007, 25, 113–124. [Google Scholar] [CrossRef]
- Oberhuber, W. Soil water availability and evaporative demand affect seasonal growth dynamics and use of stored water in co-occurring saplings and mature conifers under drought. Trees 2017, 31, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, L.; Houle, D. Modelling day-to-day stem diameter variation and annual growth of balsam fir (Abies balsamea (L.) Mill.) from daily climate. For. Ecol. Manag. 2011, 262, 863–872. [Google Scholar] [CrossRef]
- Deslauriers, A.; Morin, H.; Urbinati, C.; Carrer, M. Daily weather response of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Québec (Canada). Trees 2003, 17, 477–484. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Yang, B.; Deslauriers, A.; Qin, C.; He, M.H.; Shi, F.; Liu, J.J. Two phases of seasonal stem radius variations of Sabina przewalskii Kom. in northwestern China inferred from sub-diurnal shrinkage and expansion patterns. Trees 2012, 26, 1747–1757. [Google Scholar] [CrossRef]
- Zweifel, R.; Eugster, W.; Etzold, S.; Dobbertin, M.; Buchmann, N.; Haesler, R. Link between continuous stem radius changes and net ecosystem productivity of a subalpine Norway spruce forest in the Swiss Alps. New Phytol. 2010, 187, 819–830. [Google Scholar] [CrossRef] [PubMed]
- He, M.H.; Yang, B.; Wang, Z.Y.; Bräuning, A.; Pourtahmasi, K.; Oladi, R. Climatic forcing of xylem formation in Qilian juniper on the northeastern Tibetan Plateau. Trees 2016, 30, 923–933. [Google Scholar] [CrossRef]
- Ortuno, M.F.; Garcia-Orellana, Y.; Conejero, W.; Ruiz-Sanchez, M.C.; Mounzer, O.; Alarcon, J.J.; Torrecillas, A. Relationships between climatic variables and sap flow, stem water potential and maximum daily trunk shrinkage in lemon trees. Plant Soil 2006, 279, 229–242. [Google Scholar] [CrossRef]
- Oberhuber, W.; Hammerle, A.; Kofler, W. Tree water status and growth of saplings and mature Norway spruce (Picea abies) at a dry distribution limit. Front. Plant Sci. 2015, 6, 703. [Google Scholar] [CrossRef] [PubMed]
- Barraclough, A.D.; Zweifel, R.; Cusens, J.; Leuzinger, S. Daytime stem swelling and seasonal reversal in the peristaltic depletion of stored water along the stem of Avicennia marina (Forssk.) Vierh. Tree Physiol. 2018, 38, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Garnier, E.; Berger, A. Effect of water stress on stem diameter changes of Peach trees growing in the field. J. Appl. Ecol. 1986, 23, 193–209. [Google Scholar] [CrossRef]
- Hinckley, T.M.; Bruckerhoff, D.N. Effects of drought on water relations and stem shrinkage of Quercus alba. Can. J. Bot. 1975, 53, 62–72. [Google Scholar] [CrossRef]
- Grossiord, C.; Sevanto, S.; Borrego, I.; Chan, A.M.; Collins, A.D.; Dickman, L.T.; Hudson, P.J.; McBranch, N.; Michaletz, S.T.; Pockman, W.T.; et al. Tree water dynamics in a drying and warming world. Plant Cell Environ. 2017, 40, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Mei, T.T.; Fang, D.M.; Roll, A.; Niu, F.R.; Hendrayanto; Holscher, D. Water use patterns of four tropical bamboo species assessed with sap flux measurements. Front. Plant Sci. 2015, 6, 1202. [Google Scholar]
- Moriana, A.; Moreno, F.; Giron, I.F.; Conejero, W.; Ortuno, M.F.; Morales, D.; Corell, M.; Torrecillas, A. Seasonal changes of maximum daily shrinkage reference equations for irrigation scheduling in olive trees: Influence of fruit load. Agric. Water Manag. 2011, 99, 121–127. [Google Scholar] [CrossRef]
- Galindo, A.; Rodriguez, P.; Mellisho, C.D.; Torrecillas, E.; Moriana, A.; Cruz, Z.N.; Conejero, W.; Moreno, F.; Torrecillas, A. Assessment of discretely measured indicators and maximum daily trunk shrinkage for detecting water stress in pomegranate trees. Agric. For. Meteorol. 2013, 180, 58–65. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Castel, J.R. Evaluation of grapevine water status from trunk diameter variations. Irrig. Sci. 2007, 26, 49–59. [Google Scholar] [CrossRef]
- Abe, H.; Nakai, T.; Utsumi, Y.; Kagawa, A. Temporal water deficit and wood formation in Cryptomeria japonica. Tree Physiol. 2003, 23, 859–863. [Google Scholar] [CrossRef]
- Zweifel, R.; Haeni, M.; Buchmann, N.; Eugster, W. Are trees able to grow in periods of stem shrinkage? New Phytol. 2016, 211, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Köcher, P.; Horna, V.; Leuschner, C. Environmental control of daily stem growth patterns in five temperate broad-leaved tree species. Tree Physiol. 2012, 32, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Liang, E.Y.; Eckstein, D.; Liu, H.Y. Climate-growth relationships of relict Pinus tabulaeformis at the northern limit of its natural distribution in northern China. J. Veg. Sci. 2008, 19, 393–406. [Google Scholar] [CrossRef]
- Li, X.X.; Liang, E.Y.; Gričar, J.; Prislan, P.; Rossi, S.; Čufar, K. Age dependence of xylogenesis and its climatic sensitivity in Smith fir on the south-eastern Tibetan Plateau. Tree Physiol. 2013, 33, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Mo, X.G.; Lin, Z.H. Projections of spatial-temporal variation of drought in north China. Arid Land Geogr. 2015, 38, 239–248. (In Chinese) [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, M.; Wang, B.; Jiang, Y.; Ding, X. Environmental Controls of Diurnal and Seasonal Variations in the Stem Radius of Platycladus orientalis in Northern China. Forests 2019, 10, 784. https://doi.org/10.3390/f10090784
Dong M, Wang B, Jiang Y, Ding X. Environmental Controls of Diurnal and Seasonal Variations in the Stem Radius of Platycladus orientalis in Northern China. Forests. 2019; 10(9):784. https://doi.org/10.3390/f10090784
Chicago/Turabian StyleDong, Manyu, Bingqin Wang, Yuan Jiang, and Xinyuan Ding. 2019. "Environmental Controls of Diurnal and Seasonal Variations in the Stem Radius of Platycladus orientalis in Northern China" Forests 10, no. 9: 784. https://doi.org/10.3390/f10090784
APA StyleDong, M., Wang, B., Jiang, Y., & Ding, X. (2019). Environmental Controls of Diurnal and Seasonal Variations in the Stem Radius of Platycladus orientalis in Northern China. Forests, 10(9), 784. https://doi.org/10.3390/f10090784




