Appropriate Removal of Forest Litter is Beneficial to Pinus tabuliformis Carr. Regeneration in a Pine and Oak Mixed Forest in the Qinling Mountains, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Leaf Litter, Soil, and Seeds
2.2. Preparation of Water Extracts
2.3. Cultivation and Germination of Seeds
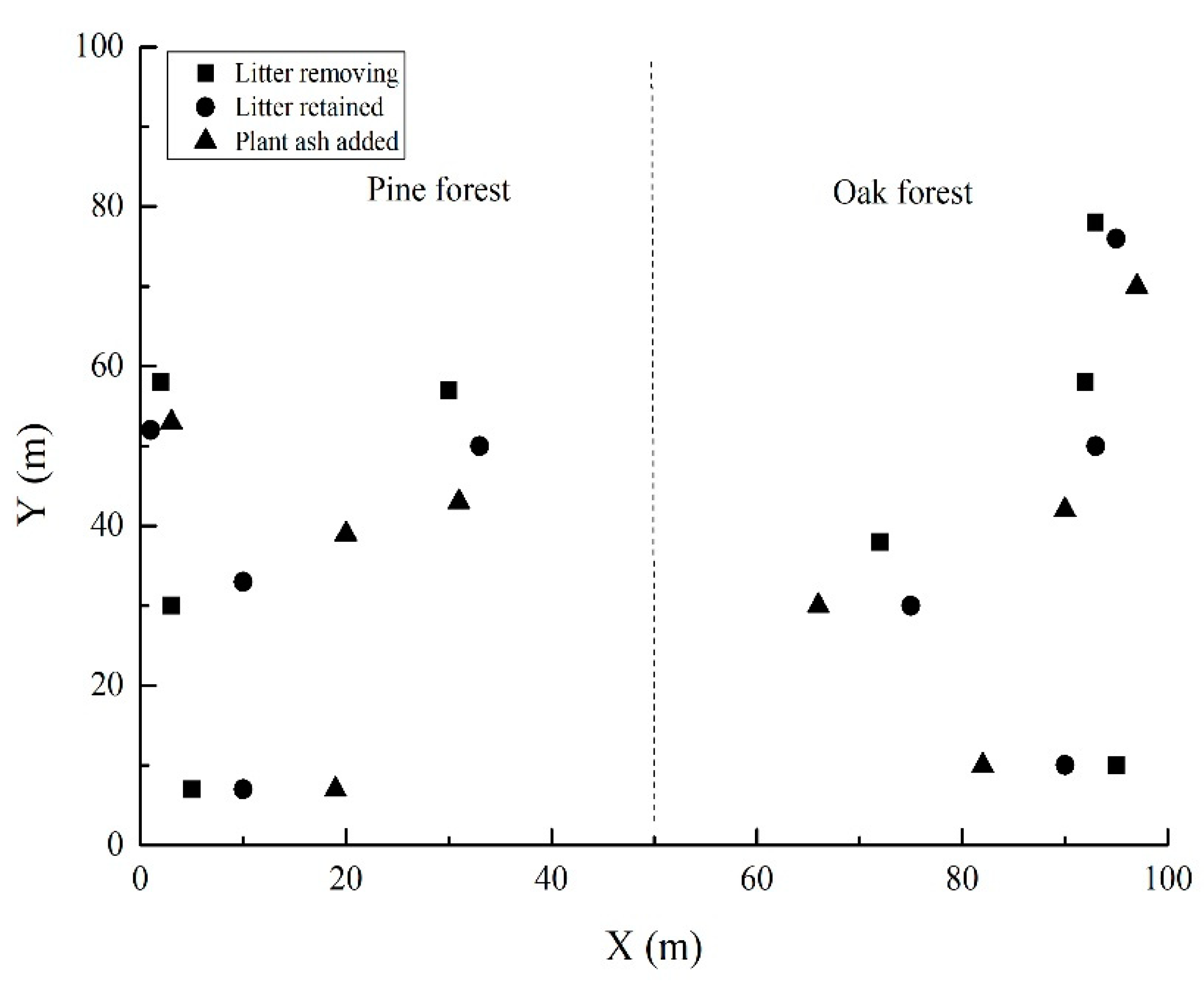
2.4. Field Seeding
2.5. Identification of Water Extracts
2.6. Statistical Analysis
3. Results
3.1. Effects of Different Treatments on P. tabuliformis and P. armandii Seeds
3.1.1. Germination Rates
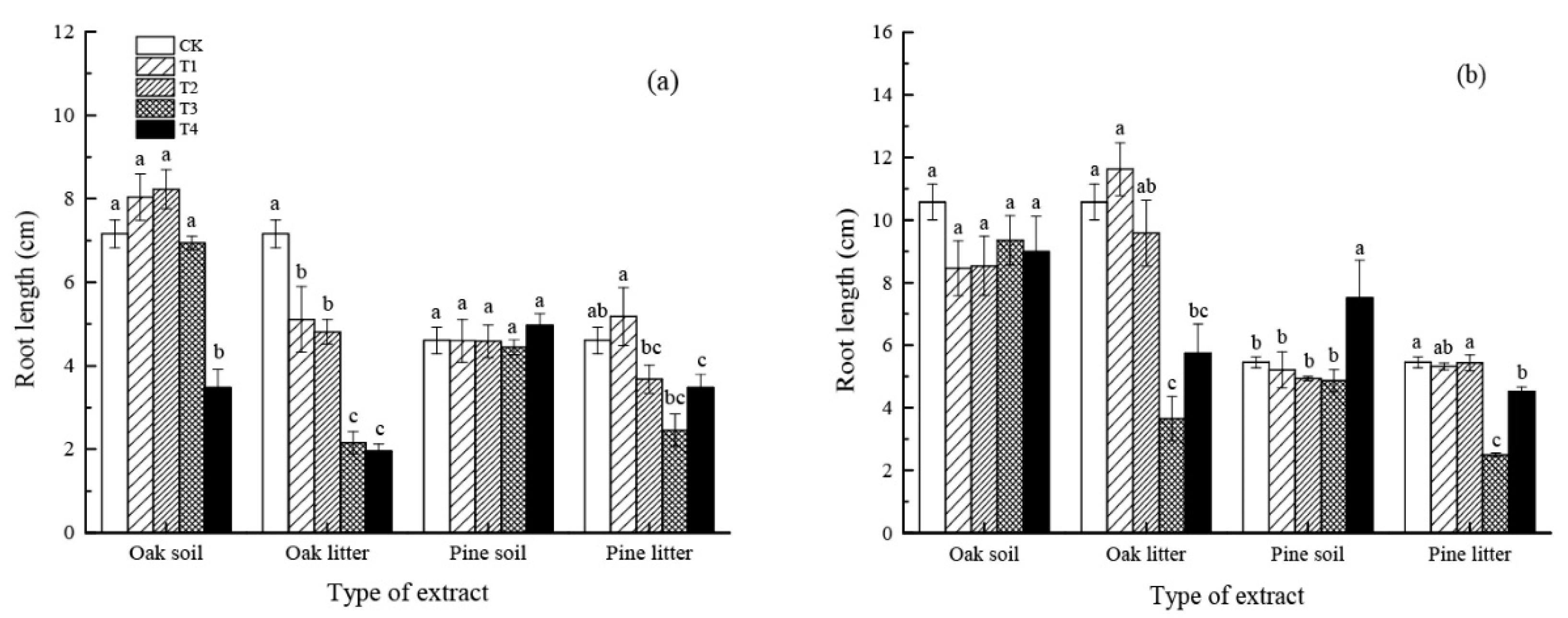
3.1.2. Root Length
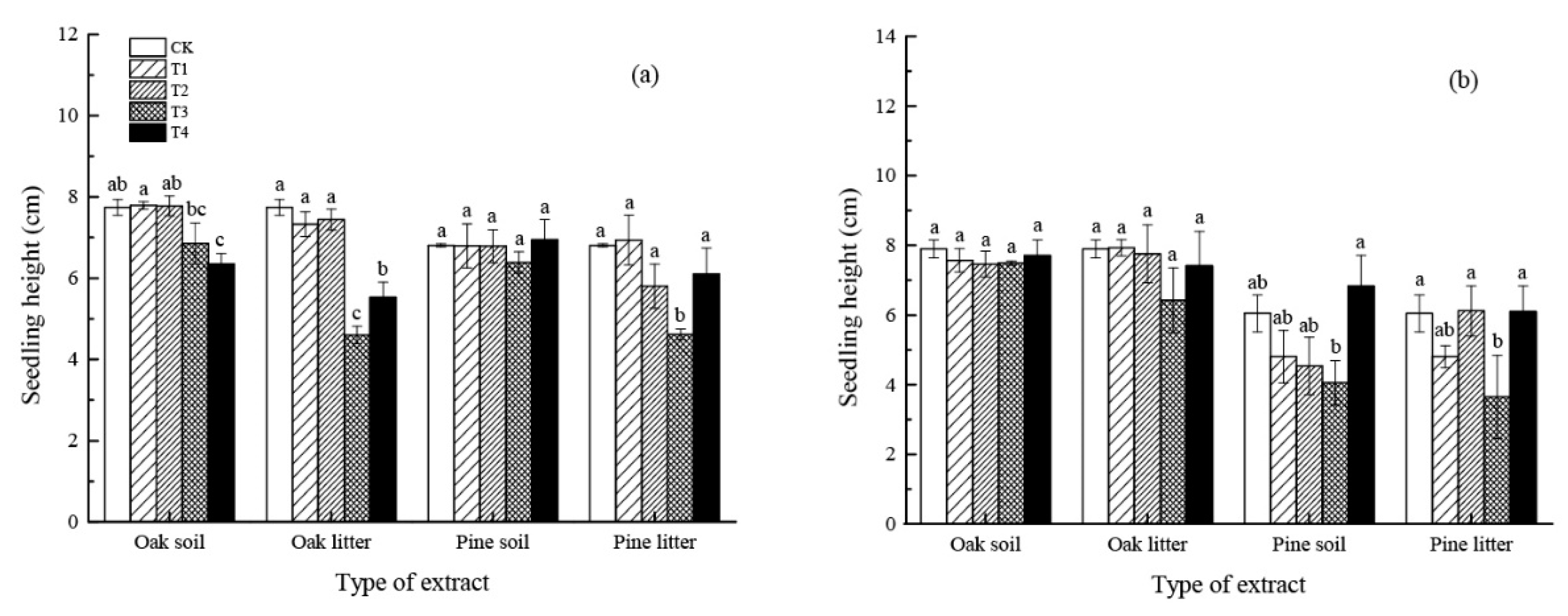
3.1.3. Seedling Height
3.1.4. Malondialdehyde Content
3.2. Seed Germination in the Field Experiment
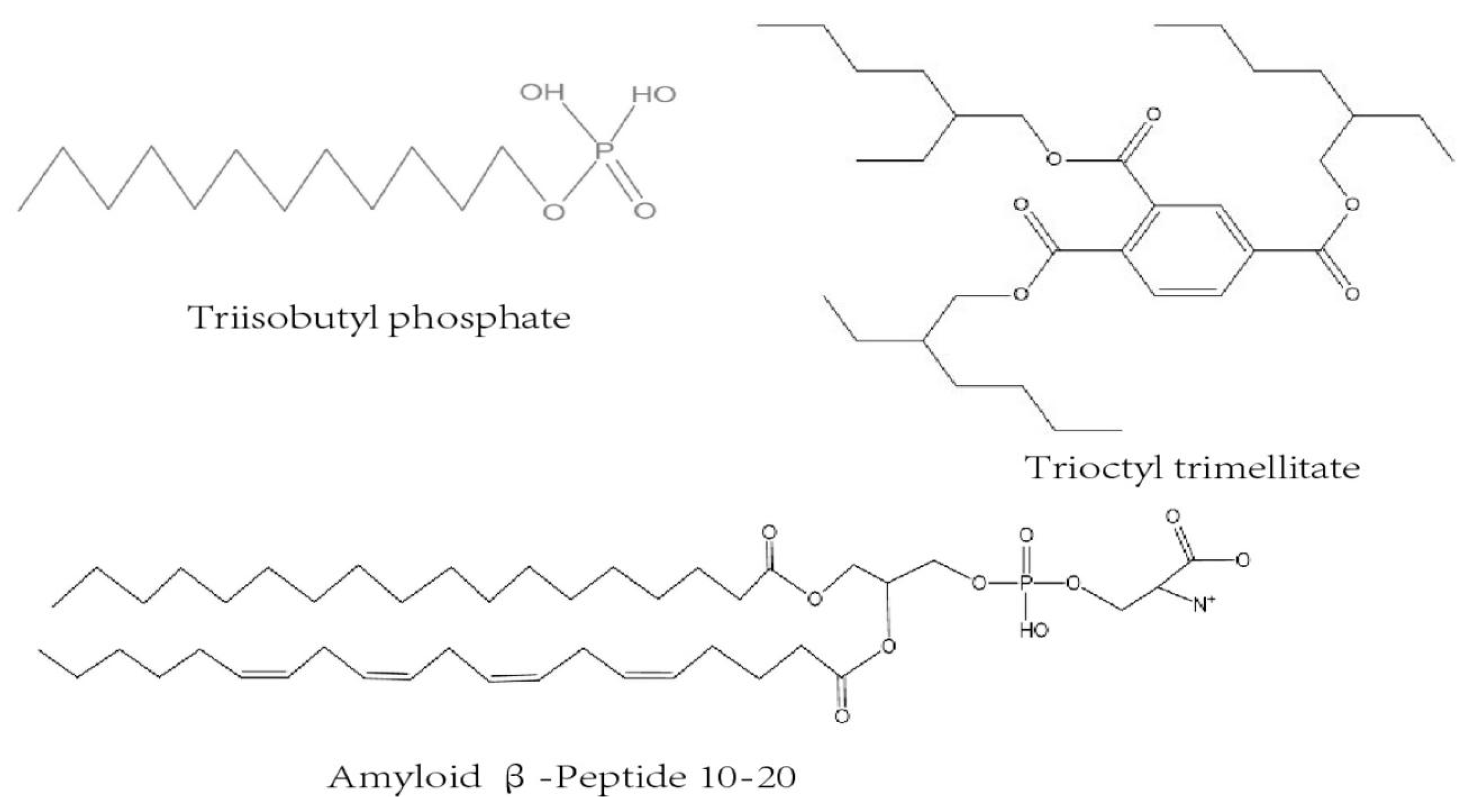
3.3. Allelochemicals in Pine Forest Litter
4. Discussion
4.1. Effect of Leaf Litter Water Extract on Seed Germination and Initial Growth
4.2. Allelopathy of Pine and Oak Forest Litter in the Field Experiment
4.3. Allelopathic Substances that Exert Allelopathic Effects in Pine Forests
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Wang, Z. Spatial heterogeneity and forest regeneration. Ying Yong Sheng Tai Xue Bao 2002, 13, 615–619. [Google Scholar] [PubMed]
- Nyland, R.D.; Bashant, A.L.; Bohn, K.K.; Verostek, J.M. Interference to hardwood regeneration in northeastern North America: Controlling effects of American beech, striped maple, and hobblebush. North J. Appl. For. 2006, 23, 122–132. [Google Scholar] [CrossRef]
- D’Amato, A.W.; Orwig, D.A.; Foster, D.R. Understory vegetation in old-growth and second-growth Tsuga canadensis forests in western Massachusetts. For. Ecol. Manag. 2009, 257, 1043–1052. [Google Scholar] [CrossRef]
- Cameron, A.D.; Mason, W.L.; Malcolm, D.C. Transformation of Plantation Forests. In the IUFRO Conference, Edinburgh, Scotland, August 29 to September 3, 1999. For. Ecol. Manag. 2001, 151, 1–5. [Google Scholar] [CrossRef]
- Broncano, M.J.; Riba, M.; Retana, J. Seed germination and seedling performance of two Mediterranean tree species, holm oak (Quercus ilex L.) and Aleppo pine (Pinus halepensis Mill.): A multifactor experimental approach. Plant Ecol. 1998, 138, 17–26. [Google Scholar] [CrossRef]
- Liu, H.Y.; Cui, H.T.; Yu, P.T.; Huang, Y.M. The origin of remnant forest stands of Pinus tabulaeformis in southeastern Inner Mongolia. Plant Ecol. 2002, 158, 139–151. [Google Scholar] [CrossRef]
- Pons, J.; Pausas, J.G. Oak regeneration in heterogeneous landscapes: The case of fragmented Quercus suber forests in the eastern Iberian Peninsula. For. Ecol. Manag. 2006, 231, 196–204. [Google Scholar] [CrossRef]
- Urbieta, I.R.; Garcia, L.V.; Zavala, M.A.; Maranon, T. Mediterranean pine and oak distribution in southern Spain: Is there a mismatch between regeneration and adult distribution? J. Veg. Sci. 2011, 22, 18–31. [Google Scholar] [CrossRef]
- Yu, F. Seed Dispersal Process and Natural Regeneration Pattern of Constructive Species in the Pine-Oak Forests of the Qinling Mountains; China College of Forestry, Northwest A & F University: Yangling, China, 2014. [Google Scholar]
- Kang, H.B.; Zheng, Y.Y.; Liu, S.T.; Chai, Z.Z.; Chang, M.J.; Hu, Y.N.; Li, G.; Wang, D.X. Population structure and spatial pattern of predominant tree species in a pine–oak mosaic mixed forest in the Qinling Mountains, China. J. Plant Interact. 2017, 12, 78–86. [Google Scholar] [CrossRef]
- Chai, Z.; Fan, D.; Wang, D. Environmental factors and underlying mechanisms of tree community assemblages of pine-oak mixed forests in the Qinling Mountains, China. J. Plant Biol. 2016, 59, 347–357. [Google Scholar] [CrossRef]
- Yu, F.; Wang, D.X.; Shi, X.X.; Yi, X.F.; Huang, Q.P.; Hu, Y.N. Effects of environmental factors on tree seedling regeneration in a pine-oak mixed forest in the Qinling Mountains, China. J. Mt. Sci. 2013, 10, 845–853. [Google Scholar] [CrossRef]
- Huo, X.Y.; Kang, H.B.; Wang, D.X.; Chang, M.J.; Yu, F. Effects of rodents on seed dispersal patterns of constructive species in the pine-oak mixed forests of the Qinling Mountains, Shaanxi Province, China. Acta Ecol. Sin. 2019, 39, 2435–2443. [Google Scholar]
- Chai, Z.Z.; Wang, D.X. Environmental influences on the successful regeneration of pine-oak mixed forests in the Qinling Mountains, China. Scand. J. For. Res. 2016, 31, 368–381. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: New York, NY, USA, 1984; pp. 1–50, 309–315. [Google Scholar]
- Walbott, M.; Gallet, C.; Corcket, E. Beech (Fagus sylvatica) germination and seedling growth under climaticand allelopathic constraints. C. R. Biol. 2018, 341, 444–453. [Google Scholar] [CrossRef]
- Hane, E.N.; Hamburg, S.P.; Barber, A.L.; Plaut, J.A. Phytotoxicity of American beech leaf leachate to sugar maple seedlings in a greenhouse experiment. Can. J. For. Res. 2003, 33, 814–821. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Chen, Y.; Zhao, J.; Wan, S.; Lin, Y.; Fu, S. Effects of Eucalyptus litter and roots on the establishment of native tree species in Eucalyptus plantations in South China. Forest Ecol. Manag. 2016, 375, 76–83. [Google Scholar] [CrossRef]
- Aguilera, N.; Guedes, L.M.; Becerra, J.; Gonzalez, L. Is autotoxicity responsible for inhibition growth of new conspecific seedlings under the canopy of the invasive Acacia dealbata Link? Gayana Bot. 2017, 74, 1–14. [Google Scholar] [CrossRef]
- Brewer, M.J.; Webster, J.A. Probing behavior of Diuraphis noxia and Rhopalosiphum maidis (Homoptera: Aphididae) affected by barley resistance to D-noxia and plant water stress. Environ. Entomol. 2001, 30, 1041–1046. [Google Scholar] [CrossRef]
- Sharma, N.K.; Samra, J.S.; Singh, H.P. Effect of leaf litter of poplar on Phalaris minor weed. Allelopath. J. 2000, 7, 243–253. [Google Scholar]
- Huang, W.W.; Hu, H.L.; Hu, T.X.; Chen, H.; Wang, Q.; Chen, G.; Tu, L.H. Impact of aqueous extracts of Cinnamomum septentrionale leaf litter on the growth and photosynthetic characteristics of Eucalyptus grandis seedlings. New For. 2015, 46, 561–576. [Google Scholar] [CrossRef]
- Garnett, E.; Jonsson, L.M.; Dighton, J.; Murnen, K. Control of pitch pine seed germination and initial growth exerted by leaf litters and polyphenolic compounds. Biol. Fertil. Soils 2004, 40, 421–426. [Google Scholar] [CrossRef]
- Kimura, F.; Sato, M.; Kato-Noguchi, H. Allelopathy of pine litter: Delivery of allelopathic substances into forest floor. J. Plant Biol. 2015, 58, 61–67. [Google Scholar] [CrossRef]
- Muturi, G.M.; Poorter, L.; Bala, P.; Mohren, G.M.J. Unleached Prosopis litter inhibits germination but leached stimulates seedling growth of dry woodland species. J. Arid Environ. 2017, 138, 44–50. [Google Scholar] [CrossRef]
- Lankau, R. Soil microbial communities alter allelopathic competition between Alliaria petiolata and a native species. Biol. Invasions 2010, 12, 2059–2068. [Google Scholar] [CrossRef]
- Prati, D.; Bossdorf, O. Allelopathic inhibition of germination by Alliaria petiolata (Brassicaceae). Am. J. Bot. 2004, 91, 285–288. [Google Scholar] [CrossRef]
- Cipollini, K.A.; McClain, G.Y.; Cipollini, D. Separating above- and belowground effects of Alliaria petiolata and Lonicera maackii on the performance of Impatiens capensis. Am. Midl. Nat. 2008, 160, 117–128. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Beard, K.H. Activated carbon as a restoration tool: Potential for control of invasive plants in abandoned agricultural fields. Restor Ecol. 2006, 14, 251–257. [Google Scholar] [CrossRef]
- Whitmore, T.C. Gaps in the forest canopy. In Tropical Trees as Living Systems; Tomlinson, P.B., Zimmerman, M.H., Eds.; Cambridge University Press: New York, NY, USA, 1978; pp. 639–655. [Google Scholar]
- Silva, R.M.G.; Brigatti, J.G.F.; Santos, V.H.M.; Mecina, G.F.; Silva, L.P. Allelopathic effect of the peel of coffee fruit. Sci. Hort. 2013, 158, 39–44. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, W.; Zuo, Z.; Li, R.; Wu, J.; Gao, Y. Inhibition effects of volatile organic compounds from Artemisia frigida Willd. on the pasture grass intake by lambs. Small Ruminant Res 2014, 121, 248–254. [Google Scholar] [CrossRef]
- Meksawat, S.; Pornprom, T. Allelopathic effect of itchgrass (Rottboellia cochinchinensis) on seed germination and plant growth. Weed Biol. Manag. 2010, 10, 16–24. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Zhang, R.; Huang, Y.; Feng, S.; Ma, X.; Zhang, Y.; Sikdar, A.; Roy, R. Allelopathic effects of aqueous leaf extracts from four shrub species on seed germination and initial growth of Amygdalus pedunculata Pall. Forests 2018, 9, 711. [Google Scholar] [CrossRef]
- Qi, J.H.; Liang, Y.L.; Liang, Z.S. Effects of root exudates of squash grafted with cucumber shoot on seed germination. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao 2005, 31, 217–220. [Google Scholar]
- Xie, D.F.; Zhang, G.C.; Xia, X.X.; Lang, Y.; Zhang, S.Y. The effects of phenolic acids on the photosynthetic characteristics and growth of Populus x euramericana cv. ‘Neva’ seedlings. Photosynthetica 2018, 56, 981–988. [Google Scholar] [CrossRef]
- Mahall, B.E.; Callaway, R.M. Root communication mechanisms and intracommunity distributions of 2 mojave desert shrubs. Ecology 1992, 73, 2145–2151. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; An, M.; Pratley, J.E.; Luckett, D.J.; Lemerle, D.; Coombes, N. The seedling root response of annual ryegrass (Lolium rigidum) to neighbouring seedlings of a highly-allelopathic canola (Brassica napus). Flora 2016, 219, 18–24. [Google Scholar] [CrossRef]
- Li-xue, Y. Effect of water extracts of larch on growth of Manchurian walnut seedlings. J. For. Res. (Harbin) 2005, 16, 285–288. [Google Scholar] [CrossRef]
- Kobayashi, K.; Koyama, H.; Shim, I.S. Relationship between behavior of dehydromatricaria ester in soil and the allelopathic activity of Solidago altissima L. in the laboratory. Plant Soil 2004, 259, 97–102. [Google Scholar] [CrossRef]
- John, Q.T.; Clifford, P.R.; Guimei, C.; Ruth, W.M. Expression of allelopathy in the soil environment: Soil concentration and activity of benzoxazinoid compounds released by rye cover crop residue. Plant Ecol. 2012, 213, 1893–1905. [Google Scholar]
- Liu, B.; Daryanto, S.; Wang, L.X.; Li, Y.J.; Liu, Q.Q.; Zhao, C.; Wang, Z.N. Excessive accumulation of Chinese fir litter inhibits its own seedling emergence and early growth-A greenhouse perspective. Forests 2017, 8, 341. [Google Scholar] [CrossRef]
- Quddus, M.S.; Bellairs, S.M.; Wurm, P.A.S. Acacia holosericea (Fabaceae) litter has allelopathic and physical effects on mission grass (Cenchrus pedicellatus and C. polystachios) (Poaceae) seedling establishment. Aust. J. Bot. 2014, 62, 189–195. [Google Scholar] [CrossRef]
- Rotundo, J.L.; Aguiar, M.R. Litter effects on plant regeneration in arid lands: A complex balance between seed retention, seed longevity and soil-seed contact. J. Ecol. 2005, 93, 829–838. [Google Scholar] [CrossRef]
- Ruprecht, E.; Donath, T.W.; Otte, A.; Eckstein, R.L. Chemical effects of a dominant grass on seed germination of four familial pairs of dry grassland species. Seed Sci. Res. 2008, 18, 239–248. [Google Scholar] [CrossRef][Green Version]
- Ruprecht, E.; Enyedi, M.Z.; Eckstein, R.L.; Donath, T.W. Restorative removal of plant litter and vegetation 40 years after abandonment enhances re-emergence of steppe grassland vegetation. Biol. Cons. 2010, 143, 449–456. [Google Scholar] [CrossRef]
- Ren, X.; He, X.F.; Zhang, Z.F.; Yan, Z.Q.; Jin, H.; Li, X.H.; Qin, B. Isolation, identification, and autotoxicity effect of allelochemicals from rhizosphere soils of flue-cured tobacco. J. Agric. Food Chem. 2015, 63, 8975–8980. [Google Scholar] [CrossRef]
- Xie, M.; Yan, Z.Q.; Ren, X.; Li, X.Z.; Qin, B. Codonopilate A, a triterpenyl ester as main autotoxin in cultivated soil of Codonopsis pilosula (Franch.) Nannf. J. Agric. Food Chem. 2017, 65, 2032–2038. [Google Scholar] [CrossRef]
- Sasamoto, H.; Mardani, H.; Sasamoto, Y.; Wasano, N.; Murashige-Baba, T.; Sato, T.; Hasegawa, A.; Fujii, Y. Evaluation of canavanine as an allelochemical in etiolated seedlings of Vicia villosa Roth: Protoplast co-culture method with digital image analysis. In Vitro Cell Dev. Biol.-Plant 2019, 55, 296–304. [Google Scholar] [CrossRef]

| Tree Species | Component | Sample | Germination Rate (%) | ||||
|---|---|---|---|---|---|---|---|
| CK | T1 | T2 | T3 | T4 | |||
| Pinus tabuliformis | Oak forest | Soil | 61.11 ± 2.94a | 63.33 ± 3.85a | 68.89 ± 1.11a | 61.11 ± 2.22a | 48.89 ± 5.88b |
| Litter | 61.11 ± 2.94a | 53.33 ± 5.09ab | 54.44 ± 4.84ab | 43.33 ± 5.77bc | 36.67 ± 1.93c | ||
| Pine forest | Soil | 52.22 ± 4.01a | 52.22 ± 4.84a | 44.45 ± 2.22a | 50.00 ± 3.85a | 51.11 ± 2.94a | |
| Litter | 52.22 ± 4.01ab | 54.45 ± 2.22a | 36.67 ± 1.93c | 41.67 ± 2.89bc | 44.44 ± 2.94bc | ||
| Pinus armandii | Oak forest | Soil | 61.11 ± 2.94a | 56.67 ± 1.93a | 51.67 ± 5.85a | 53.34 ± 3.33a | 60.00 ± 1.92a |
| Litter | 61.11 ± 2.94a | 61.67 ± 5.36a | 38.33 ± 4.40b | 38.89 ± 4.01b | 38.33 ± 1.67b | ||
| Pine forest | Soil | 37.78 ± 2.22a | 32.22 ± 6.19a | 32.22 ± 4.45a | 30.00 ± 1.92a | 37.78 ± 7.29a | |
| Litter | 37.78 ± 2.22a | 28.89 ± 4.01a | 36.67 ± 3.33a | 12.22 ± 2.94b | 36.67 ± 5.77a | ||
| Seed Categories | Pine Forest | Oak Forest | ||||
|---|---|---|---|---|---|---|
| Remove Litter | Keep Litter | Plant Ash | Remove Litter | Keep Litter | Plant Ash | |
| Pinus armandii | 85.00 ± 6.31a | 85.83 ± 3.70a | 89.17 ± 3.70a | 84.17 ± 6.85a | 75.00 ± 3.47a | 78.33 ± 7.52a |
| Pinus tabuliformis | 77.78 ± 1.11a | 50.00 ± 8.92b | 47.50 ± 7.50b | 57.50 ± 3.70a | 20.00 ± 3.60b | 23.33 ± 3.85b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, X.; Wang, D.; Bing, D.; Li, Y.; Kang, H.; Yang, H.; Wei, G.; Chao, Z. Appropriate Removal of Forest Litter is Beneficial to Pinus tabuliformis Carr. Regeneration in a Pine and Oak Mixed Forest in the Qinling Mountains, China. Forests 2019, 10, 735. https://doi.org/10.3390/f10090735
Huo X, Wang D, Bing D, Li Y, Kang H, Yang H, Wei G, Chao Z. Appropriate Removal of Forest Litter is Beneficial to Pinus tabuliformis Carr. Regeneration in a Pine and Oak Mixed Forest in the Qinling Mountains, China. Forests. 2019; 10(9):735. https://doi.org/10.3390/f10090735
Chicago/Turabian StyleHuo, Xueying, Dexiang Wang, Deye Bing, Yuanze Li, Haibin Kang, Hang Yang, Guoren Wei, and Zhi Chao. 2019. "Appropriate Removal of Forest Litter is Beneficial to Pinus tabuliformis Carr. Regeneration in a Pine and Oak Mixed Forest in the Qinling Mountains, China" Forests 10, no. 9: 735. https://doi.org/10.3390/f10090735
APA StyleHuo, X., Wang, D., Bing, D., Li, Y., Kang, H., Yang, H., Wei, G., & Chao, Z. (2019). Appropriate Removal of Forest Litter is Beneficial to Pinus tabuliformis Carr. Regeneration in a Pine and Oak Mixed Forest in the Qinling Mountains, China. Forests, 10(9), 735. https://doi.org/10.3390/f10090735




