Application of GRAS Compounds for the Control of Mould Growth on Scots Pine Sapwood Surfaces: Multivariate Modelling of Mould Grade
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Wood Specimens
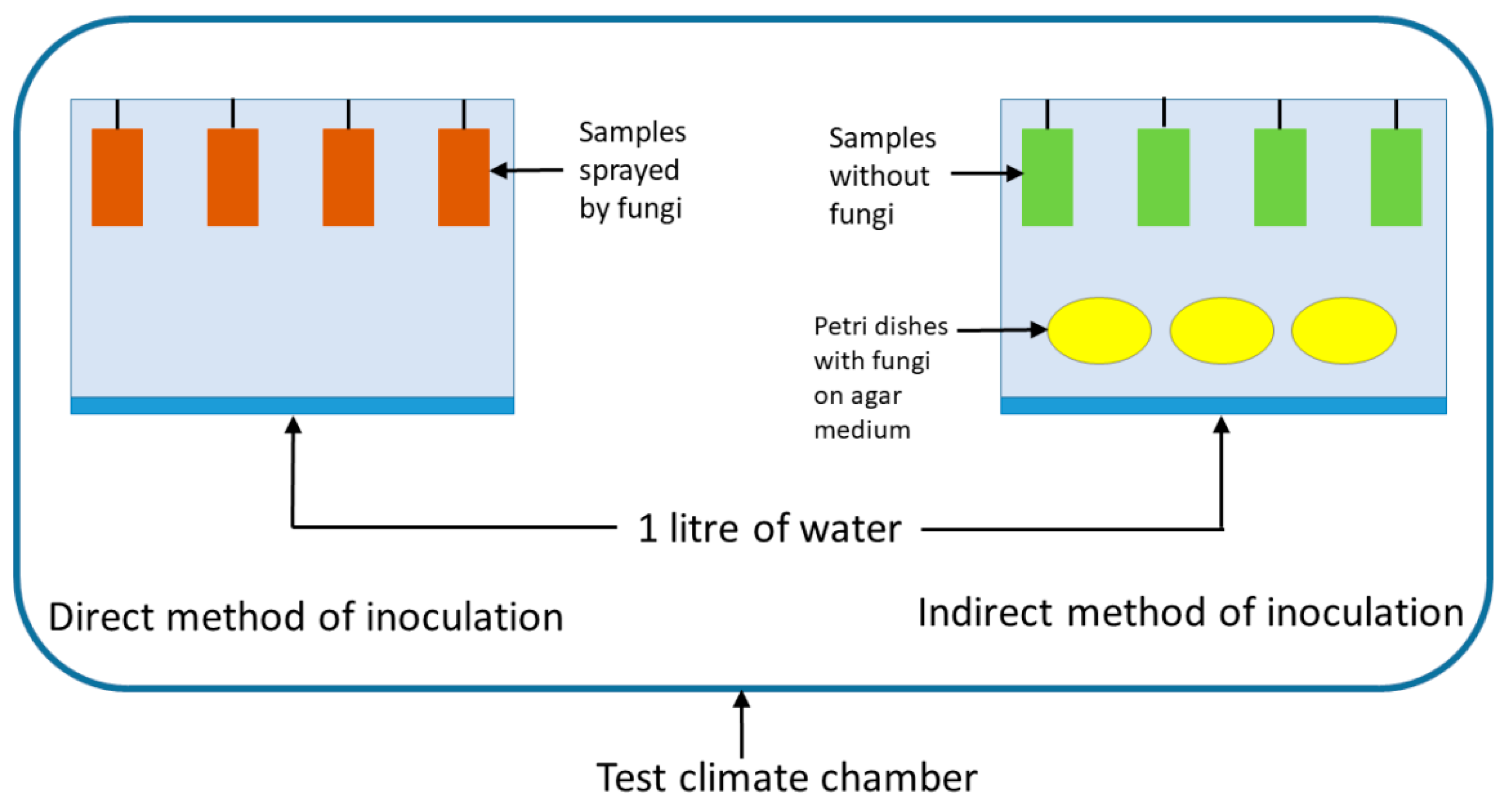
2.2. Mould Test
2.3. Mould Assessment
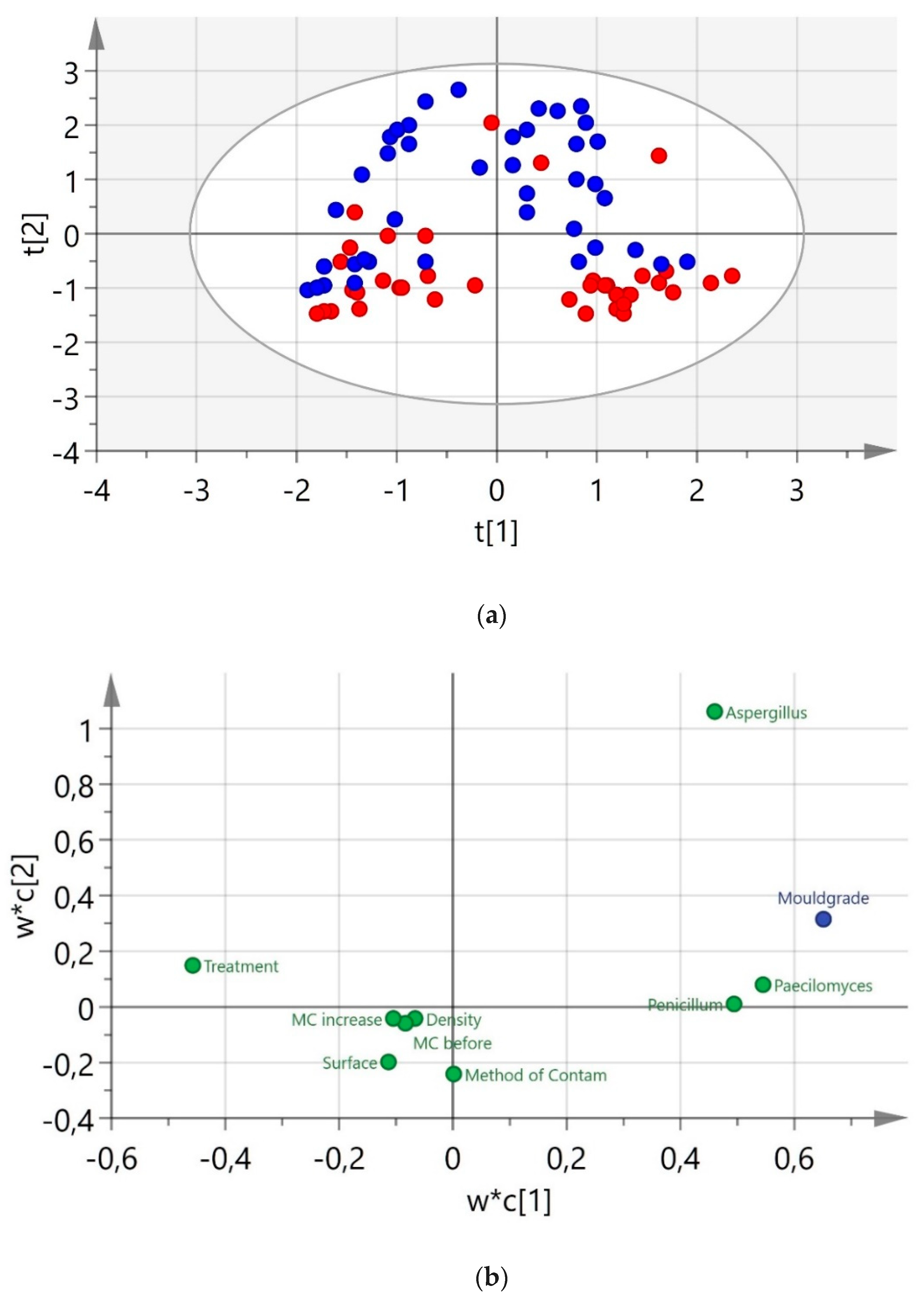
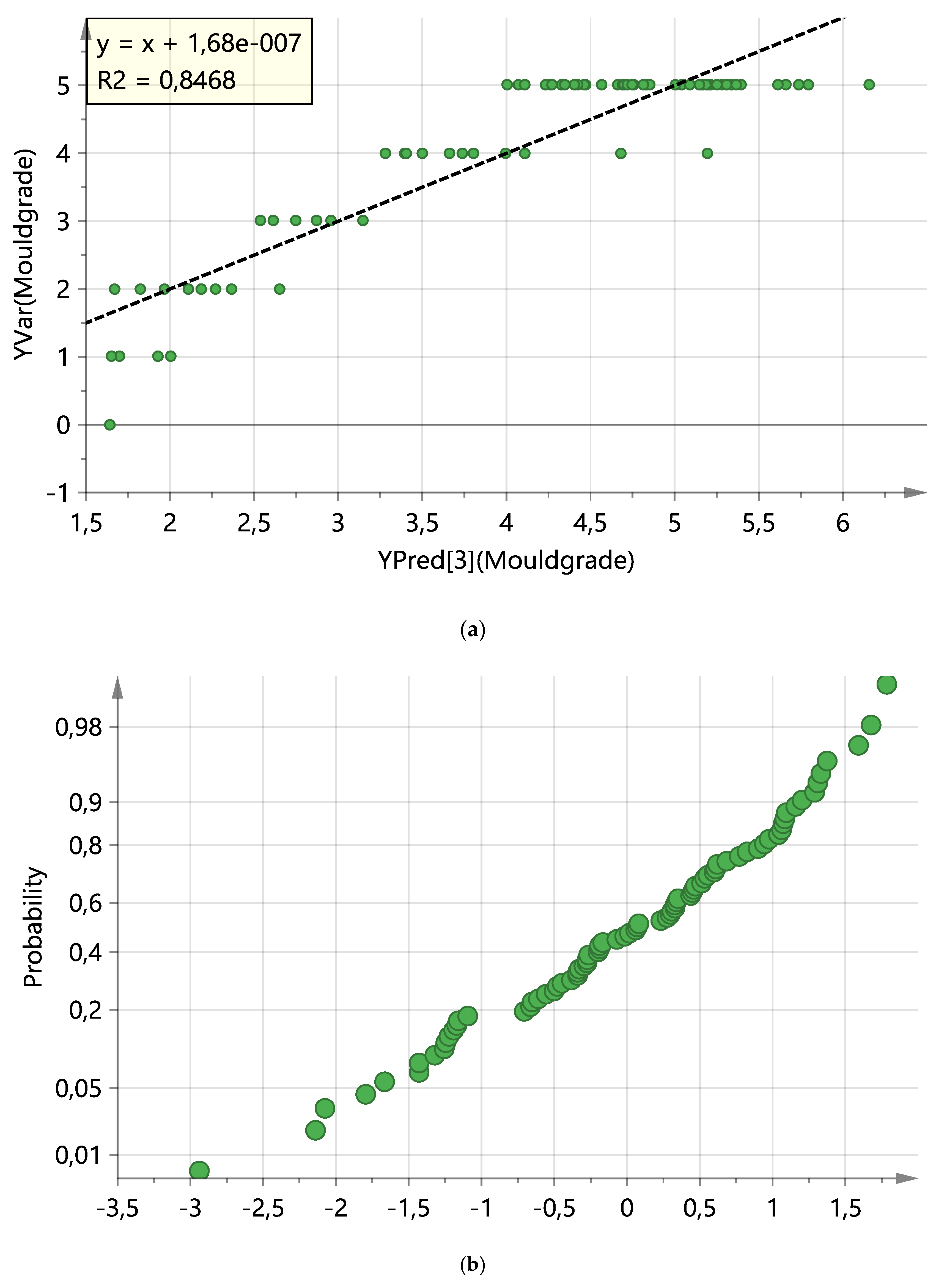
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Union. European Parliament and of the Council Directive 2004/42/CE on the Limitation of Emissions of Volatile Organic Compounds Due to the Use of Organic Solvents in Certain Paints and Varnishes and Vehicle Refinishing Products and Amending Directive 1999/13/EC; European Union: Brussels, Belgium, 2004. [Google Scholar]
- European Union. ECHA Directive 98/8/EC Concerning the Placing of Biocidal Products on the Market; European Union: Brussels, Belgium, 2011. [Google Scholar]
- Dijck, P.V.; Brown, N.A.; Goldman, G.H.; Rutherford, J.; Xue, C.; Zeebroeck, G.V. Nutrient Sensing at the Plasma Membrane of Fungal Cells. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Evans, P.D. Weathering of Wood and Wood Composites. In Handbook of Wood Chemistry and Wood Composites; Rowell, R.M., Ed.; Taylor & Francis Group: Abingdon, UK, 2013. [Google Scholar]
- Kujanpää, L.; Reiman, M.; Kujanpää, R.; Halonen, R.; Kokotti, H. The Influence of Outdoor Air Micro Flora and Season on the Indoor Surfaces of a Building. In Proceedings of the International Society of Indoor Air Quality and Climate, Beijing, China, 4–9 September 2005; pp. 1772–1776. [Google Scholar]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Wallin, S.; Gambacorta, L.; Kotova, N.; Lemming, E.W.; Nälsén, C.; Solfrizzo, M.; Olsen, M. Biomonitoring of concurrent mycotoxin exposure among adults in Sweden through urinary multi-biomarker analysis. Food Chem. Toxicol. 2015, 83, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Terziev, N. Migration of Low-Molecular Sugars and Nitrogenous Compounds in Pinus sylvestris L. During Kiln and Air Drying. Holzforsch. Int. J. Biol. Chem. Phys. Technol. Wood 2009, 49, 565–574. [Google Scholar]
- Myronycheva, O.; Karlsson, O.; Sehlstedt-Persson, M.; Öhman, M.; Sandberg, D. Distribution of low-molecular lipophilic extractives beneath the surface of air- and kiln-dried Scots pine sapwood boards. PLoS ONE 2018, 13, e0204212. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, O.; Myronycheva, O.; Sehlstedt-Persson, M.; Öhman, M.; Sandberg, D. Multivariate modeling of mould growth in relation to extractives in dried Scots pine sapwood. In Proceedings of the 48th Conference of the International Research Group on Wood Protection, IRG48, Ghent, Belgium, 4–8 June 2017. [Google Scholar]
- Guillén, Y.; Navias, D.; Machuca, Á. Tolerance to wood preservatives by copper-tolerant wood-rot fungi native to south-central Chile. Biodegradation 2009, 20, 135. [Google Scholar] [CrossRef] [PubMed]
- Myronycheva, O.; Sehlstedt-Persson, M.; Karlsson, O.; Sandberg, D. Growth of Mold and Rot Fungi on Copper-impregnated Scots Pine Sapwood: Influence of Planing Depth and Inoculation Pattern. BioResources 2018, 13, 8787–8801. [Google Scholar] [CrossRef]
- Adnan, O.C.G.; Samson, R.A. Fundamentals of Mold Growth in Indoor Environments and Strategies for Healthy Living; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011; ISBN 978-90-8686-135-4. [Google Scholar]
- U.S. Food and Drug Administration Generally Recognized as Safe (GRAS). Available online: https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/ (accessed on 16 January 2019).
- U.S. Food and Drug Administration GRAS Substances (SCOGS) Database. Available online: https://www.fda.gov/food/ingredientspackaginglabeling/gras/scogs/default.htm (accessed on 17 January 2019).
- Romanazzi, G.; Lichter, A.; Gabler, F.M.; Smilanick, J.L. Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2012, 63, 141–147. [Google Scholar] [CrossRef]
- CEN-European Committee for Standardization. EN 13183-1 Moisture Content of a Piece of Sawn Timber-Part 1: Determination by Oven Dry Method 2004; CEN-European Committee for Standardization: Brussels, Belgium, 2004. [Google Scholar]
- CEN-European Committee for Standardization. EN 16492:2014 Paints and Varnishes-Evaluation of the Surface Disfigurement Caused by Fungi and Algae on Coatings; CEN-European Committee for Standardization: Brussels, Belgium, 2014. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.A.; Datnoff, L.E. Silicon and Plant Diseases; Springer International Publishing: Basel, Switzerland, 2015; ISBN 978-3-319-22929-4. [Google Scholar]
- Okon, K.E.; Lin, F.; Chen, Y.; Huang, B. Effect of silicone oil heat treatment on the chemical composition, cellulose crystalline structure and contact angle of Chinese parasol wood. Carbohydr. Polym. 2017, 164, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Lahtela, V.; Kärki, T. Effects of impregnation and heat treatment on the physical and mechanical properties of Scots pine (Pinus sylvestris) wood. Wood Mater. Sci. Eng. 2016, 11, 217–227. [Google Scholar] [CrossRef]
- Kreber, B.; Morrell, J.J. Ability of Selected Bacterial and Fungal Bioprotectants to Limit Fungal Stain in Ponderosa Pine Sapwood. Wood Fiber Sci. 2007, 25, 23–34. [Google Scholar]
- Esping, B. Trätorkning: Grunder i Torkning; Trätek: Stockholm, Sweden, 1992; ISBN 978-91-88170-06-4. [Google Scholar]
- Nguyen Van Long, N.; Rigalma, K.; Coroller, L.; Dadure, R.; Debaets, S.; Mounier, J.; Vasseur, V. Modelling the effect of water activity reduction by sodium chloride or glycerol on conidial germination and radial growth of filamentous fungi encountered in dairy foods. Food Microbiol. 2017, 68, 7–15. [Google Scholar] [CrossRef] [PubMed]


| Code | Active Substance for Surface Treatment | Original Concentration | Concentration Used |
|---|---|---|---|
| R | Reference | ||
| B | Bacteria Bacillus amyloliquefaciens (trade company ABITEP, Germany) | 25 bln spores/mL | 1% |
| S | Silicon, potassium, natural parts of plants and water (trade company SIOO, Sweden) | 1st potassium silicate ca. 32% 2nd polymerizable alkoxylane | 100% |
| G | N-Alkylbensyldimethylammonium chloride (trade company JAPE, Sweden that) | 80 g/L | 20% |
| Grade | Description |
|---|---|
| 0 | No visable mould growth |
| 1 | Initial fungal growth consisting of scattered hyphae on the surface: less than 5% of the studied surface is covered by mould |
| 2 | Still scattered growth, but more apparent than in 1. Conidiophores may have started to develop: 6% to 20% mould growth |
| 3 | Patchily distributed, massive growth, and hyphae with developed conidiophores: 21% to 50% growth |
| 4 | Heavy growth over the entire surface: 51% to 80% mould growth |
| 5 | Very heavy growth: more than 80% mould growth |
| Variable | Range |
|---|---|
| Boards A, B, C, D, E | 1–5 |
| Method of inoculation | 1-indirect 0-direct |
| GRAS treatment | 1-R 2-B 3-S 4-G |
| Surface | 0-bark side 1-pith side |
| Mould grade | 0–5 |
| Density | 548 ± 12 kg/m3 |
| The initial moisture content of the board before the fungal test | 15.5% ± 0.4% |
| Moisture content increase | 12.1% ± 1.5% |
| Area covered by fungus Aspergillus niger | 0–87.3% |
| Area covered by fungus Penicillium commune | 0–91.9% |
| Area covered by fungus Paecilomyces variotii | 0–80.9% |
| GRAS Treatment | MC after the Test (%) | MC Increase during the Test (%) |
|---|---|---|
| Indirect Fungal-Inoculation Method | ||
| R | 27.3 ± 0.8 | 11.8 ± 1.2 |
| B | 28.0 ± 2.8 | 12.5 ± 2.6 |
| S | 27.7 ± 0.8 | 11.8 ± 1.0 |
| G | 28.9 ± 2.2 | 13.4 ± 2.0 |
| Average | 27.4 ± 1.2 | 11.8 ± 1.0 |
| Direct Fungal-Inoculation Method | ||
| R | 27.8 ± 1.1 | 12.3 ± 0.8 |
| B | 26.4 ± 1.0 * | 10.9 ± 0.8 * |
| S | 28.1 ± 1.0 | 12.6 ± 0.8 |
| G | 27.0 ± 1.0 | 11.5 ± 0.7 |
| Average | 27.9 ± 1.9 | 12.4 ± 1.9 |
| GRAS Treatment | Fungal Area of Aspergillus niger | Fungal Area of Penicillium commune | Fungal Area of Paecylomyces variotti | Mould Grade | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range (SE) | CV, % | Mean | Range (SE) | CV, % | Mean | Range (SE) | CV, % | ||
| Indirect Fungal Inoculation | ||||||||||
| R Bark side Pith side | 4.8 4.0 5.6 | 1.0–14.1 (1.6) 1.0–12.0 (2.1) 1.3–14.1 (2.6) | 105 117 104 | 71.0 71.8 70.2 | 59.0–84.5 (2.9) 59.0–81.0 (4.1) 59.2–84.5 (4.8) | 13 13 15 | 19.3 18.4 20.2 | 10.6–36.00 (2.9) 12.0–36.00 (4.6) 10.6–31.5 (4.0) | 47 55 44 | 5.0 5.0 5.0 |
| B Bark side Pith side | 10.8 3.0 18.7 | 1.4–72.8 (6.9) 2.0–4.0 (0.5) 1.4–72.8 (13.6) | 204 33 163 | 74.1 82.2 66.1 | 30.0–92.0 (5.9) 72.0–92.0 (3.9) 30.0–89.2 (10.3) | 25 11 35 | 19.2 16.4 22.1 | 12.0–33.2 (2.0) 12.0–25.0 (2.3) 15.7–33.2 (3.1) | 33 30 31 | 5.0 5.0 5.0 |
| S Bark side Pith side | 0.1 0 0.2 | 0–0.5 (0.1) 0–0.4 (0.1) 0–0.5 (0.1) | 140 143 141 | 16.1 17.2 15.0 | 0–45.0 (4.9) 5.0–33.0 (6.3) 0–45.0 (8.3) | 97 82 125 | 0.9 0.8 0.9 | 0–4.5 (0.5) 0–3.0 (0.6) 0–4.5 (3.1) | 199 179 222 | 1.9 2.2 1.6 |
| G Bark side Pith side | 30.3 36.2 24.5 | 2.0–81.0 (7.7) 2.0–81.0 (14.9) 4.5–5.3 (5.3) | 80 92 48 | 19.8 20.0 19.5 | 0–50.2 (5.5) 0–48.0 (8.4) 5.4–50.2 (8.1) | 88 95 93 | 16.1 22.6 9.7 | 1.0–81.0 (7.4) 1.0–81.0 (14.8) 4.5–19.9 (2.8) | 144 144 65 | 4.1 4.4 3.8 |
| Average permethod | 11.5 | 45.2 | 13.9 | 4.0 | ||||||
| GRAS Treatment | Aspergillus niger | Penicillium commune | Paecylomyces variotti | Mould Grade | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range (SE) | CV, % | Mean | Range (SE) | CV, % | Mean | Range (SE) | CV, % | ||
| Direct Fungal Inoculation | ||||||||||
| R Bark side Pith side | 48.74 66.20 31.28 | 11.0–87.0 (9.7) 11.0–87.0 (14.1) 11.9–56.3 (8.7) | 63 48 62 | 30.3 22.0 38.7 | 0–68.0 (8.5) 0–68.0 (12.5) 6.9–66.7 (11.6) | 89 128 67 | 12.0 4.2 19.9 | 0–29.7 (3.5) 0–15.0 (2.9) 10.5–29.7 (3.8) | 90 154 43 | 4.9 5.0 4.8 |
| B Bark side Pith side | 51.9 62.6 41.3 | 10.7–77.0 (8.1) 27.0–77.0 (9.2) 10.7–66.7 (12.4) | 49 33 67 | 27.3 19.4 35.2 | 0–60.0 (6.6) 0–43.0 (7.2) 6.5–60.0 (10.6) | 76 83 67 | 6.9 3.6 10.2 | 0–18.3 (2.3) 0–10.0 (2.3) 0–18.3 (3.7) | 106 139 80 | 5.0 5.0 5.0 |
| S Bark side Pith side | 3.0 4.4 1.5 | 0–18.0 (1.8) 0–18.0 (3.4) 0–7.4 (1.5) | 195 176 216 | 11.4 15.0 7.9 | 0–53.0 (5.0) 0–53.0 (9.9) 3.5–20.5 (3.2) | 139 147 90 | 0.2 0.4 0 | 0–2.0 (0.2) 0–2.0 (0.4) 0 | 306 223 0 | 1.8 2.0 1.6 |
| G Bark side Pith side | 61.3 69.2 53.3 | 35.0–81.0 (5.1) 54.0–81.0 (5.1) 35.0–76.4 (7.6) | 26 17 32 | 4.3 0 8.6 | 0–42.9 (4.3) 0 0–42.9 (8.6) | 316 0 224 | 2.3 0 4.6 | 0–22.8 (2.3) 0 0–22.8 (4.6) | 316 0 224 | 4.3 4.6 4.00 |
| Average per method | 41.2 | 18.3 | 5.4 | 4.0 | ||||||
| № | Model | PC | Obs. | R2X | R2Y | Q2 (cum) | |
|---|---|---|---|---|---|---|---|
| 1 | PCA | 2 | 80 | 0.448 | −0.0139 | ||
| 2 | PLS-DA | 2 | 80 | 0.404 | 0.36 | 0.319 | Treatment class |
| 3 | OPLS-DA | 2 + 1 + 0 | 80 | 0.534 | 0.394 | 0.336 | Treatment class |
| 4 | PLS | 3 | 80 | 0.49 | 0.843 | 0.75 | Y mould grade |
| 5 | PLS | 3 | 79 | 0.519 | 0.847 | 0.766 | Y mould grade |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myronycheva, O.; Poohphajai, F.; Sehlstedt-Persson, M.; Vikberg, T.; Karlsson, O.; Junge, H.; Sandberg, D. Application of GRAS Compounds for the Control of Mould Growth on Scots Pine Sapwood Surfaces: Multivariate Modelling of Mould Grade. Forests 2019, 10, 714. https://doi.org/10.3390/f10090714
Myronycheva O, Poohphajai F, Sehlstedt-Persson M, Vikberg T, Karlsson O, Junge H, Sandberg D. Application of GRAS Compounds for the Control of Mould Growth on Scots Pine Sapwood Surfaces: Multivariate Modelling of Mould Grade. Forests. 2019; 10(9):714. https://doi.org/10.3390/f10090714
Chicago/Turabian StyleMyronycheva, Olena, Faksawat Poohphajai, Margot Sehlstedt-Persson, Tommy Vikberg, Olov Karlsson, Helmut Junge, and Dick Sandberg. 2019. "Application of GRAS Compounds for the Control of Mould Growth on Scots Pine Sapwood Surfaces: Multivariate Modelling of Mould Grade" Forests 10, no. 9: 714. https://doi.org/10.3390/f10090714
APA StyleMyronycheva, O., Poohphajai, F., Sehlstedt-Persson, M., Vikberg, T., Karlsson, O., Junge, H., & Sandberg, D. (2019). Application of GRAS Compounds for the Control of Mould Growth on Scots Pine Sapwood Surfaces: Multivariate Modelling of Mould Grade. Forests, 10(9), 714. https://doi.org/10.3390/f10090714





