Estimating Crown Structure Parameters of Moso Bamboo: Leaf Area and Leaf Angle Distribution
Abstract
1. Introduction
2. Materials and Methods
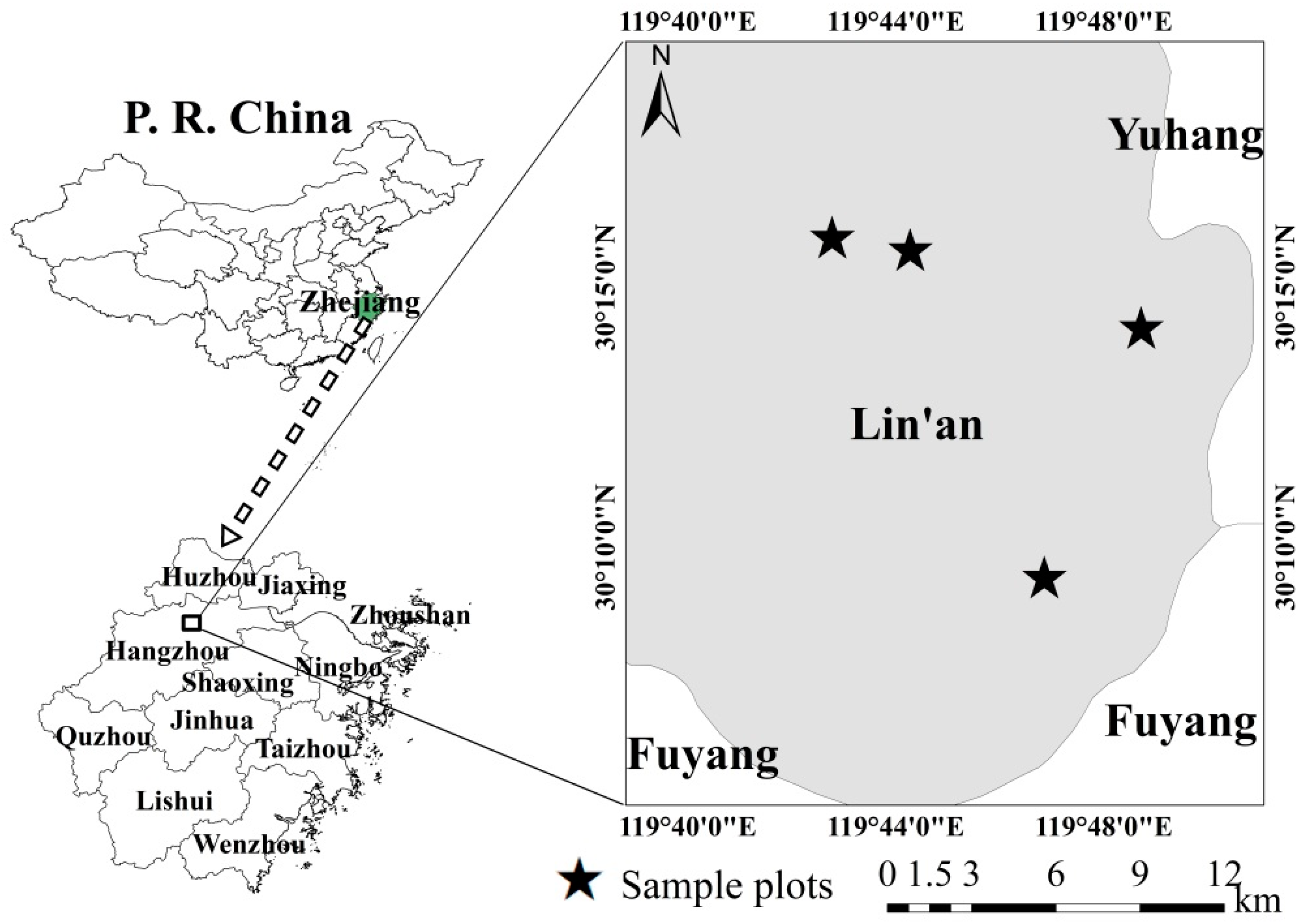
2.1. Study Area, Destructive Sampling, and Laboratory Procedures
2.2. Allometric Equations for LA Estimation
2.3. Leaf Angle Distribution and Leaf Projection Function
3. Results
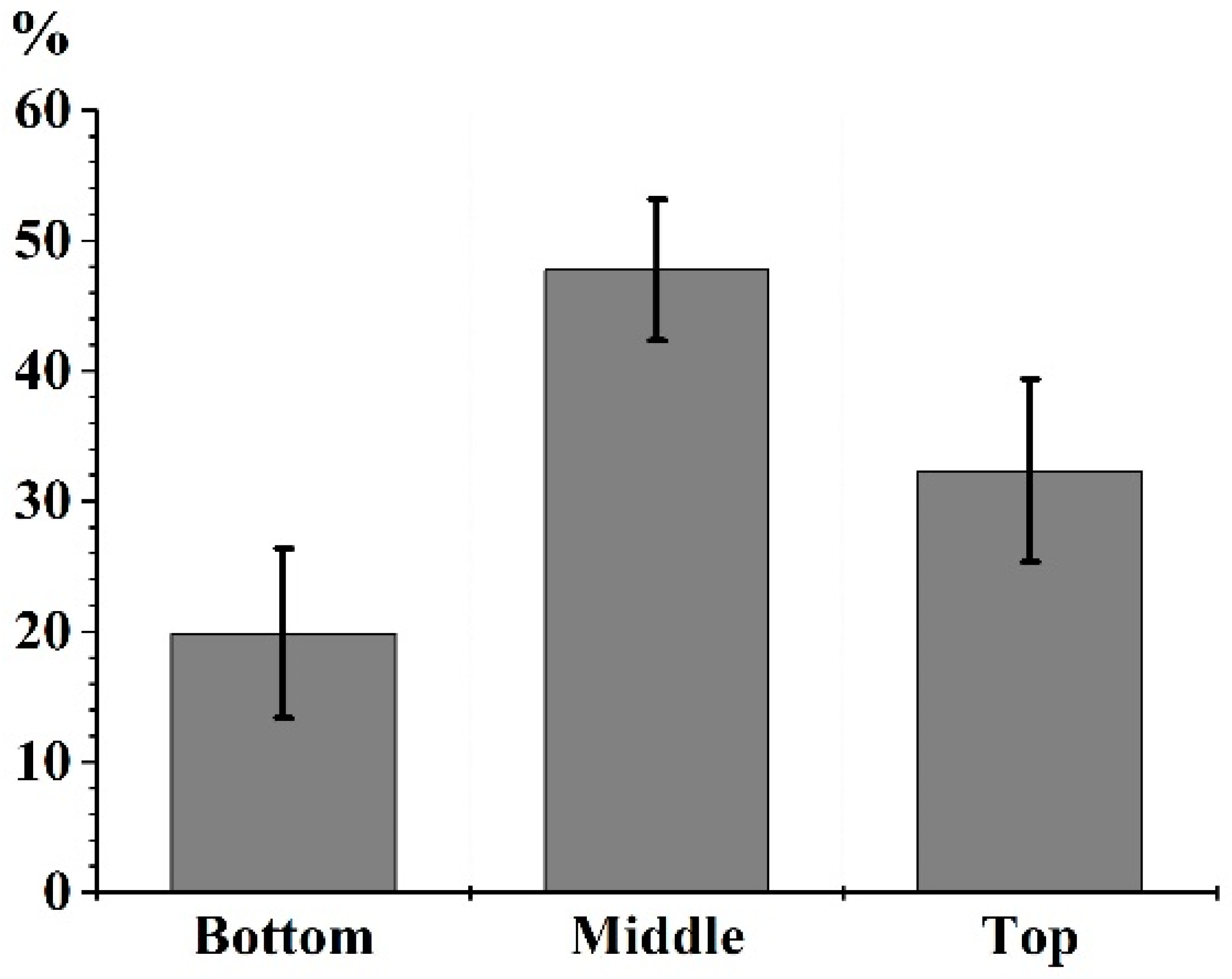
3.1. LA of a MB Crown
3.2. Allometric Equations for LA Estimation
3.3. Leaf Angle Distribution f(α) and Leaf Projection Function G(θ)
4. Discussion
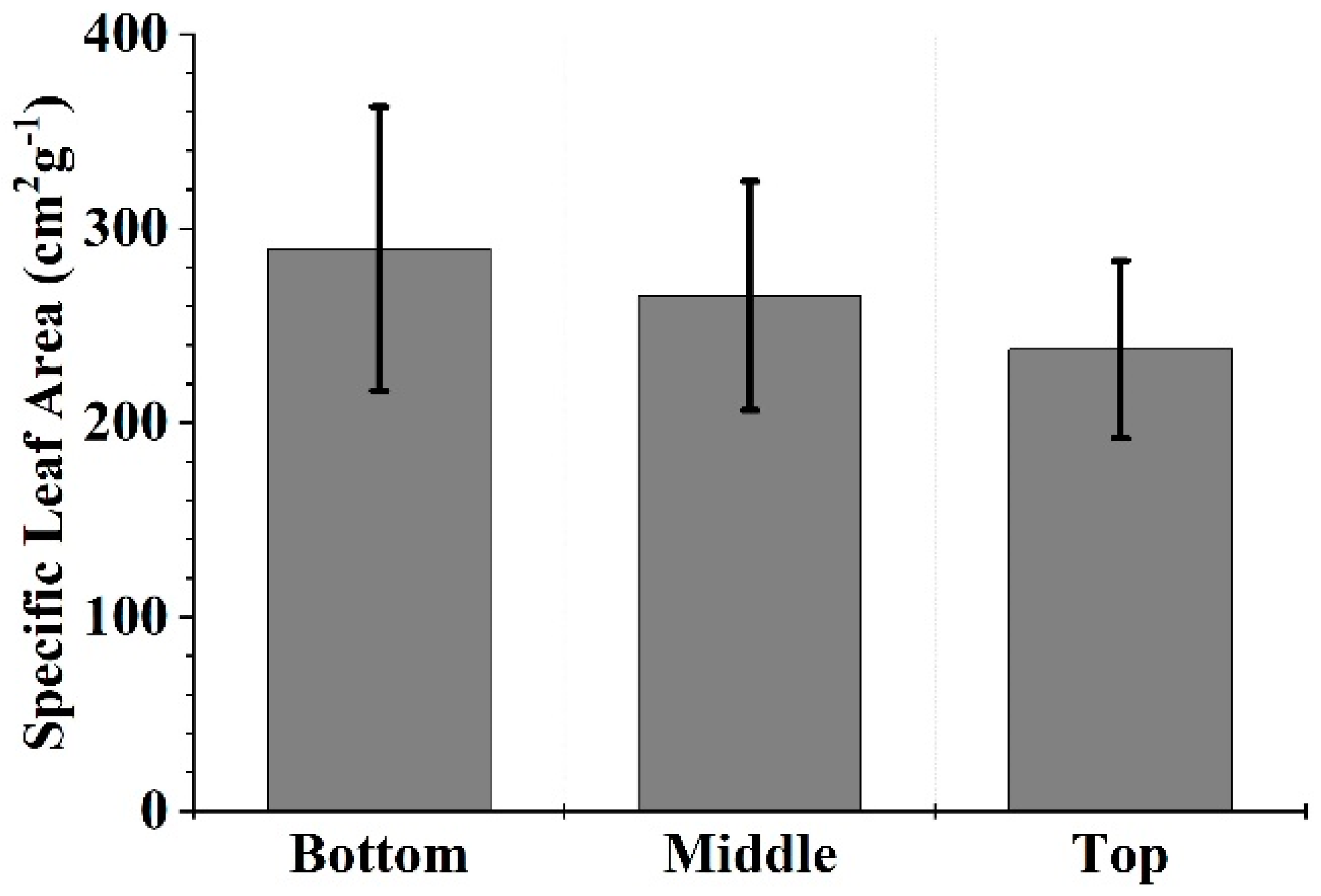
4.1. Specific Leaf Area
4.2. LA Estimation of MB
4.3. Leaf Angle Influence on LAI Estimation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Global Forest Resources Assessment 2010: Main Report; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010. [Google Scholar]
- LaPlace, S.; Komatsu, H.; Tseng, H.; Kume, T. Difference between the transpiration rates of Moso bamboo (Phyllostachys pubescens) and Japanese cedar (Cryptomeria japonica) forests in a subtropical climate in Taiwan. Ecol. Res. 2017, 32, 835–843. [Google Scholar] [CrossRef]
- SFAPRC. Forest Resources in China—The 8th National Forest Inventory; State Forestry Administration: Beijing, China, 2015. [Google Scholar]
- Komatsu, H.; Onozawa, Y.; Kume, T.; Tsuruta, K.; Kumagai, T.; Shinohara, Y.; Otsuki, K. Stand-scale transpiration estimates in a Moso bamboo forest: II. Comparison with coniferous forests. For. Ecol. Manag. 2010, 260, 1295–1302. [Google Scholar] [CrossRef]
- Xu, X.J.; Du, H.Q.; Zhou, G.M.; Mao, F.J.; Li, X.J.; Zhu, D.E.; Li, Y.G.; Cui, L. Remote estimation of canopy leaf area index and chlorophyll content in Moso bamboo (Phyllostachys edulis (Carrière) J. Houz.) forest using MODIS reflectance data. Ann. For. Sci. 2018, 75, 33. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, G.; Du, H.; Berninger, F.; Mao, F.; Li, X.; Chen, L.; Cui, L.; Li, Y.; Zhu, D. Soil respiration of a Moso bamboo forest significantly affected by gross ecosystem productivity and leaf area index in an extreme drought event. PeerJ 2018, 6, e5747. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Zhang, Y.; Booth, T.; He, X. Changes of carbon stocks in bamboo stands in China during 100 years. For. Ecol. Manag. 2009, 258, 1489–1496. [Google Scholar] [CrossRef]
- Yen, T.-M.; Lee, J.-S. Comparing aboveground carbon sequestration between moso bamboo (Phyllostachys heterocycla) and China fir (Cunninghamia lanceolata) forests based on the allometric model. For. Ecol. Manag. 2011, 261, 995–1002. [Google Scholar] [CrossRef]
- Zhou, G.M.; Meng, C.F.; Jiang, P.K.; Xu, Q.F. Review of carbon fixation in bamboo forests in China. Bot. Rev. 2011, 77, 262. [Google Scholar] [CrossRef]
- Yen, T.-M. Comparing aboveground structure and aboveground carbon storage of an age series of moso bamboo forests subjected to different management strategies. J. For. Res. 2015, 20, 1–8. [Google Scholar] [CrossRef]
- Yen, T.-M. Culm height development, biomass accumulation and carbon storage in an initial growth stage for a fast-growing moso bamboo (Phyllostachy pubescens). Bot. Stud. 2016, 57, 10. [Google Scholar] [CrossRef]
- Peter, H.; Otto, E.; Hubert, S. Leaf area of beech (Fagus sylvatica L.) from different stands in eastern Austria studied by randomized branch sampling. Eur. J. For. Res. 2010, 129, 401–408. [Google Scholar] [CrossRef]
- Sarker, S.K.; Das, N.; Chowdhury, M.Q.; Haque, M.M. Developing allometric equations for estimating leaf area and leaf biomass of Artocarpus chaplasha in Raghunandan Hill Reserve, Bangladesh. South. For. 2013, 75, 51–57. [Google Scholar] [CrossRef]
- Gonzalez-Benecke, C.A.; Flamenco, H.N.; Wightman, M.G. Effect of Vegetation Management and Site Conditions on Volume, Biomass and Leaf Area Allometry of Four Coniferous Species in the Pacific Northwest United States. Forests 2018, 9, 581. [Google Scholar] [CrossRef]
- Hagemeier, M.; Leuschner, C. Functional Crown Architecture of Five Temperate Broadleaf Tree Species: Vertical Gradients in Leaf Morphology, Leaf Angle, and Leaf Area Density. Forests 2019, 10, 265. [Google Scholar] [CrossRef]
- Xu, X.J.; Du, H.Q.; Zhou, G.M.; Li, P.H. Method for improvement of MODIS leaf area index products based on pixel-to-pixel correlations. Eur. J. Remote Sens. 2016, 49, 57–72. [Google Scholar] [CrossRef]
- Li, X.; Mao, F.; Du, H.; Zhou, G.; Xu, X.; Han, N.; Sun, S.; Gao, G.; Chen, L. Assimilating leaf area index of three typical types of subtropical forest in China from MODIS time series data based on the integrated ensemble Kalman filter and PROSAIL model. ISPRS J. Photogramm. Remote Sens. 2017, 126, 68–78. [Google Scholar] [CrossRef]
- Mao, F.; Li, X.; Du, H.; Zhou, G.; Han, N.; Xu, X.; Liu, Y.; Chen, L.; Cui, L. Comparison of Two Data Assimilation Methods for Improving MODIS LAI Time Series for Bamboo Forests. Remote Sens. 2017, 9, 401. [Google Scholar] [CrossRef]
- Li, X.J.; Du, H.Q.; Mao, F.J.; Zhou, G.M.; Chen, L.; Xing, L.Q.; Fan, W.L.; Xu, X.J.; Liu, Y.L.; Cui, L.; et al. Estimating bamboo forest aboveground biomass using EnKF-assimilated MODIS LAI spatiotemporal data and machine learning algorithms. Agric. For. Meteorol. 2018, 256–257, 445–457. [Google Scholar] [CrossRef]
- Mao, F.J.; Du, H.Q.; Zhou, G.M.; Li, X.J.; Xu, X.J.; Li, P.H.; Sun, S.B. Coupled LAI assimilation and BEPS model for analyzing the spatiotemporal pattern and heterogeneity of carbon fluxes of the bamboo forest in Zhejiang Province, China. Agric. For. Meteorol. 2017, 242, 96–108. [Google Scholar] [CrossRef]
- Xing, L.Q.; Li, X.J.; Du, H.Q.; Zhou, G.M.; Mao, F.J.; Liu, T.Y.; Zheng, J.L.; Dong, L.F.; Zhang, M.; Han, N. Assimilating Multiresolution Leaf Area Index of Moso Bamboo Forest from MODIS Time Series Data Based on a Hierarchical Bayesian Network Algorithm. Remote Sens. 2019, 11, 56. [Google Scholar] [CrossRef]
- Campbell, G.S.; Norman, J.M. The description and measurement of plant canopy structure. In Plant Canopies: Their Growth, Form and Function; Cambridge University Press: Cambridge, UK, 1989; Volume 31, pp. 1–19. [Google Scholar]
- Gower, S.T.; Norman, J.M. Rapid Estimation of Leaf Area Index in Conifer and Broad-Leaf Plantations. Ecology 1991, 72, 1896–1900. [Google Scholar] [CrossRef]
- Cutini, A.; Matteucci, G.; Mugnozza, G.S. Estimation of leaf area index with the Li-Cor LAI 2000 in deciduous forests. For. Ecol. Manag. 1998, 105, 55–65. [Google Scholar] [CrossRef]
- Van Gardingen, P.; Jackson, G.; Hernandez-Daumas, S.; Russell, G.; Sharp, L. Leaf area index estimates obtained for clumped canopies using hemispherical photography. Agric. For. Meteorol. 1999, 94, 243–257. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.M.; Miller, J.R. Determining digital hemispherical photograph exposure for leaf area index estimation. Agric. For. Meteorol. 2005, 133, 166–181. [Google Scholar] [CrossRef]
- Chianucci, F.; Cutini, A. Digital hemispherical photography for estimating forest canopy properties: Current controversies and opportunities. iForest Biogeosciences For. 2012, 5, 290–295. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ryu, Y.; Baldocchi, D.D.; Welles, J.M.; Norman, J.M. On the correct estimation of gap fraction: How to remove scattered radiation in gap fraction measurements? Agric. For. Meteorol. 2013, 174–175, 170–183. [Google Scholar] [CrossRef]
- Lang, A. Estimation of leaf area index from transmission of direct sunlight in discontinuous canopies. Agric. For. Meteorol. 1986, 37, 229–243. [Google Scholar] [CrossRef]
- Chen, J.M.; Cihlar, J. Plant canopy gap-size analysis theory for improving optical measurements of leaf-area index. Appl. Opt. 1995, 34, 6211. [Google Scholar] [CrossRef]
- Bréda, N.J.J. Ground-based measurements of leaf area index: A review of methods, instruments and current controversies. J. Exp. Bot. 2003, 54, 2403–2417. [Google Scholar] [CrossRef]
- Leblanc, S.; Fournier, R. Hemispherical photography simulations with an architectural model to assess retrieval of leaf area index. Agric. For. Meteorol. 2014, 194, 64–76. [Google Scholar] [CrossRef]
- Jonckheere, I.; Fleck, S.; Nackaerts, K.; Muys, B.; Coppin, P.; Weiss, M.; Baret, F. Review of methods for in situ leaf area index determination: Part I. Theories, sensors and hemispherical photography. Agric. For. Meteorol. 2004, 121, 19–35. [Google Scholar] [CrossRef]
- Bartelink, H. Allometric relationships on biomass and needle area of Douglas-fir. For. Ecol. Manag. 1996, 86, 193–203. [Google Scholar] [CrossRef]
- Albrektson, A. Sapwood Basal Area and Needle Mass of Scots Pine (Pinus sylvestris L.) Trees in Central Sweden. Forests 1984, 57, 35–43. [Google Scholar] [CrossRef]
- Laubhann, D.; Eckmüllner, O.; Sterba, H. Applicability of non-destructive substitutes for leaf area in different stands of Norway spruce (Picea abies L. Karst.) focusing on traditional forest crown measures. For. Ecol. Manag. 2010, 260, 1498–1506. [Google Scholar] [CrossRef]
- Jones, D.A.; O’Hara, K.L.; Battles, J.J.; Gersonde, R.F. Leaf Area Prediction Using Three Alternative Sampling Methods for Seven Sierra Nevada Conifer Species. Forests 2015, 6, 2631–2654. [Google Scholar] [CrossRef]
- Wang, W.-M.; Li, Z.-L.; Su, H.-B. Comparison of leaf angle distribution functions: Effects on extinction coefficient and fraction of sunlit foliage. Agric. For. Meteorol. 2007, 143, 106–122. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Leaf size and angle vary widely across species: What consequences for light interception? New Phytol. 2003, 158, 509–525. [Google Scholar] [CrossRef]
- Zou, X.; Mõttus, M.; Tammeorg, P.; Torres, C.L.; Takala, T.; Pisek, J.; Mäkelä, P.; Stoddard, F.; Pellikka, P.; Torres, C.I.L. Photographic measurement of leaf angles in field crops. Agric. For. Meteorol. 2014, 184, 137–146. [Google Scholar] [CrossRef]
- Vicari, M.B.; Pisek, J.; Disney, M. New estimates of leaf angle distribution from terrestrial LiDAR: Comparison with measured and modelled estimates from nine broadleaf tree species. Agric. For. Meteorol. 2019, 264, 322–333. [Google Scholar] [CrossRef]
- Mabrouk, H.; Kasemsap, P.; Sinoquet, H.; Thanisawanyangkura, S. Characterization of the Light Environment in Canopies Using 3D Digitising and Image Processing. Ann. Bot. 1998, 82, 203–212. [Google Scholar]
- Ryu, Y.; Sonnentag, O.; Nilson, T.; Vargas, R.; Kobayashi, H.; Wenk, R.; Baldocchi, D.D. How to quantify tree leaf area index in an open savanna ecosystem: A multi-instrument and multi-model approach. Agric. For. Meteorol. 2010, 150, 63–76. [Google Scholar] [CrossRef]
- Pisek, J.; Lang, M.; Nilson, T.; Korhonen, L.; Karu, H. Comparison of methods for measuring gap size distribution and canopy nonrandomness at Järvselja RAMI (RAdiation transfer Model Intercomparison) test sites. Agric. For. Meteorol. 2011, 151, 365–377. [Google Scholar] [CrossRef]
- Lang, A.; Xiang, Y.; Norman, J. Crop structure and the penetration of direct sunlight. Agric. For. Meteorol. 1985, 35, 83–101. [Google Scholar] [CrossRef]
- Pisek, J.; Sonnentag, O.; Richardson, A.D.; Mõttus, M. Is the spherical leaf inclination angle distribution a valid assumption for temperate and boreal broadleaf tree species? Agric. For. Meteorol. 2013, 169, 186–194. [Google Scholar] [CrossRef]
- Piayda, A.; Dubbert, M.; Werner, C.; Correia, A.V.; Pereira, J.S.; Cuntz, M. Influence of woody tissue and leaf clumping on vertically resolved leaf area index and angular gap probability estimates. For. Ecol. Manag. 2015, 340, 103–113. [Google Scholar] [CrossRef]
- Xiao, C.-W.; Janssens, I.A.; Yuste, J.C.; Ceulemans, R. Variation of specific leaf area and upscaling to leaf area index in mature Scots pine. Trees 2006, 20, 304–310. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.M.; Jin, G.; Qi, Y. Estimating seasonal variations of leaf area index using litterfall collection and optical methods in four mixed evergreen–deciduous forests. Agric. For. Meteorol. 2015, 209, 36–48. [Google Scholar] [CrossRef]
- Xiao, X.; White, E.P.; Hooten, M.B.; Durham, S.L. On the use of log-transformation vs. nonlinear regression for analyzing biological power laws. Ecology 2011, 92, 1887–1894. [Google Scholar] [CrossRef]
- Djomo, A.N.; Picard, N.; Fayolle, A.; Henry, M.; Ngomanda, A.; Ploton, P.; McLellan, J.; Saborowski, J.; Adamou, I.; Lejeune, P. Tree allometry for estimation of carbon stocks in African tropical forests. Forestry 2016, 89, 446–455. [Google Scholar] [CrossRef]
- He, H.; Zhang, C.; Zhao, X.; Fousseni, F.; Wang, J.; Dai, H.; Yang, S.; Zuo, Q. Allometric biomass equations for 12 tree species in coniferous and broadleaved mixed forests, Northeastern China. PLoS ONE 2018, 13, e0186226. [Google Scholar] [CrossRef]
- Basuki, T.; Van Laake, P.; Skidmore, A.; Hussin, Y. Allometric equations for estimating the above-ground biomass in tropical lowland Dipterocarp forests. For. Ecol. Manag. 2009, 257, 1684–1694. [Google Scholar] [CrossRef]
- Djomo, A.N.; Ibrahima, A.; Saborowski, J.; Gravenhorst, G. Allometric equations for biomass estimations in Cameroon and pan moist tropical equations including biomass data from Africa. For. Ecol. Manag. 2010, 260, 1873–1885. [Google Scholar] [CrossRef]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Beelen, R.; Hoek, G.; Vienneau, D.; Eeftens, M.; Dimakopoulou, K.; Pedeli, X.; Tsai, M.-Y.; Künzli, N.; Schikowski, T.; Marcon, A.; et al. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe—The ESCAPE project. Atmos. Environ. 2013, 72, 10–23. [Google Scholar] [CrossRef]
- Kilibarda, M.; Hengl, T.; Heuvelink, G.B.M.; Gräler, B.; Pebesma, E.; Tadić, M.P.; Bajat, B. Spatio-temporal interpolation of daily temperatures for global land areas at 1 km resolution. J. Geophys. Res. Atmos. 2014, 119, 2294–2313. [Google Scholar] [CrossRef]
- Wilson, J.W. Inclined point quadrats. New Phytol. 1960, 59, 1–7. [Google Scholar] [CrossRef]
- Marshall, J.D.; Monserud, R.A. Foliage height influences specific leaf area of three conifer species. Can. J. For. Res. 2003, 33, 164–170. [Google Scholar] [CrossRef]
- Konôpka, B.; Pajtík, J.; Marušák, R.; Bosela, M.; Lukac, M. Specific leaf area and leaf area index in developing stands of Fagus sylvatica L. and Picea abies Karst. For. Ecol. Manag. 2016, 364, 52–59. [Google Scholar] [CrossRef]
- Turner, D.P.; Acker, S.A.; Means, J.E.; Garman, S.L. Assessing alternative allometric algorithms for estimating leaf area of Douglas-fir trees and stands. For. Ecol. Manag. 2000, 126, 61–76. [Google Scholar] [CrossRef]
- Zhou, F.C. Studies on the structure of bamboo crown of Phyllostachys pubescens. J. Nanjing Technol. Coll. For. Prod. 1982, 3, 46–73. [Google Scholar]
- De Wit, C.T. Photosynthesis of Leaf Canopies; Centre for Agricultural Publications and Documentation: Wagenirtgen, The Netherland, 1965. [Google Scholar]
- Pan, S.; Liu, C.; Zhang, W.; Xu, S.; Wang, N.; Li, Y.; Gao, J.; Wang, Y.; Wang, G. The Scaling Relationships between Leaf Mass and Leaf Area of Vascular Plant Species Change with Altitude. PLoS ONE 2013, 8, e76872. [Google Scholar] [CrossRef]
- Ryan, M.G.; Yoder, B.J. Hydraulic Limits to Tree Height and Tree Growth. Bioscience 1997, 47, 235–242. [Google Scholar] [CrossRef]
- Hager, H.; Sterba, H. Specific leaf area and needle weight of Norway spruce (Piceaabies) in stands of different densities. Can. J. For. Res. 1985, 15, 389–392. [Google Scholar] [CrossRef]
- Abrams, M.D.; Kubiske, M.E. Leaf structural characteristics of 31 hardwood and conifer tree species in central Wisconsin: Influence of light regime and shade-tolerance rank. For. Ecol. Manag. 1990, 31, 245–253. [Google Scholar] [CrossRef]
- Bartelink, H. Allometric relationships for biomass and leaf area of beech (Fagus sylvatica L.). Ann. Sci. For. 1997, 54, 39–50. [Google Scholar] [CrossRef]
- Bond, B.J.; Farnsworth, B.T.; Coulombe, R.A.; Winner, W.E. Foliage physiology and biochemistry in response to light gradients in conifers with varying shade tolerance. Oecologia 1999, 120, 183–192. [Google Scholar] [CrossRef]
- Fellner, H.; Dirnberger, G.F.; Sterba, H. Specific leaf area of European Larch (Larix decidua Mill.). Trees 2016, 30, 1237–1244. [Google Scholar] [CrossRef]
- Chen, H.Y.; Klinka, K.; Kayahara, G.J. Effects of light on growth, crown architecture, and specific leaf area for naturally established Pinus contorta var. latifolia and Pseudotsuga menziesii var. glauca saplings. Can. J. For. Res. 1996, 26, 1149–1157. [Google Scholar] [CrossRef]
- Niinemets, U.; Ellsworth, D.S.; Lukjanova, A.; Tobias, M. Site fertility and the morphological and photosynthetic acclimation of Pinus sylvestris needles to light. Tree Physiol. 2001, 21, 1231–1244. [Google Scholar] [CrossRef]
- Fitz, F.K.; Gholz, H.L.; Waring, R.H. Leaf area differences associated with old-growth forest communities in the western Oregon Cascades. Can. J. For. Res. 1976, 6, 49–57. [Google Scholar]
- Gower, S.T.; Richards, J.H. Larches: Deciduous Conifers in an Evergreen World. Bioscience 1990, 40, 818–826. [Google Scholar] [CrossRef]
- Reich, P.B.; Ellsworth, D.S.; Walters, M.B. Leaf structure (specific leaf area) modulates photosynthesis-nitrogen relations: Evidence from within and across species and functional groups. Funct. Ecol. 1998, 12, 948–958. [Google Scholar] [CrossRef]
- Withington, J.M.; Reich, P.B.; Oleksyn, J.; Eissenstat, D.M. Comparisons of structure and life span in roots and leaves among temperate trees. Ecol. Monogr. 2006, 76, 381–397. [Google Scholar] [CrossRef]
- Kroon, H.; During, H.J.; Zhong, Z.C.; Li, R.; Werger, M.J.A. Interactions Between Shoot Age Structure, Nutrient Availability and Physiological Integration in the Giant Bamboo Phyllostachys pubescens. Plant Biol. 2000, 2, 437–446. [Google Scholar]
- Zhang, J.; Lv, J.; Li, Q.; Ying, Y.; Peng, C.; Song, X. Effects of nitrogen deposition and management practices on leaf litterfall and N and P return in a Moso bamboo forest. Biogeochemistry 2017, 134, 115–124. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Zhou, G.M.; Du, H.Q.; Shi, Y.J.; Mao, F.J.; Liu, Y.L.; Xu, L.; Li, X.J.; Xu, X.J. Biotic and abiotic influences on monthly variation in carbon fluxes in on-year and off-year Moso bamboo forest. Trees 2019, 33, 153–169. [Google Scholar] [CrossRef]
- Barna, M. Adaptation of European beech (Fagus sylvatica L.) to different ecological conditions: Leaf size variation. Pol. J. Ecol. 2004, 52, 35–45. [Google Scholar]
- Leuschner, C.; Voß, S.; Foetzki, A.; Clases, Y. Variation in leaf area index and stand leaf mass of European beech across gradients of soil acidity and precipitation. Plant Ecol. 2006, 186, 247–258. [Google Scholar] [CrossRef]
- Closa, I.; Irigoyen, J.J.; Goicoechea, N. Microclimatic conditions determined by stem density influence leaf anatomy and leaf physiology of beech (Fagus sylvatica L.) growing within stands that naturally regenerate from clear-cutting. Trees 2010, 24, 1029–1043. [Google Scholar] [CrossRef]
- Ali, A.M.; Darvishzadeh, R.; Skidmore, A.K.; Van Duren, I. Specific leaf area estimation from leaf and canopy reflectance through optimization and validation of vegetation indices. Agric. For. Meteorol. 2017, 236, 162–174. [Google Scholar] [CrossRef]
- Kwon, B.; Kim, H.-S.; Jeon, J.; Yi, M.J. Effects of Temporal and Interspecific Variation of Specific Leaf Area on Leaf Area Index Estimation of Temperate Broadleaved Forests in Korea. Forests 2016, 7, 215. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Li, F.; Jin, G. Non-destructively predicting leaf area, leaf mass and specific leaf area based on a linear mixed-effect model for broadleaf species. Ecol. Indic. 2017, 78, 340–350. [Google Scholar] [CrossRef]
- Mencuccini, M.; Bonosi, L. Leaf/sapwood area ratios in Scots pine show acclimation across Europe. Can. J. For. Res. 2001, 31, 442–456. [Google Scholar] [CrossRef]
- Perrin, P.M.; Mitchell, F.J. Effects of shade on growth, biomass allocation and leaf morphology in European yew (Taxus baccata L.). Eur. J. For. Res. 2013, 132, 211–218. [Google Scholar] [CrossRef]
- Yen, T.-M.; Ji, Y.-J.; Lee, J.-S. Estimating biomass production and carbon storage for a fast-growing makino bamboo (Phyllostachys makinoi) plant based on the diameter distribution model. For. Ecol. Manag. 2010, 260, 339–344. [Google Scholar] [CrossRef]
- Djomo, A.N.; Chimi, C.D. Tree allometric equations for estimation of above, below and total biomass in a tropical moist forest: Case study with application to remote sensing. For. Ecol. Manag. 2017, 391, 184–193. [Google Scholar] [CrossRef]
- Avalos, G.; Sylvester, O. Allometric estimation of total leaf area in the neotropical palm Euterpe oleracea at La Selva, Costa Rica. Trees 2010, 24, 969–974. [Google Scholar] [CrossRef]
- Das, N. Modeling Develops to Estimate Leaf Area and Leaf Biomass of Lagerstroemia speciosa in West Vanugach Reserve Forest of Bangladesh. ISRN For. 2014, 2014, 486478. [Google Scholar]
- Shinozaki, Y.; Shaw, R.; Lichtin, N.N. Reactions of Active Nitrogen with Organic Substrates. II. Molecular Origins of Products of Reaction with Propene. J. Am. Chem. Soc. 1964, 86, 341–344. [Google Scholar] [CrossRef]
- Leblanc, S.G.; Chen, J.M.; Fernandes, R.; Deering, D.W.; Conley, A. Methodology comparison for canopy structure parameters extraction from digital hemispherical photography in boreal forests. Agric. For. Meteorol. 2005, 129, 187–207. [Google Scholar] [CrossRef]
- Bao, Y.; Ni, W.; Wang, D.; Yue, C.; He, H.; Verbeeck, H. Effects of Tree Trunks on Estimation of Clumping Index and LAI from HemiView and Terrestrial LiDAR. Forests 2018, 9, 144. [Google Scholar] [CrossRef]
- Chen, J.M.; Black, T.A. Defining leaf area index for non-flat leaves. Plant Cell Environ. 1992, 15, 421–429. [Google Scholar] [CrossRef]
- Chen, J.M.; Mo, G.; Pisek, J.; Liu, J.; Deng, F.; Ishizawa, M.; Chan, D. Effects of foliage clumping on the estimation of global terrestrial gross primary productivity. Glob. Biogeochem. Cycles 2012, 26, 26. [Google Scholar] [CrossRef]
- Baret, F.; De Solan, B.; López-Lozano, R.; Ma, K.; Weiss, M. GAI estimates of row crops from downward looking digital photos taken perpendicular to rows at 57.5° zenith angle: Theoretical considerations based on 3D architecture models and application to wheat crops. Agric. For. Meteorol. 2010, 150, 1393–1401. [Google Scholar] [CrossRef]
- Culvenor, D.S.; Newnham, G.J.; Mellor, A.; Sims, N.C.; Haywood, A. Automated In-Situ Laser Scanner for Monitoring Forest Leaf Area Index. Sensors 2014, 14, 14994–15008. [Google Scholar] [CrossRef]
- Orlando, F.; Movedi, E.; Paleari, L.; Gilardelli, C.; Foi, M.; Dell’Oro, M.; Confalonieri, R. Estimating leaf area index in tree species using the PocketLAI smart app. Appl. Veg. Sci. 2015, 18, 716–723. [Google Scholar] [CrossRef]
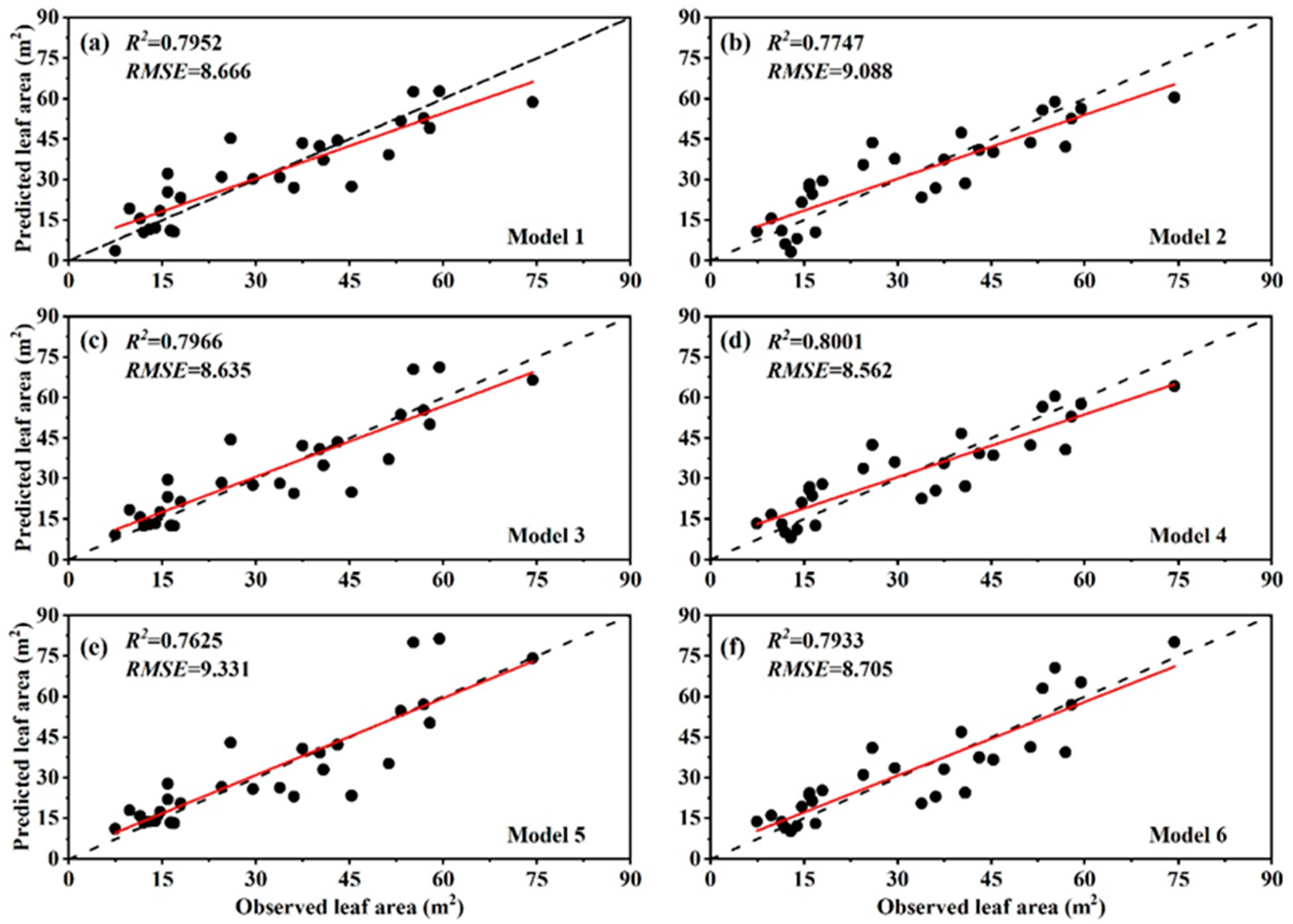
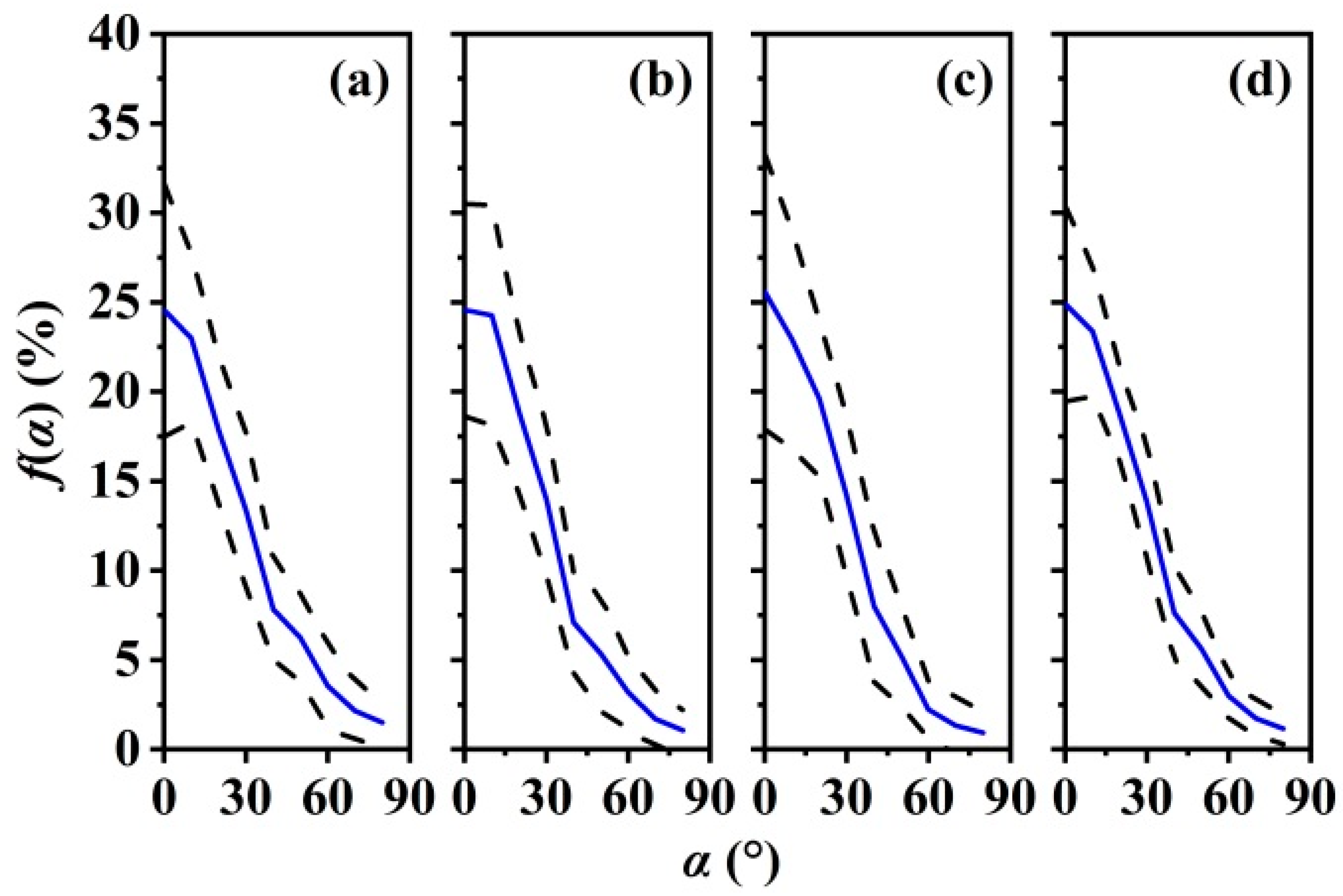
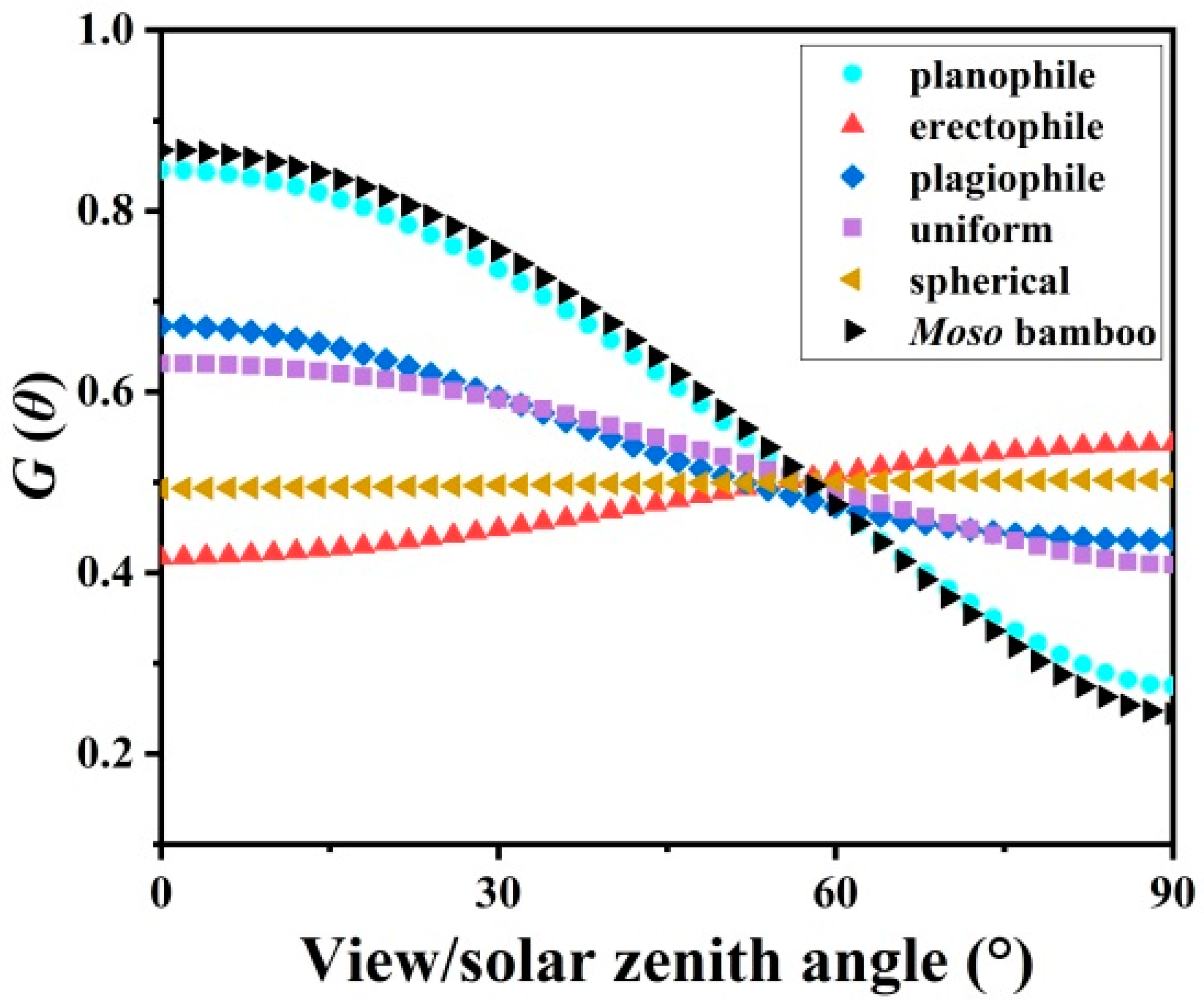
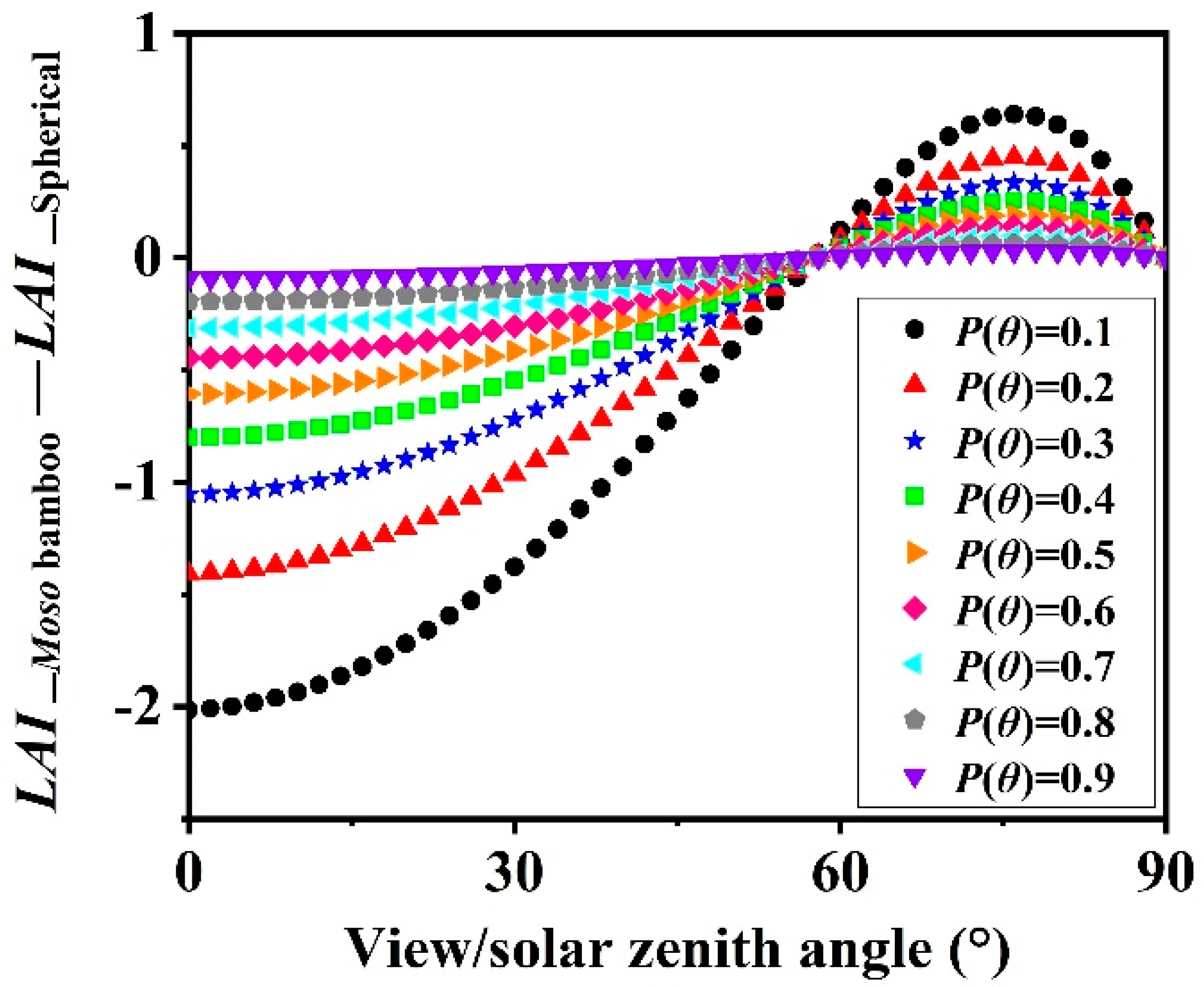
| Sample | H (m) | DBH (cm) | Biomass of Sampled Leaves (g) | Area of Sampled Leaves (cm2) | Leaf Biomass of a Crown (g) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Top | Middle | Bottom | Top | Middle | Bottom | Top | Middle | Bottom | |||
| 1 | 17.00 | 14.1 | 4.78 | 4.94 | 0.90 | 804.0 | 857.3 | 137.2 | 1488.68 | 2174.24 | 760.80 |
| 2 | 7.55 | 5.4 | 2.40 | 5.59 | 4.31 | 483.1 | 1025.8 | 1079.8 | 143.20 | 156.37 | 66.75 |
| 3 | 12.00 | 9.5 | 1.62 | 3.04 | 2.58 | 256.4 | 575.6 | 555.1 | 371.27 | 519.10 | 407.95 |
| 4 | 12.14 | 8.1 | 2.69 | 2.82 | 2.49 | 569.7 | 575.6 | 574.8 | 220.74 | 345.28 | 181.02 |
| 5 | 14.23 | 10.8 | 3.03 | 3.18 | 3.36 | 551.6 | 633.3 | 735.0 | 394.99 | 547.29 | 358.43 |
| 6 | 9.98 | 6.2 | 3.24 | 3.35 | 3.48 | 695.0 | 775.3 | 774.5 | 107.59 | 189.38 | 138.11 |
| 7 | 9.89 | 7.2 | 2.05 | 2.17 | 2.96 | 442.0 | 525.6 | 704.7 | 131.58 | 363.51 | 127.48 |
| 8 | 15.72 | 10.8 | 3.12 | 3.31 | 4.25 | 743.2 | 842.7 | 931.3 | 593.76 | 1176.86 | 582.36 |
| 9 | 8.73 | 5.5 | 2.63 | 2.44 | 1.62 | 600.8 | 606.0 | 451.5 | 149.15 | 368.04 | 154.52 |
| 10 | 10.70 | 8.5 | 2.03 | 1.93 | 1.66 | 538.1 | 570.7 | 494.0 | 242.12 | 278.24 | 110.12 |
| 11 | 8.63 | 4.8 | 1.25 | 1.13 | 0.83 | 405.8 | 407.4 | 313.1 | 132.00 | 158.25 | 52.66 |
| 12 | 11.02 | 8.3 | 1.88 | 1.94 | 1.69 | 524.3 | 550.1 | 502.9 | 220.56 | 245.63 | 91.83 |
| 13 | 14.06 | 9.9 | 2.63 | 1.86 | 1.49 | 683.5 | 543.5 | 524.8 | 534.57 | 686.80 | 98.17 |
| 14 | 8.80 | 7.7 | 1.45 | 1.40 | 1.08 | 418.8 | 436.2 | 401.2 | 180.26 | 260.23 | 81.02 |
| 15 | 12.02 | 7.65 | 3.62 | 2.68 | 2.43 | 817.5 | 813.0 | 717.6 | 502.99 | 461.39 | 287.60 |
| 16 | 13.10 | 8.5 | 3.89 | 3.44 | 3.36 | 760.3 | 731.1 | 788.7 | 594.87 | 962.32 | 373.98 |
| 17 | 13.47 | 11 | 3.48 | 3.33 | 2.68 | 842.3 | 949.1 | 766.9 | 496.16 | 999.01 | 376.50 |
| 18 | 8.91 | 5.1 | 3.99 | 3.90 | 3.83 | 863.1 | 856.2 | 807.3 | 197.61 | 302.08 | 142.41 |
| 19 | 9.40 | 5.5 | 2.62 | 2.24 | 2.42 | 643.5 | 593.1 | 654.3 | 178.86 | 181.14 | 82.77 |
| 20 | 11.42 | 8.2 | 3.63 | 3.10 | 2.99 | 944.6 | 912.2 | 896.4 | 479.59 | 598.05 | 200.05 |
| 21 | 11.90 | 9.9 | 4.58 | 5.48 | 4.42 | 844.9 | 956.6 | 1025.5 | 450.63 | 909.38 | 228.99 |
| 22 | 8.80 | 4.4 | 2.67 | 2.38 | 2.75 | 761.7 | 809.6 | 731.4 | 145.27 | 202.86 | 69.31 |
| 23 | 14.26 | 10.5 | 2.33 | 1.73 | 1.51 | 545.3 | 510.0 | 528.2 | 632.20 | 748.51 | 179.50 |
| 24 | 11.55 | 10.4 | 5.02 | 4.28 | 3.63 | 919.6 | 1002.4 | 1086.5 | 1016.58 | 863.68 | 216.92 |
| 25 | 13.91 | 11.5 | 2.79 | 2.15 | 1.37 | 596.4 | 561.1 | 399.9 | 677.71 | 684.62 | 269.64 |
| 26 | 17.23 | 13.1 | 2.04 | 1.80 | 1.34 | 614.1 | 612.6 | 552.5 | 601.80 | 923.84 | 240.40 |
| 27 | 15.15 | 12.5 | 1.94 | 1.74 | 1.15 | 645.7 | 638.4 | 538.4 | 848.05 | 646.07 | 126.65 |
| 28 | 15.50 | 12.9 | 1.86 | 1.59 | 1.33 | 545.9 | 607.6 | 566.2 | 832.48 | 659.52 | 86.68 |
| 29 | 17.12 | 13.4 | 2.61 | 2.82 | 1.87 | 639.5 | 710.6 | 619.5 | 709.64 | 929.70 | 434.50 |
| Model | a or a′ (SE) | b or b′ (SE) | R2 | RMSE | CF | |
|---|---|---|---|---|---|---|
| 1 | LA = a·H + b | 6.0030 (0.5429) *** | −41.25 (6.8015) *** | 0.8191 | 8.1440 | - |
| 2 | LA = a·DBH + b | 5.9902 (0.5748) *** | −21.92 (5.4180) ** | 0.8009 | 8.5445 | - |
| 3 | ln LA = a′·ln H + b′ | 2.5056 (0.2251) *** | −2.925 (0.5597) *** | 0.8211 | 0.2808 | 1.040 |
| 4 | ln LA = a′·ln DBH + b′ | 1.7165 (0.1760) *** | −0.407 (0.3823) | 0.7789 | 0.3122 | 1.050 |
| 5 | ln LA = a′·H + b′ | 0.2067 (0.0194) *** | 0.754 (0.2433) * | 0.8075 | 0.2913 | 1.043 |
| 6 | ln LA = a′·DBH + b′ | 0.2072 (0.0201) *** | 1.411 (0.1897) *** | 0.7970 | 0.2992 | 1.046 |
| Broadleaf | SLA (cm2·g−1) | References |
|---|---|---|
| Moso bamboo (Phyllostachys edulis (Carrière) J. Houz.) | 152–466 | This study |
| European beech (Fagus sylvatica L.) | 120–480 | [60,80,81,82] |
| Goat willow (Salix caprea L.) | 113–203 | [83] |
| Sargent’s cherry (Prunus sargentii Rehder) | 182.0 ± 4.1 * | [84] |
| Korean birch (Betula costata Trautv.) | 214.8 ± 3.3 * | [85] |
| Needleleaf | SLA (cm2·g−1) | References |
|---|---|---|
| Scots pine (Pinus sylvestris L.) | 29–55 | [48,86] |
| Norway spruce (Picea abies (L.) H. Karst.) | 30–70 | [60,66] |
| European yew (Taxus baccata L.) | 100–200 | [87] |
| Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) | 34.3 ± 1.0 * | [59] |
| European larch (Larix decidua Mill.) | 117 ± 27.9 * | [70] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Fan, W.; Du, H.; Ge, H.; Huang, F.; Xu, X. Estimating Crown Structure Parameters of Moso Bamboo: Leaf Area and Leaf Angle Distribution. Forests 2019, 10, 686. https://doi.org/10.3390/f10080686
Wu X, Fan W, Du H, Ge H, Huang F, Xu X. Estimating Crown Structure Parameters of Moso Bamboo: Leaf Area and Leaf Angle Distribution. Forests. 2019; 10(8):686. https://doi.org/10.3390/f10080686
Chicago/Turabian StyleWu, Xuhan, Weiliang Fan, Huaqiang Du, Hongli Ge, Feilong Huang, and Xiaojun Xu. 2019. "Estimating Crown Structure Parameters of Moso Bamboo: Leaf Area and Leaf Angle Distribution" Forests 10, no. 8: 686. https://doi.org/10.3390/f10080686
APA StyleWu, X., Fan, W., Du, H., Ge, H., Huang, F., & Xu, X. (2019). Estimating Crown Structure Parameters of Moso Bamboo: Leaf Area and Leaf Angle Distribution. Forests, 10(8), 686. https://doi.org/10.3390/f10080686





