Genome-Wide Analysis of NAC Gene Family in Betula pendula
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification and Basic Information Statistics of BpNAC Gene Family
2.2. Phylogenetic Analysis based on the BpNAC Domain
2.3. Functional Analysis of the BpNAC Gene Family
2.4. Differential Expression Profile of BpNAC Gene Family
2.5. Quantitative RT-PCR
2.6. Co-Expression Network Construction and GO Enrichment Analysis of BpNAC Gene Family
3. Results
3.1. Genome-Wide Identification of the BpNAC Gene Family
3.2. Sequence-Structure Features of BpNAC Gene Family
3.3. Chromosomal Location and Gene Duplication of the BpNAC Gene Family
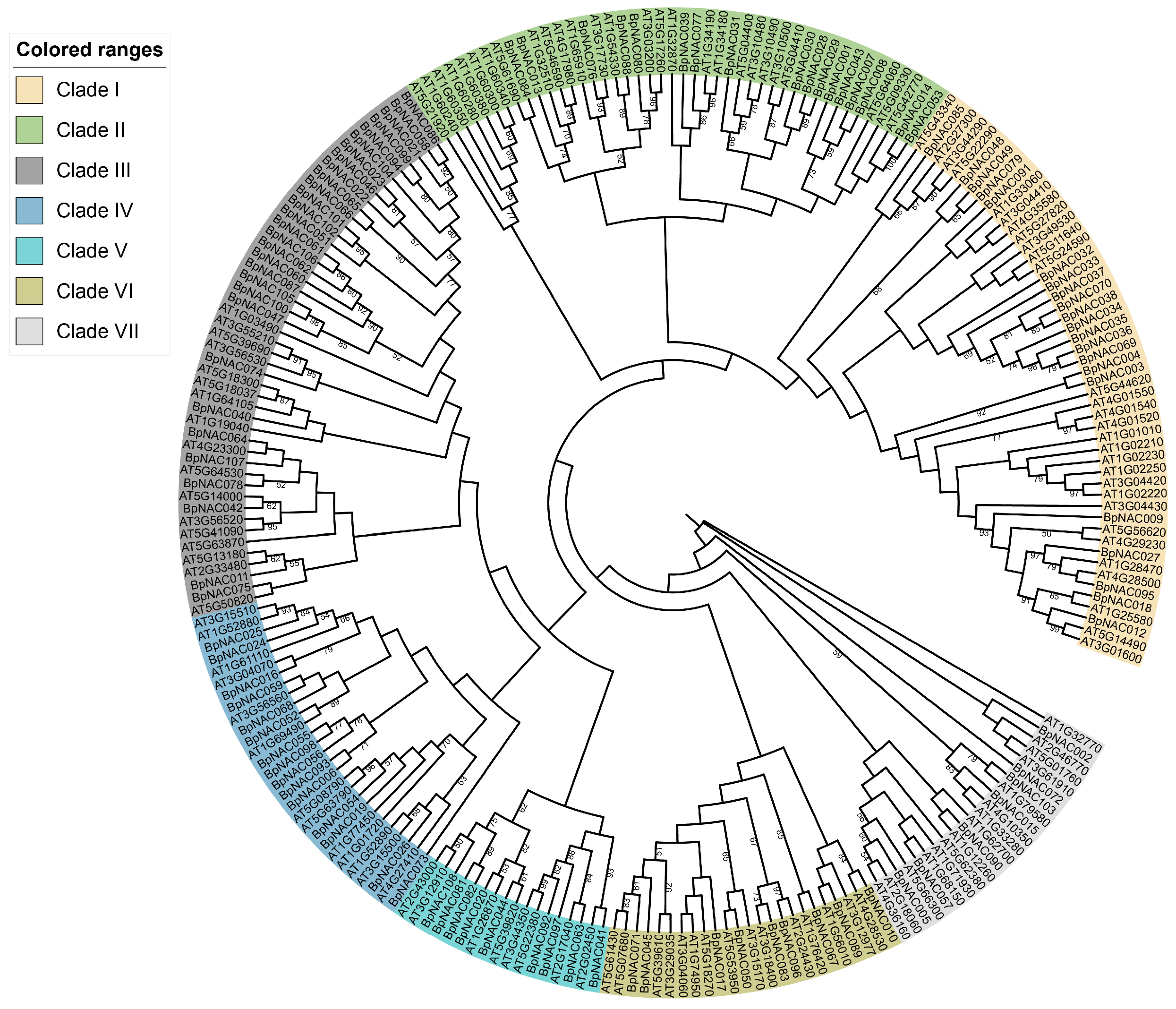
3.4. Phylogenetic Analysis of the BpNAC Gene Family
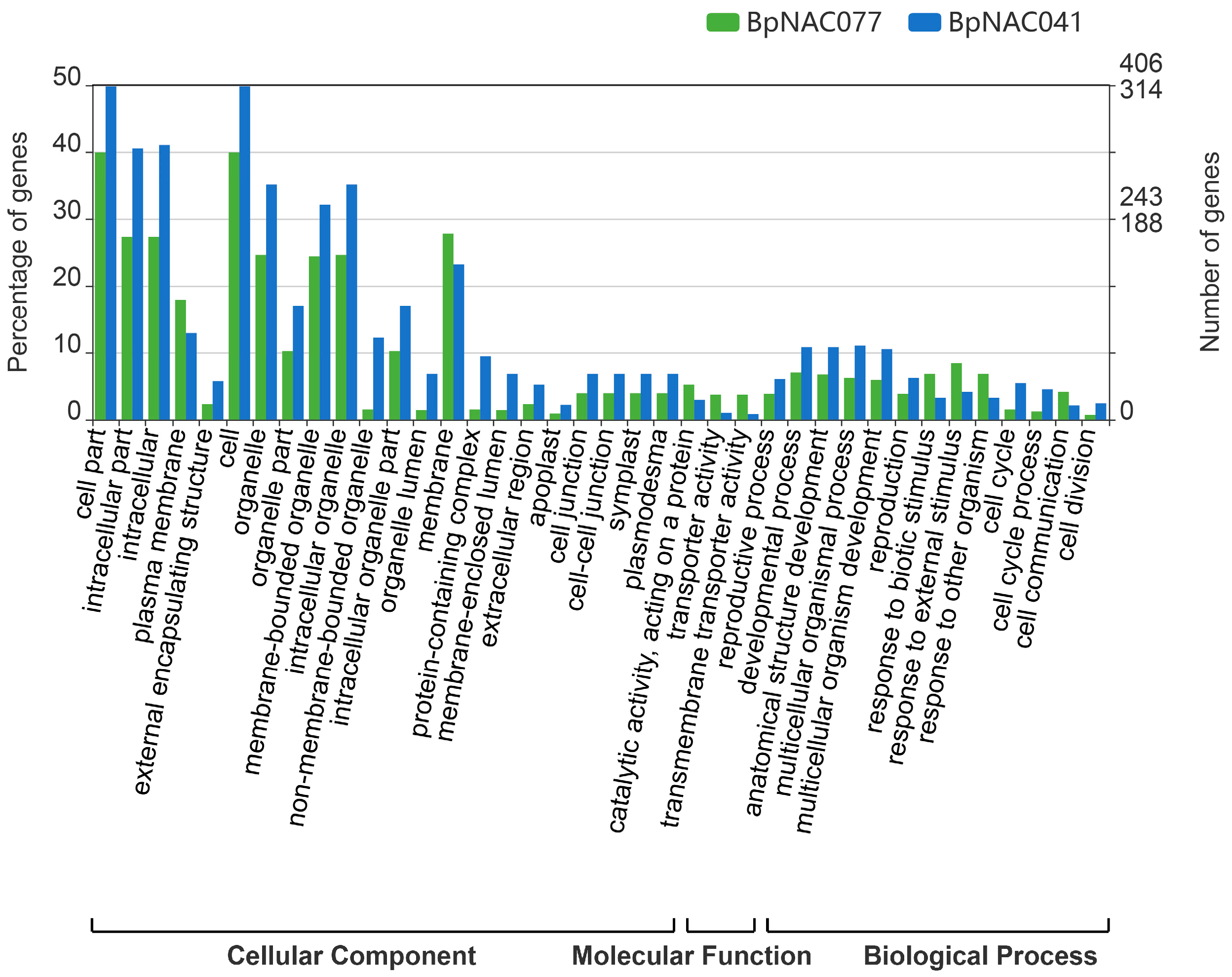
3.5. Functional Analysis of the BpNAC Gene Family
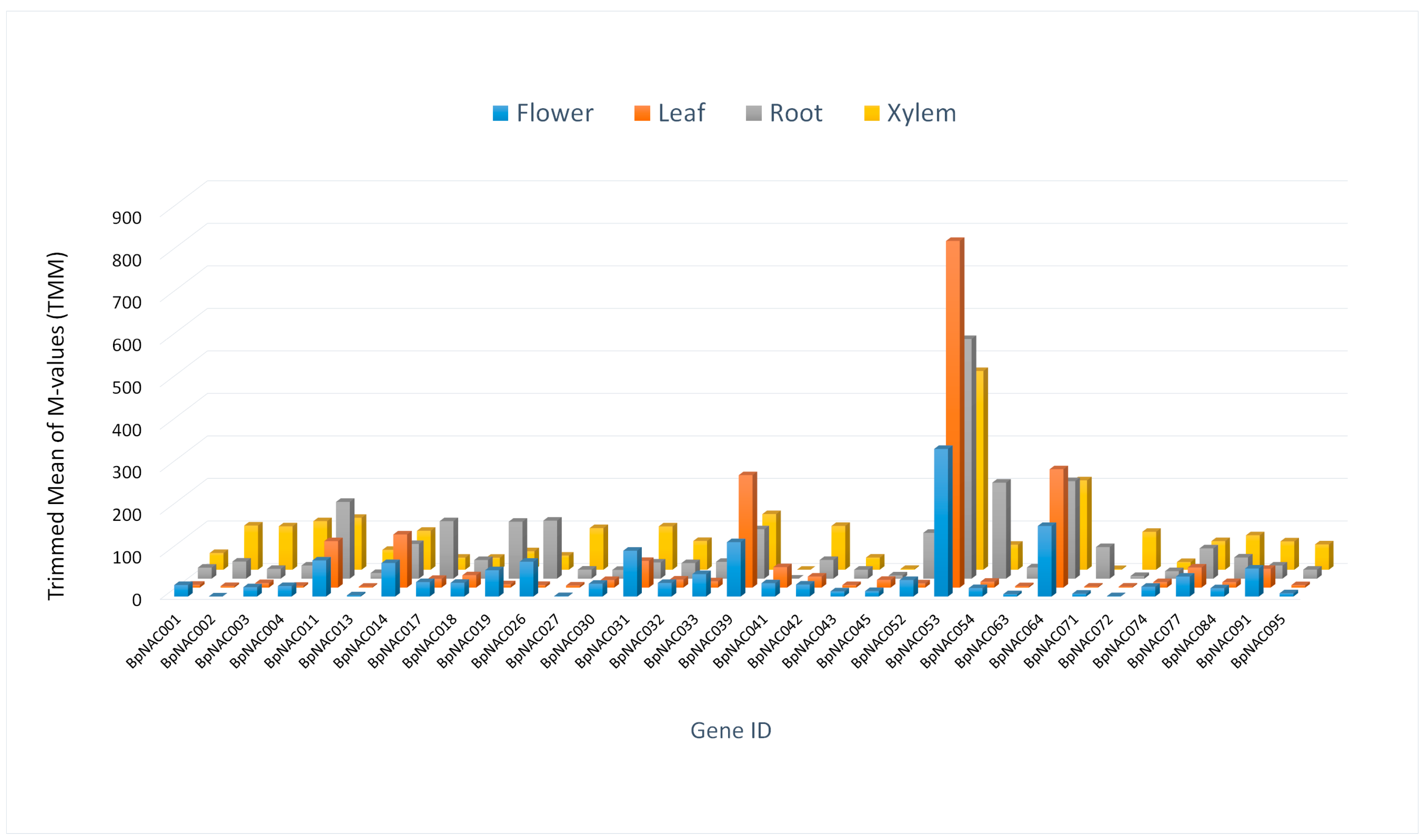
3.6. Tissue-Specific Expression Profiles of BpNACs
3.7. Differential Expression Profiles of the BpNAC Gene Family under Cold Stress
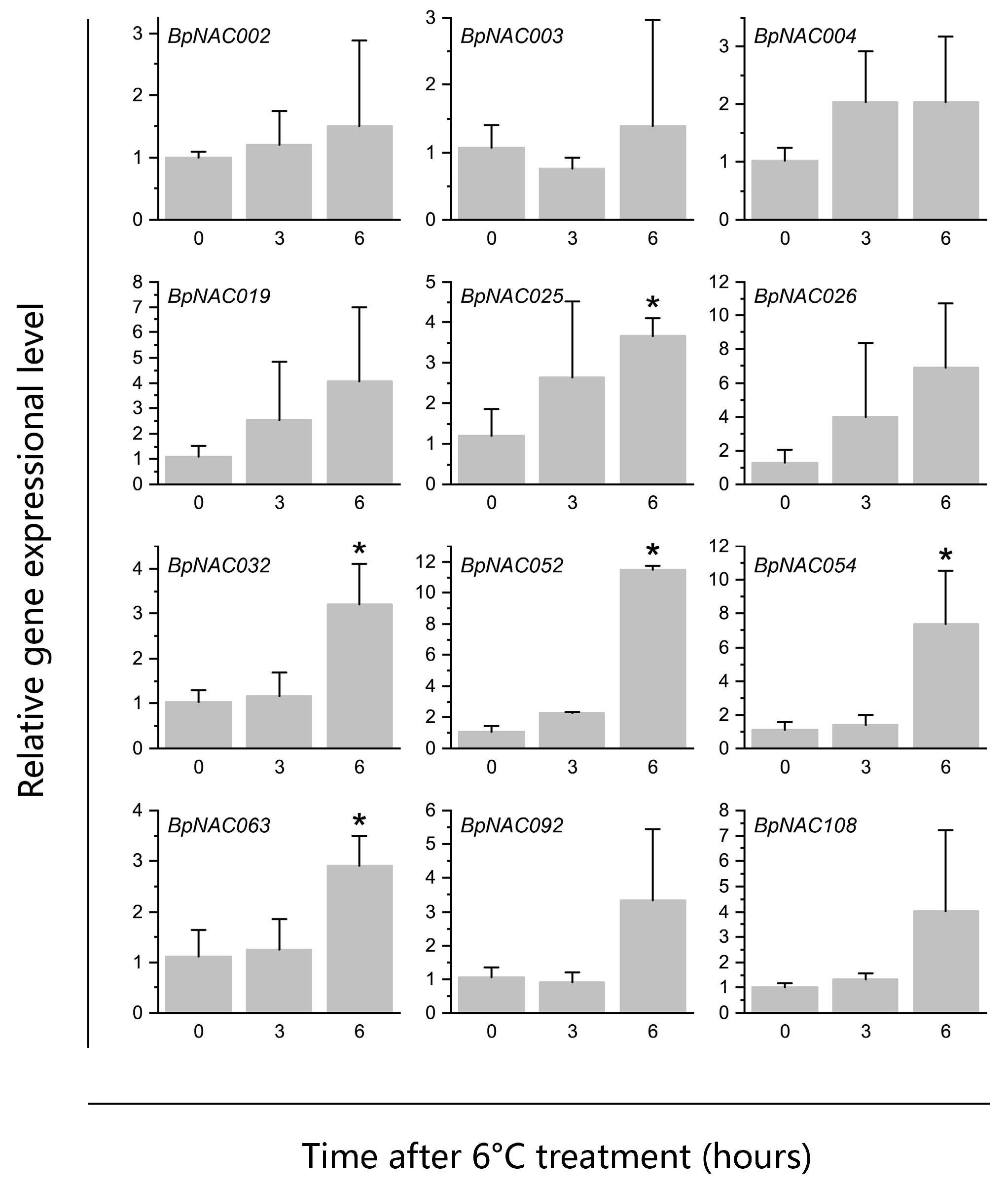
3.8. Validation of DEGs Identified by RNA-seq Using qRT-PCR
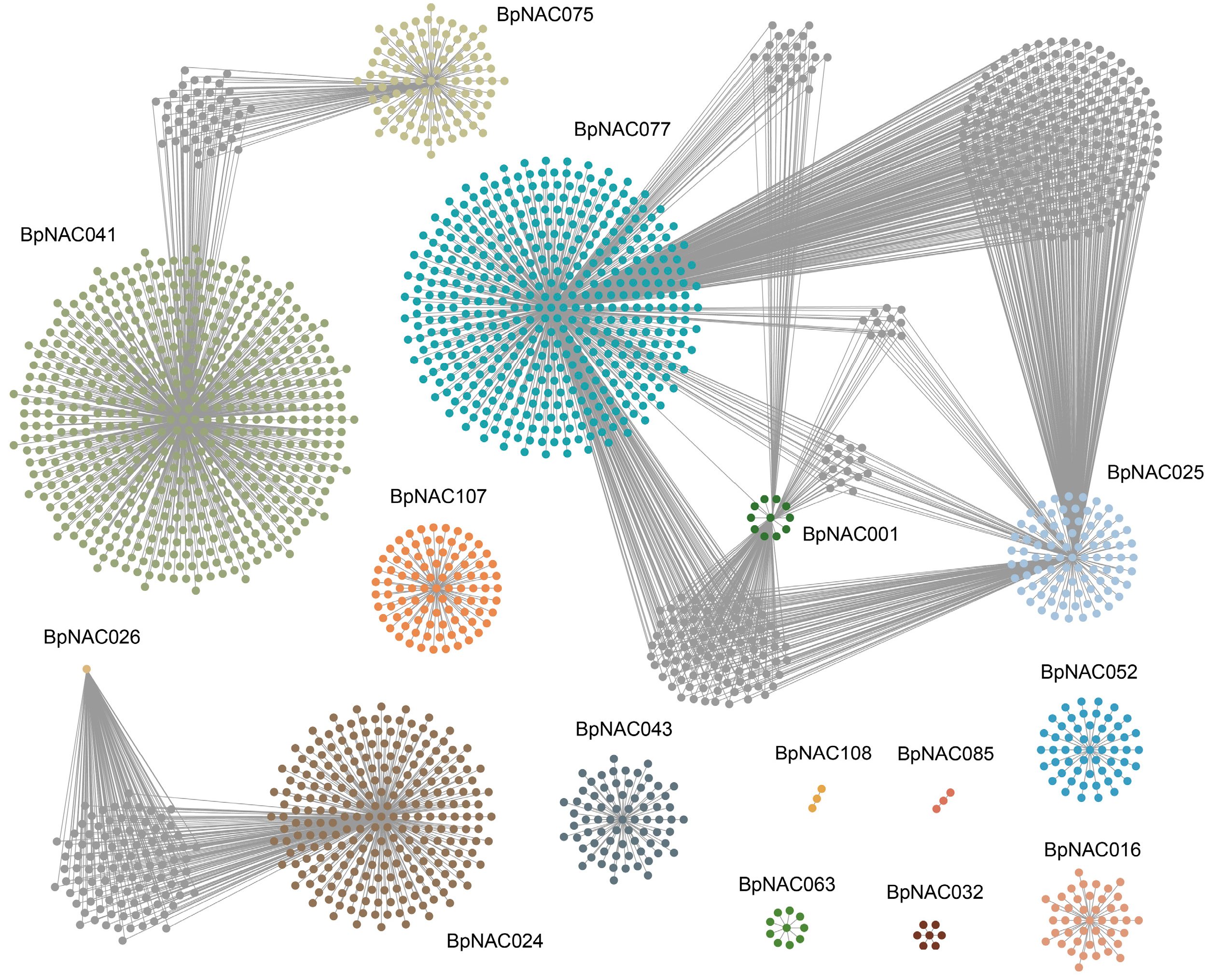
3.9. Co-Expression of Low-Temperature Stress Genes in the BpNAC Gene Family
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pearson, H. Genetics: What Is a Gene? Nature Publishing Group: London, UK, 2006. [Google Scholar]
- Die, J.V.; Gil, J.; Millan, T. Genome-wide identification of the auxin response factor gene family in Cicer arietinum. BMC Genom. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Initiative, A.G. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, R.; Zhang, Z.; Li, L.; Gu, X.; Fan, W.; Lucas, W.J.; Wang, X.; Xie, B.; Ni, P. The genome of the cucumber, Cucumis sativus L. Nat. Genet. 2009, 41, 1275–1281. [Google Scholar] [CrossRef]
- Shulaev, V.; Sargent, D.J.; Crowhurst, R.N.; Mockler, T.C.; Folkerts, O.; Delcher, A.L.; Jaiswal, P.; Mockaitis, K.; Liston, A.; Mane, S.P. The genome of woodland strawberry (Fragaria vesca). Nat. Genet. 2011, 43, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Tuskan, G.A.; Difazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D. The genome of the domesticated apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Salojärvi, J.; Smolander, O.-P.; Nieminen, K.; Rajaraman, S.; Safronov, O.; Safdari, P.; Lamminmäki, A.; Immanen, J.; Lan, T.; Tanskanen, J. Genome sequencing and population genomic analyses provide insights into the adaptive landscape of silver birch. Nat. Genet. 2017, 49, 904–912. [Google Scholar] [CrossRef]
- Bolger, M.E.; Weisshaar, B.; Scholz, U.; Stein, N.; Usadel, B.; Mayer, K.F.X. Plant genome sequencing-applications for crop improvement. Curr. Opin. Biotechnol. 2014, 26, 31–37. [Google Scholar] [CrossRef]
- Latchman, D.S. Transcription factors: An overview. Int. J. Exp. Pathol. 1993, 74, 417–422. [Google Scholar] [CrossRef]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2016, 45, gkw982. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.-Z.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.; Samaha, R. Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Bokowiec, M.T.; Han, S.; Zhang, H.; Brannock, J.F.; Chen, X.; Laudeman, T.W.; Timko, M.P. Tobacco transcription factors: Novel insights into transcriptional regulation in the Solanaceae. Plant Physiol. 2008, 147, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.B.; Qi, G.A.; Kong, Y.Z.; Kong, D.J.; Gao, Q.A.; Zhou, G.K. Comprehensive Analysis of NAC Domain Transcription Factor Gene Family in Populus trichocarpa. BMC Plant Biol. 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Souer, E.; van Houwelingen, A.; Kloos, D.; Mol, J.; Koes, R. The no apical meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell 1996, 85, 159–170. [Google Scholar] [CrossRef]
- Aida, M.; Ishida, T.; Fukaki, H.; Fujisawa, H.; Tasaka, M. Genes involved in organ separation in Arabidopsis: An analysis of the cup-shaped cotyledon mutant. Plant Cell 1997, 9, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Nevo, E.; Sun, D.; Peng, J. Phylogenetic analyses unravel the evolutionary history of NAC proteins in plants. Evol. Int. J. Org. Evol. 2012, 66, 1833–1848. [Google Scholar] [CrossRef]
- Mitsuda, N.; Iwase, A.; Yamamoto, H.; Yoshida, M.; Seki, M.; Shinozaki, K.; Ohme-Takagi, M. NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell 2007, 19, 270–280. [Google Scholar] [CrossRef]
- Zhong, R.; Demura, T.; Ye, Z.-H. SND1, a NAC domain transcription factor, is a key regulator of secondary wall synthesis in fibers of Arabidopsis. Plant Cell 2006, 18, 3158–3170. [Google Scholar] [CrossRef]
- Zhao, C.; Avci, U.; Grant, E.H.; Haigler, C.H.; Beers, E.P. XND1, a member of the NAC domain family in Arabidopsis thaliana, negatively regulates lignocellulose synthesis and programmed cell death in xylem. Plant J. 2008, 53, 425–436. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Kubo, M.; Fukuda, H.; Demura, T. VASCULAR-RELATED NAC-DOMAIN7 is involved in the differentiation of all types of xylem vessels in Arabidopsis roots and shoots. Plant J. 2008, 55, 652–664. [Google Scholar] [CrossRef]
- Ambavaram, M.M.; Krishnan, A.; Trijatmiko, K.R.; Pereira, A. Coordinated activation of cellulose and repression of lignin biosynthesis pathways in rice. Plant Physiol. 2011, 155, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, W.; Zeng, J.; Zhang, J.; Grierson, D.; Li, X.; Yin, X.; Chen, K. A NAC transcription factor, EjNAC1, affects lignification of loquat fruit by regulating lignin. Postharvest Biol. Technol. 2015, 102, 25–31. [Google Scholar] [CrossRef]
- Li, W.; Huang, G.-Q.; Zhou, W.; Xia, X.-C.; Li, D.-D.; Li, X.-B. A cotton (Gossypium hirsutum) gene encoding a NAC transcription factor is involved in negative regulation of plant xylem development. Plant Physiol. Biochem. 2014, 83, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Lee, C.; Ye, Z.-H. Functional characterization of poplar wood-associated NAC domain transcription factors. Plant Physiol. 2010, 152, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Mathew, I.E.; Agarwal, P. May the Fittest Protein Evolve: Favoring the Plant-Specific Origin and Expansion of NAC Transcription Factors. Bioessays 2018, 40, 1800018. [Google Scholar] [CrossRef]
- He, X.J.; Mu, R.L.; Cao, W.H.; Zhang, Z.G.; Zhang, J.S.; Chen, S.Y. AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. Plant J. 2005, 44, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Fujita, Y.; Maruyama, K.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Tran, L.S.P.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J. 2004, 39, 863–876. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Kim, Y.; Kim, S.Y.; Lee, J.S.; Ahn, J.H. Control of flowering time and cold response by a NAC-domain protein in Arabidopsis. PLoS ONE 2007, 2, e642. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, B.; Lu, G.; Han, B. Overexpression of a NAC transcription factor enhances rice drought and salt tolerance. Biochem. Biophys. Res. Commun. 2009, 379, 985–989. [Google Scholar] [CrossRef]
- Shan, W.; Kuang, J.F.; Lu, W.J.; Chen, J.Y. Banana fruit NAC transcription factor MaNAC 1 is a direct target of MaICE 1 and involved in cold stress through interacting with MaCBF 1. Plant Cell Environ. 2014, 37, 2116–2127. [Google Scholar] [CrossRef]
- Jin, C.; Li, K.-Q.; Xu, X.-Y.; Zhang, H.-P.; Chen, H.-X.; Chen, Y.-H.; Hao, J.; Wang, Y.; Huang, X.-S.; Zhang, S.-L. A novel NAC transcription factor, PbeNAC1, of Pyrus betulifolia confers cold and drought tolerance via interacting with PbeDREBs and activating the expression of stress-responsive genes. Front. Plant Sci. 2017, 8, 1049. [Google Scholar] [CrossRef] [PubMed]
- Ooka, H.; Satoh, K.; Doi, K.; Nagata, T.; Otomo, Y.; Murakami, K.; Matsubara, K.; Osato, N.; Kawai, J.; Carninci, P. Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res. 2003, 10, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.N.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bryant, S.H. CD-Search: Protein domain annotations on the fly. Nucleic Acids Res. 2004, 32, W327–W331. [Google Scholar] [CrossRef]
- Singh, S.; Brueffer, C.; Cock, P. Biopython Project Update 2017. In Proceedings of the 18th Bioinformatics Open Source Conference, Prague, Czech Republic, 22–23 July 2017. [Google Scholar]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Lyons, E.; Freeling, M. How to usefully compare homologous plant genes and chromosomes as DNA sequences. Plant J. 2008, 53, 661–673. [Google Scholar] [CrossRef]
- Chen, C.; Xia, R.; Chen, H.; He, Y. TBtools, a Toolkit for Biologists integrating various biological data handling tools with a user-friendly interface. BioRxiv 2018, 289660. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Tang, H.B.; DeBarry, J.D.; Tan, X.; Li, J.P.; Wang, X.Y.; Lee, T.H.; Jin, H.Z.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Cepas, J.; Forslund, K.; Coelho, L.P.; Szklarczyk, D.; Jensen, L.J.; von Mering, C.; Bork, P. Fast Genome-Wide Functional Annotation through Orthology Assignment by eggNOG-Mapper. Mol. Biol. Evol. 2017, 34, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Cepas, J.; Szklarczyk, D.; Forslund, K.; Cook, H.; Heller, D.; Walter, M.C.; Rattei, T.; Mende, D.R.; Sunagawa, S.; Kuhn, M. eggNOG 4.5: A hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Res. 2015, 44, D286–D293. [Google Scholar] [CrossRef] [PubMed]
- Lamesch, P.; Berardini, T.Z.; Li, D.H.; Swarbreck, D.; Wilks, C.; Sasidharan, R.; Muller, R.; Dreher, K.; Alexander, D.L.; Garcia-Hernandez, M.; et al. The Arabidopsis Information Resource (TAIR): Improved gene annotation and new tools. Nucleic Acids Res. 2012, 40, D1202–D1210. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Alonso-Serra, J.; Safronov, O.; Lim, K.J.; Fraser-Miller, S.J.; Blokhina, O.B.; Campilho, A.; Chong, S.L.; Fagerstedt, K.; Haavikko, R.; Helariutta, Y. Tissue-specific study across the stem reveals the chemistry and transcriptome dynamics of birch bark. New Phytol. 2019, 222, 1816–1831. [Google Scholar] [CrossRef]
- Chang, S.; Puryear, J.; Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Yang, C.; Wei, H. Designing microarray and RNA-Seq experiments for greater systems biology discovery in modern plant genomics. Mol. Plant 2015, 8, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ruotti, V.; Stewart, R.M.; Thomson, J.A.; Dewey, C.N. RNA-Seq gene expression estimation with read mapping uncertainty. Bioinformatics 2009, 26, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhang, Y.; Cui, H.; Liu, J.; Wu, Y.; Cheng, Y.; Xu, H.; Huang, X.; Li, S.; Zhou, A. WEGO 2.0: A web tool for analyzing and plotting GO annotations, 2018 update. Nucleic Acids Res. 2018, 46, W71–W75. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.B. Endless forms: The evolution of gene regulation and morphological diversity. Cell 2000, 101, 577–580. [Google Scholar] [CrossRef]
- Coordinators, N.R. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2016, 44, D7–D19. [Google Scholar]
- Kamiuchi, Y.; Yamamoto, K.; Furutani, M.; Tasaka, M.; Aida, M. The CUC1 and CUC2 genes promote carpel margin meristem formation during Arabidopsis gynoecium development. Front. Plant Sci. 2014, 5, 165. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, V.; Hanlon, M.R.; Contrino, S.; Ferlanti, E.S.; Karamycheva, S.; Kim, M.; Rosen, B.D.; Cheng, C.-Y.; Moreira, W.; Mock, S.A. Araport: The Arabidopsis information portal. Nucleic Acids Res. 2014, 43, D1003–D1009. [Google Scholar] [CrossRef] [PubMed]
- Hibara, K.-I.; Karim, M.R.; Takada, S.; Taoka, K.-i.; Furutani, M.; Aida, M.; Tasaka, M. Arabidopsis CUP-SHAPED COTYLEDON3 regulates postembryonic shoot meristem and organ boundary formation. Plant Cell 2006, 18, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Wang, H.; Guo, C.; Zhang, N.; Zeng, L.; Chen, Y.; Ma, H.; Qi, J. Widespread whole genome duplications contribute to genome complexity and species diversity in angiosperms. Mol. Plant 2018, 11, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.K.; Kjaersgaard, T.; Nielsen, M.M.; Galberg, P.; Petersen, K.; O’shea, C.; Skriver, K. The Arabidopsis thaliana NAC transcription factor family: Structure–function relationships and determinants of ANAC019 stress signalling. Biochem. J. 2010, 426, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ren, J.; Nevo, E.; Yin, X.; Sun, D.; Peng, J. Divergent evolutionary patterns of NAC transcription factors are associated with diversification and gene duplications in angiosperm. Front. Plant Sci. 2017, 8, 1156. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yin, Y.; Chen, F.; Xu, Y.; Dixon, R.A. A bioinformatic analysis of NAC genes for plant cell wall development in relation to lignocellulosic bioenergy production. BioEnergy Res. 2009, 2, 217. [Google Scholar] [CrossRef]
- Harris, M.A.; Clark, J.; Ireland, A.; Lomax, J.; Ashburner, M.; Foulger, R.; Eilbeck, K.; Lewis, S.; Marshall, B.; Mungall, C.; et al. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004, 32, D258–D261. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Kiryutin, B.; Koonin, E.V.; Krylov, D.M.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; et al. The COG database: An updated version includes eukaryotes. BMC Bioinform. 2003, 4. [Google Scholar] [CrossRef]
- Botia, J.A.; Vandrovcova, J.; Forabosco, P.; Guelfi, S.; D’Sa, K.; Hardy, J.; Lewis, C.M.; Ryten, M.; Weale, M.E.; Co, U.K.B.E. An additional k-means clustering step improves the biological features of WGCNA gene co-expression networks. BMC Syst. Biol. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Foley, R.C.; Oñate-Sánchez, L. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 2002, 5, 430–436. [Google Scholar] [CrossRef]
- Liu, Z.; Shao, F.; Tang, G. The Research Progress of Structure, Function and Regulation of Plant NAC Transcription Factors. Acta Bot. Boreali-Occident. Sin. 2007, 9, 1915–1920. [Google Scholar]
- Argout, X.; Salse, J.; Aury, J.-M.; Guiltinan, M.J.; Droc, G.; Gouzy, J.; Allegre, M.; Chaparro, C.; Legavre, T.; Maximova, S.N. The genome of Theobroma cacao. Nat. Genet. 2011, 43, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ming, R.; Hou, S.; Feng, Y.; Yu, Q.; Dionne-Laporte, A.; Saw, J.H.; Senin, P.; Wang, W.; Ly, B.V.; Lewis, K.L. The draft genome of the transgenic tropical fruit tree papaya (Carica papaya Linnaeus). Nature 2008, 452, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Yang, S.H.; Park, A.H.; Lerouxel, O.; Han, K.H. ANAC012, a member of the plant-specific NAC transcription factor family, negatively regulates xylary fiber development in Arabidopsis thaliana. Plant J. 2007, 50, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Kim, S.-G.; Kim, Y.-S.; Seo, P.J.; Bae, M.; Yoon, H.-K.; Park, C.-M. Exploring membrane-associated NAC transcription factors in Arabidopsis: Implications for membrane biology in genome regulation. Nucleic Acids Res. 2006, 35, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gan, S. AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J. 2006, 46, 601–612. [Google Scholar] [CrossRef]
- Pitaksaringkarn, W.; Matsuoka, K.; Asahina, M.; Miura, K.; Sage-Ono, K.; Ono, M.; Yokoyama, R.; Nishitani, K.; Ishii, T.; Iwai, H. XTH 20 and XTH 19 regulated by ANAC 071 under auxin flow are involved in cell proliferation in incised Arabidopsis inflorescence stems. Plant J. 2014, 80, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-C.; Reca, I.-B.; Kim, Y.; Park, S.; Thomashow, M.F.; Keegstra, K.; Han, K.-H. Transcription factors that directly regulate the expression of CSLA9 encoding mannan synthase in Arabidopsis thaliana. Plant Mol. Biol. 2014, 84, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Goubet, F.; Barton, C.J.; Mortimer, J.C.; Yu, X.; Zhang, Z.; Miles, G.P.; Richens, J.; Liepman, A.H.; Seffen, K.; Dupree, P. Cell wall glucomannan in Arabidopsis is synthesised by CSLA glycosyltransferases, and influences the progression of embryogenesis. Plant J. 2009, 60, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Ye, Z.-H. The Arabidopsis NAC transcription factor NST2 functions together with SND1 and NST1 to regulate secondary wall biosynthesis in fibers of inflorescence stems. Plant Signal. Behav. 2015, 10, e989746. [Google Scholar] [CrossRef] [PubMed]
- Hussey, S.G.; Mizrachi, E.; Spokevicius, A.V.; Bossinger, G.; Berger, D.K.; Myburg, A.A. SND2, a NAC transcription factor gene, regulates genes involved in secondary cell wall development in Arabidopsis fibres and increases fibre cell area in Eucalyptus. BMC Plant Biol. 2011, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, B.O.; Yoo, J.H.; Jung, M.S.; Lee, S.M.; Han, H.J.; Kim, K.E.; Kim, S.H.; Lim, C.O.; Yun, D.-J. Identification of a calmodulin-binding NAC protein as a transcriptional repressor in Arabidopsis. J. Biol. Chem. 2007, 282, 36292–36302. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yin, Y.; Ma, Q.; Tang, X.; Hao, D.; Xu, Y. Genome-scale identification of cell-wall related genes in Arabidopsis based on co-expression network analysis. BMC Plant Biol. 2012, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Ohtani, M.; Mitsuda, N.; Kubo, M.; Ohme-Takagi, M.; Fukuda, H.; Demura, T. VND-INTERACTING2, a NAC domain transcription factor, negatively regulates xylem vessel formation in Arabidopsis. Plant Cell 2010, 22, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Close, T.J. Dehydrins: A commonalty in the response of plants to dehydration and low temperature. Physiol. Plant. 1997, 100, 291–296. [Google Scholar] [CrossRef]
- Huner, N.P.; Öquist, G.; Hurry, V.M.; Krol, M.; Falk, S.; Griffith, M. Photosynthesis, photoinhibition and low temperature acclimation in cold tolerant plants. Photosynth. Res. 1993, 37, 19–39. [Google Scholar] [CrossRef]
- Alborzi, S.Z.; Devignes, M.D.; Ritchie, D.W. ECDomainMiner: Discovering hidden associations between enzyme commission numbers and Pfam domains. BMC Bioinform. 2017, 18. [Google Scholar] [CrossRef]
- Ng, S.; Ivanova, A.; Duncan, O.; Law, S.R.; Van Aken, O.; De Clercq, I.; Wang, Y.; Carrie, C.; Xu, L.; Kmiec, B. A membrane-bound NAC transcription factor, ANAC017, mediates mitochondrial retrograde signaling in Arabidopsis. Plant Cell 2013, 25, 3450–3471. [Google Scholar] [CrossRef]
- Shao, H.; Wang, H.; Tang, X. NAC transcription factors in plant multiple abiotic stress responses: Progress and prospects. Front. Plant Sci. 2015, 6, 902. [Google Scholar] [CrossRef] [PubMed]
- Nuruzzaman, M.; Sharoni, A.M.; Kikuchi, S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Front. Microbiol. 2013, 4, 248. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Gene Locus | CDS Length (bp) | AA 1 | pI 2 | MW (Da) 3 |
|---|---|---|---|---|---|
| BpNAC001 | Bpev01.c0000.g0143 | 1056 | 351 | 4.58 | 39,004.80 |
| BpNAC002 | Bpev01.c0001.g0043 | 1158 | 385 | 6.40 | 43,779.76 |
| BpNAC003 | Bpev01.c0001.g0069 | 1512 | 503 | 6.56 | 57,125.50 |
| BpNAC004 | Bpev01.c0001.g0070 | 1542 | 513 | 6.02 | 58,179.28 |
| BpNAC005 | Bpev01.c0022.g0005 | 1185 | 394 | 5.14 | 45,193.43 |
| BpNAC006 | Bpev01.c0029.g0103 | 567 | 188 | 8.30 | 22,497.58 |
| BpNAC007 | Bpev01.c0039.g0006 | 1041 | 346 | 4.48 | 38,679.40 |
| BpNAC008 | Bpev01.c0039.g0015 | 1284 | 427 | 4.93 | 47,875.71 |
| BpNAC009 | Bpev01.c0044.g0097 | 1368 | 455 | 6.25 | 51,160.80 |
| BpNAC010 | Bpev01.c0050.g0126 | 804 | 267 | 5.73 | 30,466.32 |
| BpNAC011 | Bpev01.c0052.g0056 | 786 | 261 | 9.42 | 29,539.17 |
| BpNAC012 | Bpev01.c0052.g0182 | 1095 | 364 | 6.01 | 40,524.81 |
| BpNAC013 | Bpev01.c0055.g0027 | 1023 | 340 | 5.19 | 38,238.63 |
| BpNAC014 | Bpev01.c0080.g0077 | 474 | 157 | 7.92 | 17,308.59 |
| BpNAC015 | Bpev01.c0080.g0092 | 1086 | 361 | 7.65 | 41,283.20 |
| BpNAC016 | Bpev01.c0082.g0005 | 1047 | 348 | 8.47 | 39,276.47 |
| BpNAC017 | Bpev01.c0082.g0007 | 1092 | 363 | 6.27 | 40,947.88 |
| BpNAC018 | Bpev01.c0088.g0022 | 1314 | 437 | 5.02 | 49,013.50 |
| BpNAC019 | Bpev01.c0088.g0098 | 876 | 291 | 7.61 | 33,171.68 |
| BpNAC020 | Bpev01.c0101.g0069 | 1251 | 416 | 6.67 | 46,717.66 |
| BpNAC021 | Bpev01.c0129.g0029 | 918 | 305 | 4.64 | 34,313.49 |
| BpNAC022 | Bpev01.c0135.g0042 | 963 | 320 | 4.62 | 36,457.72 |
| BpNAC023 | Bpev01.c0135.g0072 | 921 | 306 | 4.67 | 34,460.78 |
| BpNAC024 | Bpev01.c0155.g0038 | 1059 | 352 | 8.34 | 38,836.59 |
| BpNAC025 | Bpev01.c0162.g0012 | 996 | 331 | 8.92 | 36,700.26 |
| BpNAC026 | Bpev01.c0162.g0014 | 1023 | 340 | 7.09 | 38,715.32 |
| BpNAC027 | Bpev01.c0167.g0004 | 813 | 270 | 8.73 | 30,315.34 |
| BpNAC028 | Bpev01.c0191.g0026 | 1359 | 452 | 4.72 | 50,997.03 |
| BpNAC029 | Bpev01.c0191.g0027 | 1551 | 516 | 5.53 | 56,743.10 |
| BpNAC030 | Bpev01.c0191.g0028 | 1914 | 637 | 4.71 | 70,689.54 |
| BpNAC031 | Bpev01.c0191.g0029 | 1398 | 465 | 5.82 | 51,879.04 |
| BpNAC032 | Bpev01.c0210.g0037 | 1743 | 580 | 4.91 | 63,826.53 |
| BpNAC033 | Bpev01.c0210.g0038 | 447 | 148 | 9.16 | 17,122.73 |
| BpNAC034 | Bpev01.c0210.g0039 | 1554 | 517 | 4.61 | 56,841.20 |
| BpNAC035 | Bpev01.c0210.g0040 | 1281 | 426 | 5.23 | 47,771.98 |
| BpNAC036 | Bpev01.c0210.g0041 | 1428 | 475 | 4.93 | 53,076.79 |
| BpNAC037 | Bpev01.c0210.g0042 | 1551 | 516 | 4.89 | 57,647.89 |
| BpNAC038 | Bpev01.c0210.g0043 | 3804 | 1268 | 5.02 | 139,552.01 |
| BpNAC039 | Bpev01.c0210.g0048 | 657 | 218 | 4.28 | 23,950.57 |
| BpNAC040 | Bpev01.c0224.g0004 | 576 | 191 | 4.52 | 21,962.39 |
| BpNAC041 | Bpev01.c0245.g0072 | 1155 | 384 | 6.66 | 43,548.68 |
| BpNAC042 | Bpev01.c0261.g0059 | 684 | 227 | 9.65 | 25,959.43 |
| BpNAC043 | Bpev01.c0275.g0039 | 1005 | 334 | 4.72 | 37,105.64 |
| BpNAC044 | Bpev01.c0281.g0055 | 1200 | 399 | 6.13 | 44,787.28 |
| BpNAC045 | Bpev01.c0281.g0084 | 1023 | 340 | 6.57 | 38,567.56 |
| BpNAC046 | Bpev01.c0281.g0092 | 924 | 307 | 4.64 | 34,661.06 |
| BpNAC047 | Bpev01.c0330.g0003 | 915 | 304 | 5.51 | 34,776.84 |
| BpNAC048 | Bpev01.c0344.g0002 | 510 | 169 | 9.49 | 19,234.75 |
| BpNAC049 | Bpev01.c0344.g0004 | 3090 | 1029 | 5.37 | 117,765.80 |
| BpNAC050 | Bpev01.c0346.g0003 | 1065 | 354 | 8.18 | 39,093.88 |
| BpNAC051 | Bpev01.c0349.g0059 | 276 | 91 | 4.74 | 9914.98 |
| BpNAC052 | Bpev01.c0357.g0068 | 855 | 284 | 8.54 | 32,795.40 |
| BpNAC053 | Bpev01.c0376.g0004 | 483 | 160 | 7.92 | 17,263.55 |
| BpNAC054 | Bpev01.c0390.g0007 | 891 | 296 | 6.61 | 34,034.73 |
| BpNAC055 | Bpev01.c0404.g0003 | 1077 | 358 | 4.90 | 41,609.59 |
| BpNAC056 | Bpev01.c0404.g0007 | 966 | 321 | 5.94 | 37,486.94 |
| BpNAC057 | Bpev01.c0411.g0006 | 960 | 319 | 6.35 | 36,457.90 |
| BpNAC058 | Bpev01.c0428.g0005 | 1398 | 465 | 4.84 | 50,270.40 |
| BpNAC059 | Bpev01.c0450.g0023 | 987 | 328 | 5.78 | 37,235.70 |
| BpNAC060 | Bpev01.c0452.g0001 | 837 | 278 | 5.02 | 32,793.02 |
| BpNAC061 | Bpev01.c0452.g0002 | 717 | 238 | 8.14 | 28,149.23 |
| BpNAC062 | Bpev01.c0452.g0003 | 1047 | 348 | 4.64 | 40,257.88 |
| BpNAC063 | Bpev01.c0455.g0003 | 885 | 294 | 7.07 | 34,051.03 |
| BpNAC064 | Bpev01.c0457.g0009 | 1356 | 451 | 5.31 | 50,761.01 |
| BpNAC065 | Bpev01.c0477.g0025 | 891 | 296 | 4.87 | 33,263.97 |
| BpNAC066 | Bpev01.c0477.g0027 | 834 | 277 | 5.06 | 31,589.50 |
| BpNAC067 | Bpev01.c0480.g0034 | 1128 | 375 | 6.52 | 42,094.62 |
| BpNAC068 | Bpev01.c0483.g0016 | 1092 | 363 | 6.27 | 41,571.91 |
| BpNAC069 | Bpev01.c0514.g0006 | 756 | 251 | 4.61 | 28,414.49 |
| BpNAC070 | Bpev01.c0514.g0007 | 1575 | 524 | 5.08 | 57,993.34 |
| BpNAC071 | Bpev01.c0517.g0013 | 1074 | 357 | 8.23 | 40,150.47 |
| BpNAC072 | Bpev01.c0522.g0030 | 1281 | 426 | 6.16 | 48,038.53 |
| BpNAC073 | Bpev01.c0531.g0007 | 897 | 298 | 6.68 | 34,698.52 |
| BpNAC074 | Bpev01.c0555.g0002 | 906 | 301 | 5.99 | 33,702.72 |
| BpNAC075 | Bpev01.c0557.g0054 | 750 | 249 | 8.72 | 28,285.83 |
| BpNAC076 | Bpev01.c0568.g0011 | 726 | 241 | 4.85 | 27,749.94 |
| BpNAC077 | Bpev01.c0652.g0024 | 1692 | 563 | 4.80 | 63,738.88 |
| BpNAC078 | Bpev01.c0656.g0002 | 558 | 185 | 4.63 | 21,053.47 |
| BpNAC079 | Bpev01.c0726.g0004 | 1032 | 343 | 5.11 | 38,869.14 |
| BpNAC080 | Bpev01.c0727.g0012 | 1995 | 664 | 5.19 | 75,547.32 |
| BpNAC081 | Bpev01.c0751.g0007 | 858 | 285 | 8.60 | 32,919.09 |
| BpNAC082 | Bpev01.c0751.g0009 | 864 | 287 | 6.64 | 33,211.28 |
| BpNAC083 | Bpev01.c0755.g0001 | 942 | 313 | 8.72 | 35,486.93 |
| BpNAC084 | Bpev01.c0781.g0009 | 1152 | 383 | 5.29 | 42,218.92 |
| BpNAC085 | Bpev01.c0796.g0007 | 1236 | 411 | 6.02 | 46,275.75 |
| BpNAC086 | Bpev01.c0803.g0020 | 1395 | 464 | 4.86 | 51,157.26 |
| BpNAC087 | Bpev01.c0863.g0019 | 477 | 158 | 8.90 | 18,172.77 |
| BpNAC088 | Bpev01.c1058.g0003 | 1065 | 354 | 5.02 | 40,826.35 |
| BpNAC089 | Bpev01.c1071.g0002 | 564 | 187 | 6.18 | 20,761.41 |
| BpNAC090 | Bpev01.c1155.g0007 | 339 | 112 | 5.84 | 12,868.12 |
| BpNAC091 | Bpev01.c1185.g0017 | 2094 | 697 | 5.33 | 77,759.91 |
| BpNAC092 | Bpev01.c1189.g0015 | 744 | 247 | 6.34 | 28,484.74 |
| BpNAC093 | Bpev01.c1204.g0001 | 1089 | 362 | 5.74 | 40,418.56 |
| BpNAC094 | Bpev01.c1218.g0003 | 327 | 109 | 7.63 | 12,565.44 |
| BpNAC095 | Bpev01.c1228.g0010 | 930 | 309 | 6.74 | 35,236.58 |
| BpNAC096 | Bpev01.c1250.g0009 | 1008 | 335 | 8.72 | 37,415.34 |
| BpNAC097 | Bpev01.c1272.g0001 | 783 | 260 | 6.55 | 29,666.12 |
| BpNAC098 | Bpev01.c1539.g0003 | 1191 | 396 | 5.88 | 46,313.98 |
| BpNAC099 | Bpev01.c1580.g0002 | 924 | 307 | 4.59 | 34,644.71 |
| BpNAC100 | Bpev01.c1670.g0008 | 735 | 244 | 8.72 | 28,528.46 |
| BpNAC101 | Bpev01.c1773.g0003 | 621 | 206 | 9.13 | 23,494.36 |
| BpNAC102 | Bpev01.c1773.g0004 | 582 | 193 | 9.48 | 22,043.09 |
| BpNAC103 | Bpev01.c1899.g0001 | 1014 | 337 | 7.07 | 38,269.85 |
| BpNAC104 | Bpev01.c1913.g0005 | 945 | 314 | 4.67 | 35,530.73 |
| BpNAC105 | Bpev01.c1933.g0004 | 675 | 224 | 8.54 | 26,398.37 |
| BpNAC106 | Bpev01.c2186.g0001 | 609 | 202 | 5.92 | 23,661.86 |
| BpNAC107 | Bpev01.c2413.g0002 | 591 | 196 | 4.88 | 22,210.41 |
| BpNAC108 | Bpev01.c2439.g0003 | 846 | 281 | 8.75 | 32,581.73 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Lin, X.; Zhang, D.; Li, Q.; Zhao, X.; Chen, S. Genome-Wide Analysis of NAC Gene Family in Betula pendula. Forests 2019, 10, 741. https://doi.org/10.3390/f10090741
Chen S, Lin X, Zhang D, Li Q, Zhao X, Chen S. Genome-Wide Analysis of NAC Gene Family in Betula pendula. Forests. 2019; 10(9):741. https://doi.org/10.3390/f10090741
Chicago/Turabian StyleChen, Song, Xin Lin, Dawei Zhang, Qi Li, Xiyang Zhao, and Su Chen. 2019. "Genome-Wide Analysis of NAC Gene Family in Betula pendula" Forests 10, no. 9: 741. https://doi.org/10.3390/f10090741
APA StyleChen, S., Lin, X., Zhang, D., Li, Q., Zhao, X., & Chen, S. (2019). Genome-Wide Analysis of NAC Gene Family in Betula pendula. Forests, 10(9), 741. https://doi.org/10.3390/f10090741





