Nutrient Allocation to Different Compartments of Age-Sequence Larch Plantations in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Biomass Determination and Sample Extraction
2.3. Soil Sampling
2.4. Chemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Soil Chemical Properties
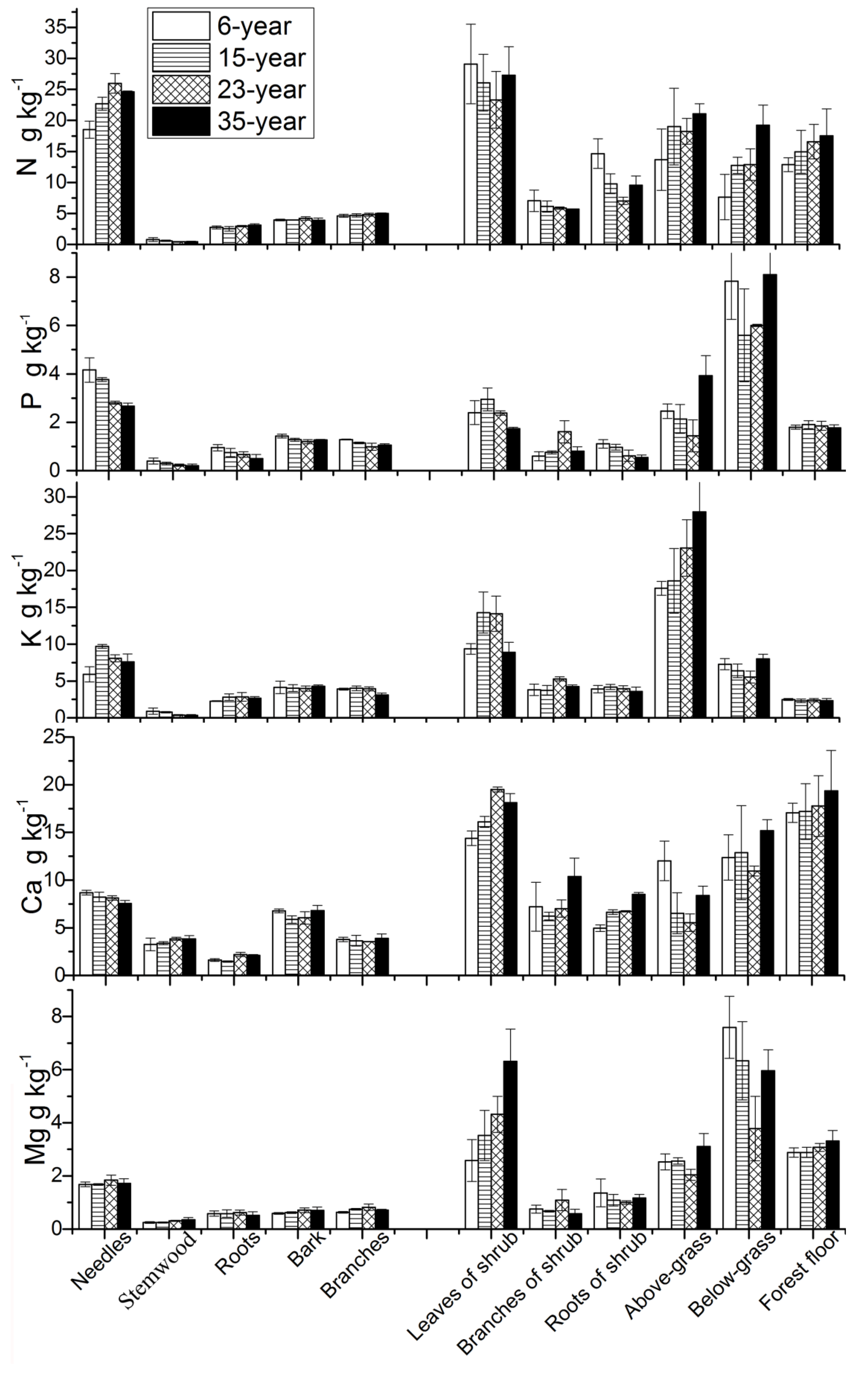
3.2. Nutrient Concentrations
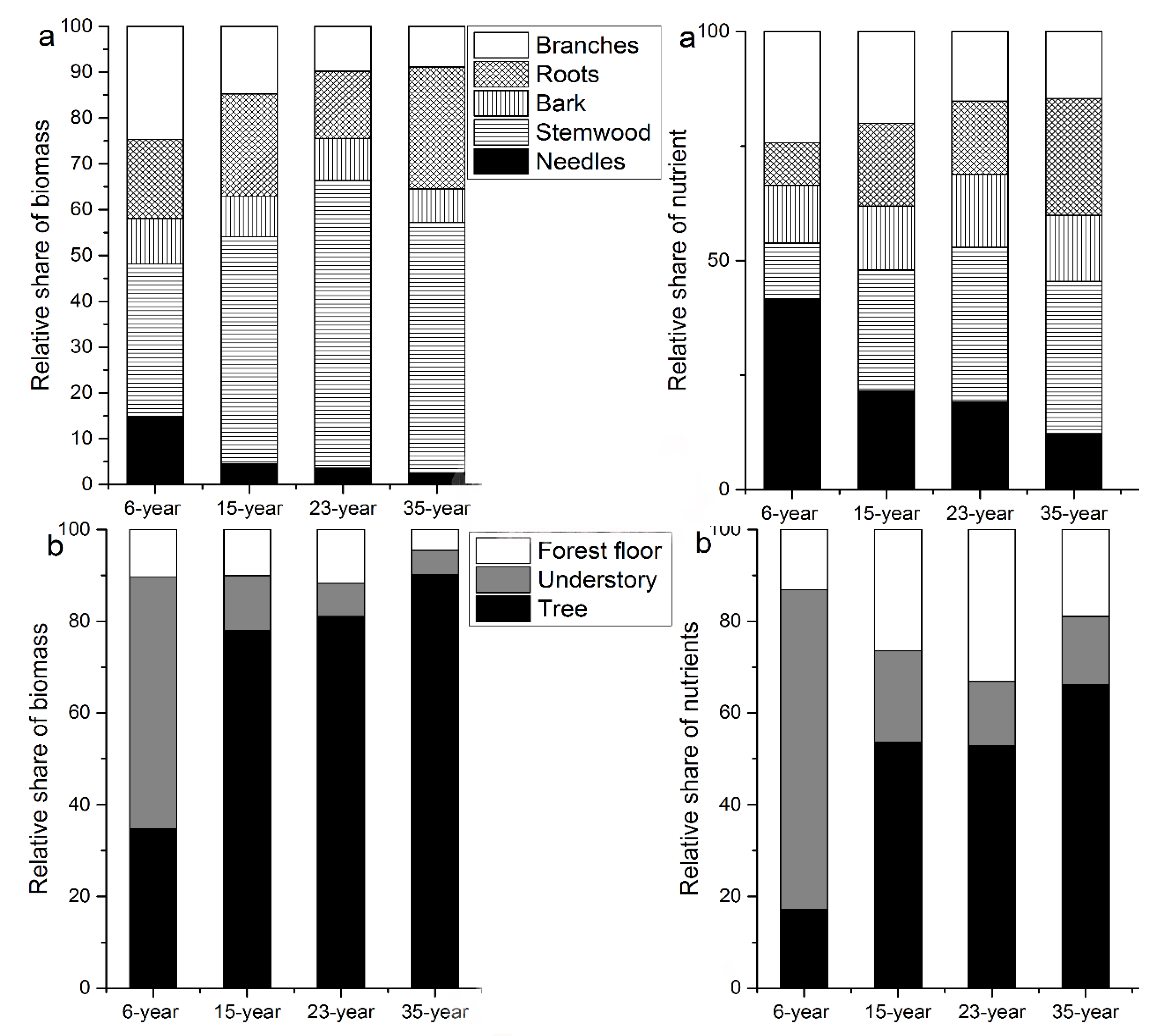
3.3. Nutrient Allocation
4. Discussion
4.1. Nutrient Concentrations
4.2. Nutrient Allocation to Different Compartments
4.3. Nutrient Exports through Harvesting
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hytönen, J. Biomass, nutrient content and energy yield of short-rotation hybrid aspen (P. tremula × P. tremuloides) coppice. For. Ecol. Manag. 2018, 413, 21–31. [Google Scholar] [CrossRef]
- Picchio, R.; Verani, S.; Sperandio, G.; Spina, R.; Marchi, E. Stump grinding on a poplar plantation: Working time, productivity, and economic and energetic inputs. Ecol. Eng. 2012, 40, 117–120. [Google Scholar] [CrossRef]
- Brandtberg, P.O.; Olsson, B.A. Changes in the effects of whole-tree harvesting on soil chemistry during 10 years of stand development. For. Ecol. Manag. 2012, 277, 150–162.4. [Google Scholar] [CrossRef]
- Kaarakka, L.; Tamminen, P.; Saarsalmi, A.; Kukkola, M.; Helmisaari, H.S.; Burton, A.J. Effects of repeated whole-tree harvesting on soil properties and tree growth in a Norway spruce (Picea abies (L.) Karst.) stand. For. Ecol. Manag. 2014, 313, 180–187. [Google Scholar] [CrossRef]
- Mushinski, R.M.; Gentry, T.J.; Dorosky, R.J.; Boutton, T.W. Forest harvest intensity and soil depth alter inorganic nitrogen pool sizes and ammonia oxidizer community composition. Soil Biol. Biochem. 2017, 112, 216–227. [Google Scholar] [CrossRef]
- Nieminen, T.M.; Finér, L.; Laiho, R.; Ukonmaanaho, L.; Laurén, A.; Sarkkola, S.; Nieminen, M. Should harvest residues be left on site in peatland forests to decrease the risk of potassium depletion? For. Ecol. Manag. 2016, 374, 136–145. [Google Scholar]
- Nieminen, M.; Sarkkola, S.; Laurén, A. Impacts of forest harvesting on nutrient, sediment and dissolved organic carbon exports from drained peatlands: A literature review, synthesis and suggestions for the future. For. Ecol. Manag. 2017, 392, 13–20. [Google Scholar] [CrossRef]
- Wall, A. Effect of removal of logging residue on nutrient leaching and nutrient pools in the soil after clearcutting in a Norway spruce stand. For. Ecol. Manag. 2008, 256, 1372–1383. [Google Scholar] [CrossRef]
- Tamminen, P.; Saarsalmi, A.; Smolander, A.; Kukkola, M.; Helmisaari, H.S. Effects of logging residue harvest in thinnings on amounts of soil carbon and nutrients in Scots pine and Norway spruce stands. For. Ecol. Manag. 2012, 263, 31–38. [Google Scholar] [CrossRef]
- Hornbeck, J.W.; Smith, C.T.; Martin, Q.W.; Tritton, L.M.; Pierce, R.S. Effects of intensive harvesting on nutrient capitals of three forest types in New England. For. Ecol. Manag. 1990, 30, 55–64. [Google Scholar] [CrossRef]
- Mendham, D.S.; O’Connell, A.M.; Grove, T.S.; Rance, S.J. Residue management effects on soil carbon and nutrient contents and growth of second rotation eucalypts. For. Ecol. Manag. 2003, 181, 357–372. [Google Scholar] [CrossRef]
- Phillips, T.; Watmough, S.A. A nutrient budget for a selection harvest: Implications for long-term sustainability. Can. J. For. Res. 2012, 42, 2064–2077. [Google Scholar] [CrossRef]
- Gómez-García, E.; Diéguez-Aranda, U.; Cunha, M.; Rodríguez-Soalleiro, R. Comparison of harvest-related removal of aboveground biomass, carbon and nutrients in pedunculate oak stands and in fast-growing tree stands in NW Spain. For. Ecol. Manag. 2016, 365, 119–127. [Google Scholar] [CrossRef]
- Duchesne, L.; Houle, D. Impact of nutrient removal through harvesting on the sustainability of the boreal forest. Ecol. Appl. 2008, 18, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Menegale, M.L.C.; Rocha, J.H.T.; Harrison, R.; Goncalves, J.L.d.M.; Almeida, R.F.; Goncalves, J.L.d.M.; Piccolo, M.d.C.; Hubner, A.; Arthur Junior, J.C.; De Vicente Ferraz, A.; et al. Effect of timber harvest intensities and fertilizer application on stocks of soil C, N, P, and S. Forests 2016, 7, 319. [Google Scholar] [CrossRef]
- Pyttel, P.L.; Köhn, M.; Bauhus, J. Effects of different harvesting intensities on the macro nutrient pools in aged oak coppice forests. For. Ecol. Manag. 2015, 349, 94–105. [Google Scholar] [CrossRef]
- McMahon, D.E.; Vergütz, L.; Valadares, S.V.; da Silva, I.R.; Jackson, R.B. Soil nutrient stocks are maintained over multiple rotations in Brazilian Eucalyptus plantations. For. Ecol. Manag. 2019, 448, 364–375. [Google Scholar] [CrossRef]
- Premer, M.I.; Froese, R.E.; Vance, E.D. Whole-tree harvest and residue recovery in commercial aspen: Implications to forest growth and soil productivity across a rotation. For. Ecol. Manag. 2019, 447, 130–138. [Google Scholar] [CrossRef]
- Ma, X.; Heal, K.V.; Liu, A.; Jarvis, P.G. Nutrient cycling and distribution in different-aged plantations of Chinese fir in southern China. For. Ecol. Manag. 2007, 243, 61–74. [Google Scholar] [CrossRef]
- Jang, W.; Keyes, C.R.; Page-Dumroese, D.S. Long-term effects on distribution of forest biomass following different harvesting levels in the northern Rocky Mountains. For. Ecol. Manag. 2015, 358, 281–290. [Google Scholar] [CrossRef]
- Helmisaari, H.S.; Hanssen, K.H.; Jacobson, S.; Kukkola, M.; Luiro, J.; Saarsalmi, A.; Tamminen, P.; Tveite, B. Logging residue removal after thinning in Nordic boreal forests: Long-term impact on tree growth. For. Ecol. Manag. 2011, 261, 1919–1927. [Google Scholar] [CrossRef]
- Merilä, P.; Mustajärvi, K.; Helmisaari, H.S.; Hilli, S.; Lindroos, A.J.; Nieminen, T.M.; Nöjd, P.; Rautio, P.; Salemaa, M.; Ukonmaanaho, L. Above- and below-ground N stocks in coniferous boreal forests in Finland: Implications for sustainability of more intensive biomass utilization. For. Ecol. Manag. 2014, 311, 17–28. [Google Scholar] [CrossRef]
- Gower, S.T.; Richards, J.H. Larches: Deciduous Conifers in an Evergreen World: In their harsh environments, these unique conifers support a net carbon gain similar to evergreens. Bioscience 1990, 40, 818–826. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, K.; Yan, Q.; Liu, Z.; Yu, L.; Wang, H. Feasibility of implementing thinning in even-aged Larix olgensis plantations to develop uneven-aged larch–broadleaved mixed forests. J. For. Res. 2010, 15, 71–80. [Google Scholar] [CrossRef]
- Yan, T.; Lü, X.T.; Zhu, J.J.; Yang, K.; Yu, L.Z.; Gao, T. Changes in nitrogen and phosphorus cycling suggest a transition to phosphorus limitation with the stand development of larch plantations. Plant Soil 2018, 422, 385–396. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Zu, Y. Temporal changes in SOM, N, P, K, and their stoichiometric ratios during reforestation in China and interactions with soil depths: Importance of deep-layer soil and management implications. For. Ecol. Manag. 2014, 325, 8–17. [Google Scholar] [CrossRef]
- Yang, K.; Yu, L.; Zhang, J.; Zhu, J.; Yan, T. Nutrient removal under different harvesting scenarios for larch plantations in northeast China: Implications for nutrient conservation and management. For. Ecol. Manag. 2017, 400, 150–158. [Google Scholar]
- Liu, S. Nitrogen cycling and dynamic analysis of man made larch forest ecosystem. Plant Soil 1995, 168–169, 391–397. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.Y.; Zeng, D.H. Aboveground biomass and nutrient allocation in an age-sequence of Larix olgensis plantations. J. For. Res. 2011, 22, 71–76. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, Z.; Yang, K.; Zhu, J. Impacts of conversion from secondary forests to larch plantations on the structure and function of microbial communities. Appl. Soil Ecol. 2017, 111, 73–83. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, M.A. Above- and belowground ecosystem biomass and carbon pools in an age-sequence of temperate pine plantation forests. Agric. For. Meteorol. 2006, 140, 51–63. [Google Scholar] [CrossRef]
- Ponette, Q.; Ranger, J.; Ottorini, J.M.; Ulrich, E. Aboveground biomass and nutrient content of five Douglas-fir stands in France. For. Ecol. Manag. 2001, 142, 109–127. [Google Scholar] [CrossRef]
- Turner, J.; Lambert, M.J. Analysis of nutrient depletion in a radiata pine plantation. For. Ecol. Manag. 2011, 262, 1327–1336. [Google Scholar] [CrossRef]
- Johnson, D.W.; Trettin, C.C.; Todd, D.E. Changes in forest floor and soil nutrients in a mixed oak forest 33 years after stem only and whole-tree harvest. For. Ecol. Manag. 2016, 361, 56–68. [Google Scholar] [CrossRef]
- Santos, F.M.; Chaer, G.M.; Diniz, A.R.; de Carvalho Balieiro, F. Nutrient cycling over five years of mixed-species plantations of Eucalyptus and Acacia on a sandy tropical soil. For. Ecol. Manag. 2017, 384, 110–121. [Google Scholar] [CrossRef]
- Alifragis, D.; Smiris, P.; Maris, F.; Kavvadias, V.; Konstantinidou, E.; Stamou, N. The effect of stand age on the accumulation of nutrients in the aboveground components of an Aleppo pine ecosystem. For. Ecol. Manag. 2001, 141, 259–269. [Google Scholar] [CrossRef]
- Peri, P.L.; Gargaglione, V.; Pastur, G.M. Dynamics of above- and below-ground biomass and nutrient accumulation in an age sequence of Nothofagus antarctica forest of Southern Patagonia. For. Ecol. Manag. 2006, 233, 85–99. [Google Scholar] [CrossRef]
- Zhou, L.; Shalom, A.D.D.; Wu, P.; He, Z.; Liu, C.; Ma, X. Biomass production, nutrient cycling and distribution in age-sequence Chinese fir (Cunninghamia lanceolate) plantations in subtropical China. J. For. Res. 2016, 27, 357–368. [Google Scholar] [CrossRef]
- Turner, J.; Lambert, M.; Turner, S. Long term carbon and nutrient dynamics within two small radiata pine catchments. For. Ecol. Manag. 2017, 389, 1–14. [Google Scholar] [CrossRef]
- Chang, Y.; Li, N.; Wang, W.; Liu, X.; Du, F.; Yao, D. Nutrients resorption and stoichiometry characteristics of different-aged plantations of Larix kaempferi in the Qinling Mountains, central China. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Peri, P.L.; Gargaglione, V.; Pastur, G.M. Above- and belowground nutrients storage and biomass accumulation in marginal Nothofagus antarctica forests in Southern Patagonia. For. Ecol. Manag. 2008, 255, 2502–2511. [Google Scholar] [CrossRef]
- Ares, A.; Neill, A.R.; Puettmann, K.J. Understory abundance, species diversity and functional attribute response to thinning in coniferous stands. For. Ecol. Manag. 2010, 260, 1104–1113. [Google Scholar] [CrossRef]
- Son, Y.; Gower, S.T. Nitrogen and phosphorus distribution for five plantation species in southwestern Wisconsin. For. Ecol. Manag. 1992, 53, 175–193. [Google Scholar] [CrossRef]
- Caldeira, D.R.M.; Gonçalves, J.L.M.; Leite, F.P.; Brandani, C.B.; Brunet, D.; Moreira, M.Z.; Bouillet, J.-P.; Paula, R.R.; Tardy, F.; Voigtlaender, M.; et al. Nitrogen cycling in monospecific and mixed-species plantations of Acacia mangium and Eucalyptus at 4 sites in Brazil. For. Ecol. Manag. 2019, 436, 56–67. [Google Scholar]
- Palviainen, M.; Finér, L. Estimation of nutrient removals in stem-only and whole-tree harvesting of Scots pine, Norway spruce, and birch stands with generalized nutrient equations. Eur. J. For. Res. 2012, 131, 945–964. [Google Scholar] [CrossRef]
- Hernández, J.; del Pino, A.; Salvo, L.; Arrarte, G. Nutrient export and harvest residue decomposition patterns of a Eucalyptus dunnii Maiden plantation in temperate climate of Uruguay. For. Ecol. Manag. 2009, 258, 92–99. [Google Scholar] [CrossRef]
- Bravo-Oviedo, A.; Ruiz-Peinado, R.; Onrubia, R.; del Río, M. Thinning alters the early-decomposition rate and nutrient immobilization-release pattern of foliar litter in Mediterranean oak-pine mixed stands. For. Ecol. Manag. 2017, 391, 309–320. [Google Scholar] [CrossRef]
- Schumacher, M.V.; Rodríguez-Soalleiro, R.; de Oliveira Ramos, L.O.; Viera, M.; Bonacina, D.M. Biomass and nutrient allocation to aboveground components in fertilized Eucalyptus saligna and E. urograndis plantations. New For. 2017, 48, 445–462. [Google Scholar]
- Qiao, Y.; Miao, S.; Silva, L.C.R.; Horwath, W.R. Understory species regulate litter decomposition and accumulation of C and N in forest soils: A long-term dual-isotope experiment. For. Ecol. Manag. 2014, 329, 318–327. [Google Scholar] [CrossRef]
- Turner, J.; Lambert, M. Pattern of carbon and nutrient cycling in a small Eucalyptus forest catchment, NSW. For. Ecol. Manag. 2016, 372, 258–268. [Google Scholar] [CrossRef]
- Larson, A.J.; Goodburn, J.M.; Belote, R.T.; Page-Dumroese, D.S.; Schaedel, M.S.; Affleck, D.L.R. Early forest thinning changes aboveground carbon distribution among pools, but not total amount. For. Ecol. Manag. 2017, 389, 187–198. [Google Scholar]
- Nunes, A.; Oliveira, G.; Cabral, M.S.; Branquinho, C.; Correia, O. Beneficial effect of pine thinning in mixed plantations through changes in the understory functional composition. Ecol. Eng. 2014, 70, 387–396. [Google Scholar] [CrossRef]
- Trentini, C.P.; Campanello, P.I.; Villagra, M.; Ritter, L.; Ares, A.; Goldstein, G. Thinning of loblolly pine plantations in subtropical Argentina: Impact on microclimate and understory vegetation. For. Ecol. Manag. 2017, 384, 236–247. [Google Scholar] [CrossRef]
- Dagley, C.M.; Berrill, J.P.; Leonard, L.P.; Kim, Y.G. Restoration thinning enhances growth and diversity in mixed redwood/Douglas-fir stands in northern California, U.S.A. Restor. Ecol. 2018, 26, 1170–1179. [Google Scholar] [CrossRef]
- Carneiro, M.; Serrão, V.; Fabião, A.; Madeira, M.; Balsemão, I.; Hilário, L. Does harvest residue management influence biomass and nutrient accumulation in understory vegetation of Eucalyptus globulus Labill. plantations in a Mediterranean environment? For. Ecol. Manag. 2009, 257, 527–535. [Google Scholar] [CrossRef][Green Version]
- Van Cleemput, O.; Muhindo, D.; Boeckx, P.; Cizungu, L.; Staelens, J.; Walangululu, J.; Huygens, D. Litterfall and leaf litter decomposition in a central African tropical mountain forest and Eucalyptus plantation. For. Ecol. Manag. 2014, 326, 109–116. [Google Scholar]
- Seiwa, K.; Etoh, Y.; Hisita, M.; Masaka, K.; Imaji, A.; Ueno, N.; Hasegawa, Y.; Konno, M.; Kanno, H.; Kimura, M. Roles of thinning intensity in hardwood recruitment and diversity in a conifer, Criptomeria japonica plantation: A 5-year demographic study. For. Ecol. Manag. 2012, 269, 177–187. [Google Scholar] [CrossRef]
- Dang, P.; Gao, Y.; Liu, J.; Yu, S.; Zhao, Z. Effects of thinning intensity on understory vegetation and soil microbial communities of a mature Chinese pine plantation in the Loess Plateau. Sci. Total Environ. 2018, 630, 171–180. [Google Scholar] [CrossRef]
- Piirainen, S.; Finér, L.; Starr, M. Changes in forest floor and mineral soil carbon and nitrogen stocks in a boreal forest after clear-cutting and mechanical site preparation. Eur. J. Soil Sci. 2015, 66, 735–743. [Google Scholar] [CrossRef]
- Briggs, R.D.; Hornbeck, J.W.; Smith, C.T.; Lemin, R.C.; McCormack, M.L. Long-term effects of forest management on nutrient cycling in spruce-fir forests. For. Ecol. Manag. 2000, 138, 285–299. [Google Scholar] [CrossRef]
- Stark, N.M. Nutrient Losses from Timber Harvesting in a Larch/Douglas-Fir Forest; USDA Forest Service Research Pap. INT-231; Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1979. [Google Scholar]
- O’Connell, A.M.; Sankaran, K.V.; Grove, T.S.; Mendham, D.S.; Rance, S.J.; Kumaraswamy, S. Harvest residue effects on soil organic matter, nutrients and microbial biomass in eucalypt plantations in Kerala, India. For. Ecol. Manag. 2014, 328, 140–149. [Google Scholar]
- Achat, D.L.; Deleuze, C.; Landmann, G.; Pousse, N.; Ranger, J.; Augusto, L. Quantifying consequences of removing harvesting residues on forest soils and tree growth - A meta-analysis. For. Ecol. Manag. 2015, 348, 124–141. [Google Scholar] [CrossRef]
- Nieminen, M.; Laiho, R.; Sarkkola, S.; Penttilä, T. Whole-tree, stem-only, and stump harvesting impacts on site nutrient capital of a Norway spruce-dominated peatland forest. Eur. J. For. Res. 2016, 135, 531–538. [Google Scholar] [CrossRef]
- Turner, J.; Lambert, M.J. Nutrient cycling in age sequences of two Eucalyptus plantation species. For. Ecol. Manag. 2008, 255, 1701–1712. [Google Scholar] [CrossRef]
- Hopmans, P.; Stewart, H.T.L.; Flinn, D.W. Impacts of harvesting on nutrients in a eucalypt ecosystem in southeastern Australia. For. Ecol. Manag. 1993, 59, 29–51. [Google Scholar] [CrossRef]
- Picchio, R.; Sirna, A.; Spina, R.; Sperandio, G.; Verani, S. Mechanized harvesting of eucalypt coppicefor biomass production using high mechanization level. Croat. J. For. Eng. 2012, 33, 15–24. [Google Scholar]
- Hume, A.M.; Chen, H.Y.H.; Taylor, A.R. Intensive forest harvesting increases susceptibility of northern forest soils to carbon, nitrogen and phosphorus loss. J. Appl. Ecol. 2018, 55, 246–255. [Google Scholar] [CrossRef]

| Ages | Plots Number | DBH (cm) | Tree Height (m) | Density (N ha−1) | Elevation (m) | Soil Depth (cm) | Slope (°) | Year (s) Thinned |
|---|---|---|---|---|---|---|---|---|
| 6-year | 6 | 3.3 ± 0.4 | 3.8 ± 0.3 | 3092 ± 252 | 1552 | 63.9 ± 3.8 | 23 | - |
| 15-year | 6 | 11.1 ± 1.0 | 11.5 ± 0.6 | 2316 ± 150 | 1786 | 70.0 ± 2.3 | 19 | 2008 |
| 23-year | 6 | 17.5 ± 1.3 | 17.1 ± 1.6 | 697 ± 68 | 1580 | 73.0 ± 3.2 | 19 | 1997, 2003, 2008 |
| 35-year | 6 | 21.4 ± 1.8 | 18.8 ± 1.1 | 558 ± 65 | 1584 | 63.6 ± 3.2 | 26 | 1987, 1992, 1999, 2004 |
| No. | Parameter | Stand Age | Soil Depth | Interaction | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| 1 | pH | 22.63 | 0.00 | 0.89 | 0.42 | 0.27 | 0.95 |
| 2 | SOM | 88.34 | 0.00 | 48.03 | 0.00 | 11.04 | 0.00 |
| 3 | TN | 29.86 | 0.00 | 74.15 | 0.00 | 5.41 | 0.00 |
| 4 | AN | 106.98 | 0.00 | 27.11 | 0.00 | 7.23 | 0.00 |
| 5 | TP | 15.91 | 0.00 | 0.62 | 0.54 | 1.10 | 0.17 |
| 6 | AP | 36.81 | 0.00 | 114.03 | 0.00 | 14.07 | 0.00 |
| 7 | TK | 2.41 | 0.06 | 0.43 | 0.65 | 0.06 | 1.00 |
| 8 | AK | 8.13 | 0.00 | 17.25 | 0.00 | 5.68 | 0.00 |
| 9 | Ca | 0.81 | 0.50 | 0.01 | 0.99 | 0.29 | 0.94 |
| 10 | Mg | 0.46 | 0.71 | 0.36 | 0.70 | 0.47 | 0.83 |
| Ages | Components | Biomass | Nutrient Stocks (kg ha−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (Mg ha−1) | Percentage (%) | N | P | K | Ca | Mg | Total | Percentage (%) | ||
| 6-year | Needles | 1.2 | 14.8 | 24.6 | 4.6 | 6.4 | 10.5 | 2.0 | 48.1 | 41.6 |
| Stemwood | 2.7 | 33.3 | 2.8 | 1.2 | 2.5 | 6.8 | 0.7 | 13.9 | 12.1 | |
| Bark | 0.8 | 9.9 | 3.2 | 1.1 | 4.0 | 5.7 | 0.5 | 14.5 | 12.6 | |
| Roots | 1.4 | 17.3 | 3.4 | 1.3 | 3.2 | 2.0 | 0.8 | 10.7 | 9.3 | |
| Branches | 2.0 | 24.7 | 9.4 | 2.5 | 7.5 | 7.4 | 1.2 | 28.0 | 24.3 | |
| Total | 8.1 | 100 | 43.4 | 10.6 | 23.7 | 32.4 | 5.2 | 115.2 | 100 | |
| Percentage (%) | - | - | 37.7 | 9.2 | 20.5 | 28.1 | 4.5 | 100 | - | |
| 15-year | Needles | 2.9 | 4.5 | 69.3 | 11.0 | 28.4 | 25.4 | 4.9 | 139.0 | 21.5 |
| Stemwood | 32.0 | 49.6 | 20.2 | 11.1 | 27.9 | 103.0 | 8.9 | 171.1 | 26.4 | |
| Bark | 5.7 | 8.8 | 22.7 | 7.3 | 23.0 | 34.0 | 3.6 | 90.7 | 14 | |
| Roots | 14.4 | 22.3 | 34.6 | 13.1 | 41.4 | 20.4 | 7.5 | 117.0 | 18.1 | |
| Branches | 9.5 | 14.7 | 44.8 | 10.8 | 37.3 | 29.8 | 6.8 | 129.4 | 20 | |
| Total | 64.5 | 100 | 191.5 | 53.2 | 157.9 | 212.7 | 31.7 | 647.0 | 100 | |
| Percentage (%) | - | - | 29.6 | 8.2 | 24.4 | 32.9 | 4.9 | 100 | - | |
| 23-year | Needles | 3.1 | 3.6 | 86.7 | 8.8 | 26.2 | 27.1 | 5.2 | 154.0 | 19.1 |
| Stemwood | 53.6 | 62.7 | 24.7 | 11.2 | 25.7 | 196.7 | 15.5 | 273.8 | 33.9 | |
| Bark | 7.9 | 9.2 | 35.8 | 9.9 | 34.7 | 42.0 | 5.8 | 128.1 | 15.8 | |
| Roots | 12.5 | 14.6 | 38.7 | 9.9 | 41.5 | 30.6 | 9.1 | 129.6 | 16 | |
| Branches | 8.4 | 9.8 | 42.6 | 8.3 | 35.4 | 30.0 | 6.6 | 123.0 | 15.2 | |
| Total | 85.5 | 100 | 228.5 | 48.0 | 163.5 | 326.4 | 42.1 | 808.5 | 100 | |
| Percentage (%) | - | - | 28.3 | 5.9 | 20.2 | 40.4 | 5.2 | 100 | - | |
| 35-year | Needles | 3.0 | 2.6 | 67.9 | 8.5 | 21.8 | 24.2 | 5.1 | 127.5 | 12.2 |
| Stemwood | 63.6 | 54.6 | 22.6 | 13.9 | 20.4 | 261.3 | 27.8 | 346.1 | 33.2 | |
| Bark | 8.6 | 7.4 | 32.1 | 10.7 | 38.0 | 62.3 | 7.2 | 150.3 | 14.4 | |
| Roots | 31.0 | 26.6 | 103.8 | 20.3 | 57.7 | 64.8 | 18.6 | 265.2 | 25.5 | |
| Branches | 10.3 | 8.4 | 51.8 | 11.5 | 33.1 | 48.3 | 7.6 | 152.3 | 14.6 | |
| Total | 116.5 | 100 | 278.2 | 65.0 | 171.0 | 460.9 | 66.3 | 1041.4 | 100 | |
| Percentage (%) | - | - | 26.7 | 6.2 | 16.4 | 44.3 | 6.4 | 100 | - | |
| Ages | Compartments | Biomass | Nutrient Stocks (kg ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (Mg ha−1) | Percentage (%) | N | P | K | Ca | Mg | Total | ||
| 6-year | Tree | 8.1 | 34.8 | 43.4 | 10.6 | 23.7 | 32.4 | 5.2 | 115.3 |
| Understory | 12.8 | 54.9 | 158.2 | 36.9 | 109.9 | 124.6 | 37.7 | 467.3 | |
| Forest floor | 2.4 | 10.3 | 30.6 | 4.3 | 5.9 | 40.5 | 6.9 | 88.1 | |
| Soil | - | - | 4570 | 1560 | 84,470 | 36,980 | 42,790 | 170,370 | |
| 15-year | Tree | 64.5 | 78.0 | 191.5 | 53.2 | 157.9 | 212.7 | 31.7 | 647.1 |
| Understory | 9.9 | 12.0 | 92.8 | 12.2 | 50.6 | 72.8 | 12.9 | 241.3 | |
| Forest floor | 8.3 | 10.0 | 120.8 | 15.7 | 19.0 | 140.1 | 23.8 | 319.3 | |
| Soil | - | - | 5690 | 1690 | 87,870 | 35,100 | 42,610 | 172,960 | |
| 23-year | Tree | 85.5 | 81.0 | 228.5 | 48.0 | 163.5 | 326.4 | 42.1 | 808.5 |
| Understory | 7.7 | 7.3 | 84.6 | 12.2 | 49.0 | 57.6 | 10.7 | 214.2 | |
| Forest floor | 12.3 | 11.7 | 201.1 | 22.7 | 29.3 | 215.9 | 37.6 | 506.5 | |
| Soil | - | - | 6970 | 1590 | 84,990 | 34,670 | 41,430 | 169,650 | |
| 35-year | Tree | 116.5 | 90.2 | 278.2 | 65.0 | 171.0 | 460.9 | 66.4 | 1041.5 |
| Understory | 6.9 | 5.3 | 81.4 | 17.4 | 44.2 | 76.5 | 14.3 | 233.8 | |
| Forest floor | 5.8 | 4.5 | 117.3 | 11.9 | 15.7 | 130.1 | 22.4 | 297.4 | |
| Soil | - | - | 7720 | 1790 | 85,560 | 37,340 | 40,400 | 172,810 | |
| Species | Location | Layer | Biomass | Nutrient Stocks (kg ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (Mg ha−1) | N | P | K | Ca | Mg | References | ||||
| Larch | 34°13′ N | soil | - | 7720 | 1830 | 85,560 | 37,340 | 38,400 | This study | |
| 105°48′ E | tree | 116.5 | 278.22 | 65.01 | 170.98 | 460.9 | 66.35 | |||
| Eucalyptus | 33°24′ S | soil | - | 4188.2 | 30.4(AP) | 1063.6 | 1289.5 | 595.7 | [50] | |
| 105°00′ E | tree | 149.8 | 220.6 | 18.15 | 215.4 | 687.3 | 115.3 | |||
| Chinese fir | 25.8° N | soil | - | 6740 | 1770 | 125,000 | 21,900 | 21,900 | [19] | |
| 117°8 E | tree | 142.3 | 295.3 | 33.72 | 234.4 | 281.1 | 59.85 | |||
| Spruce-fir | 45°57′ N | soil | - | 5833 | 2697 | 10,001 | 10,332 | 36,450 | [60] | |
| 69°19′ W | tree | - | 376 | 54 | 224 | 494 | 52 | |||
| Mixed oak | - | soil | - | - | - | - | - | - | [34] | |
| tree | 149.3 | 230 | 18 | 40 | 480 | 38 | ||||
| Ages | Scenarios | Biomass | Nutrient Stocks (kg ha−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (Mg ha−1) | Percentage (%) | N | P | K | Ca | Mg | Total | Percentage (%) | |||
| 15-year | 30% thinning | SOH | 11.3 | 58.5 | 12.9 | 5.5 | 15.3 | 41.1 | 3.8 | 78.5 | 40.4 |
| WTH | 19.4 | 100 | 57.4 | 16.0 | 47.4 | 63.8 | 9.5 | 194.1 | 100 | ||
| 23-year | Clear-cutting | SOH | 61.5 | 71.9 | 60.5 | 21.1 | 60.4 | 238.7 | 21.3 | 402 | 49.7 |
| WTH | 85.5 | 100 | 228.5 | 48.1 | 163.5 | 326.4 | 42.2 | 808.7 | 100 | ||
| 35-year | Clear-cutting | SOH | 72.2 | 62.0 | 54.7 | 24.6 | 58.4 | 323.6 | 35 | 496.3 | 47.6 |
| WTH | 116.5 | 100 | 278.2 | 64.9 | 171 | 460.9 | 66.3 | 1041.3 | 100 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Chen, D.; Sun, X. Nutrient Allocation to Different Compartments of Age-Sequence Larch Plantations in China. Forests 2019, 10, 759. https://doi.org/10.3390/f10090759
Wang H, Chen D, Sun X. Nutrient Allocation to Different Compartments of Age-Sequence Larch Plantations in China. Forests. 2019; 10(9):759. https://doi.org/10.3390/f10090759
Chicago/Turabian StyleWang, Hongxing, Dongsheng Chen, and Xiaomei Sun. 2019. "Nutrient Allocation to Different Compartments of Age-Sequence Larch Plantations in China" Forests 10, no. 9: 759. https://doi.org/10.3390/f10090759
APA StyleWang, H., Chen, D., & Sun, X. (2019). Nutrient Allocation to Different Compartments of Age-Sequence Larch Plantations in China. Forests, 10(9), 759. https://doi.org/10.3390/f10090759





