Influence of Cambial Age and Axial Height on the Spatial Patterns of Xylem Traits in Catalpa bungei, a Ring-Porous Tree Species Native to China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Radial Strip Sampling
2.2. Wood Sampling and Light Microscopy Observation
2.3. Scanning Electron Microscopy Observation
2.4. Statistical Analyses
3. Results
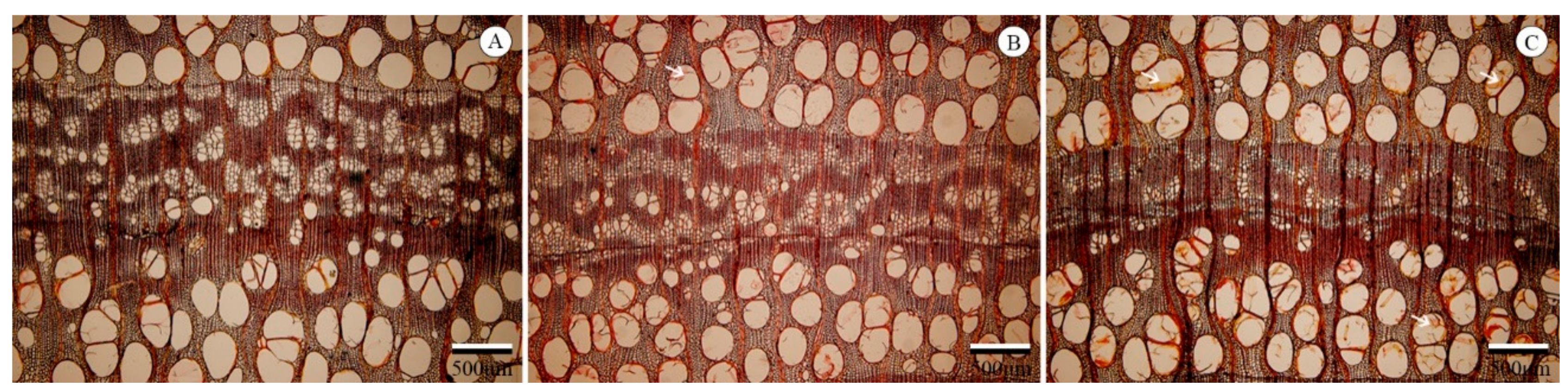
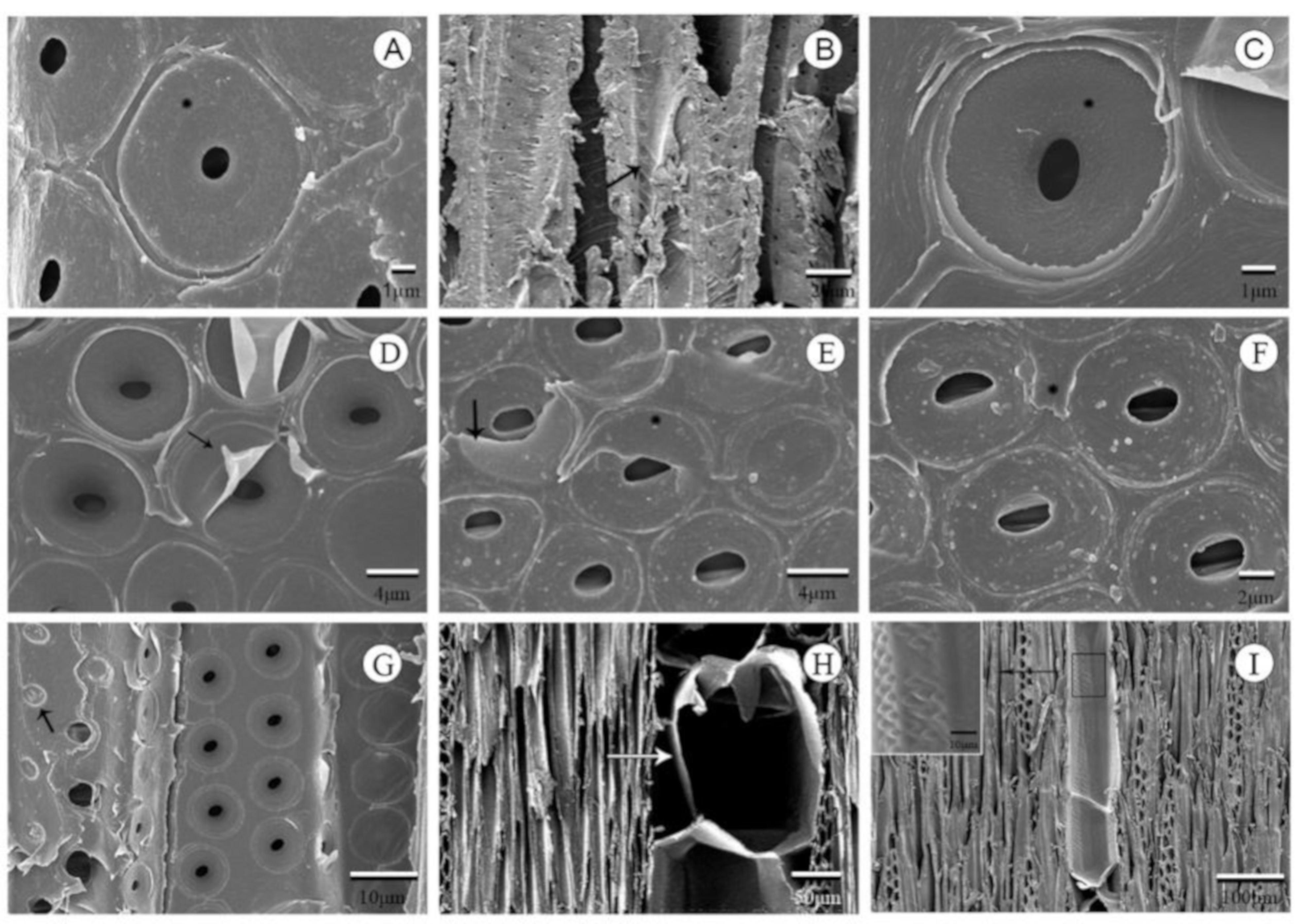
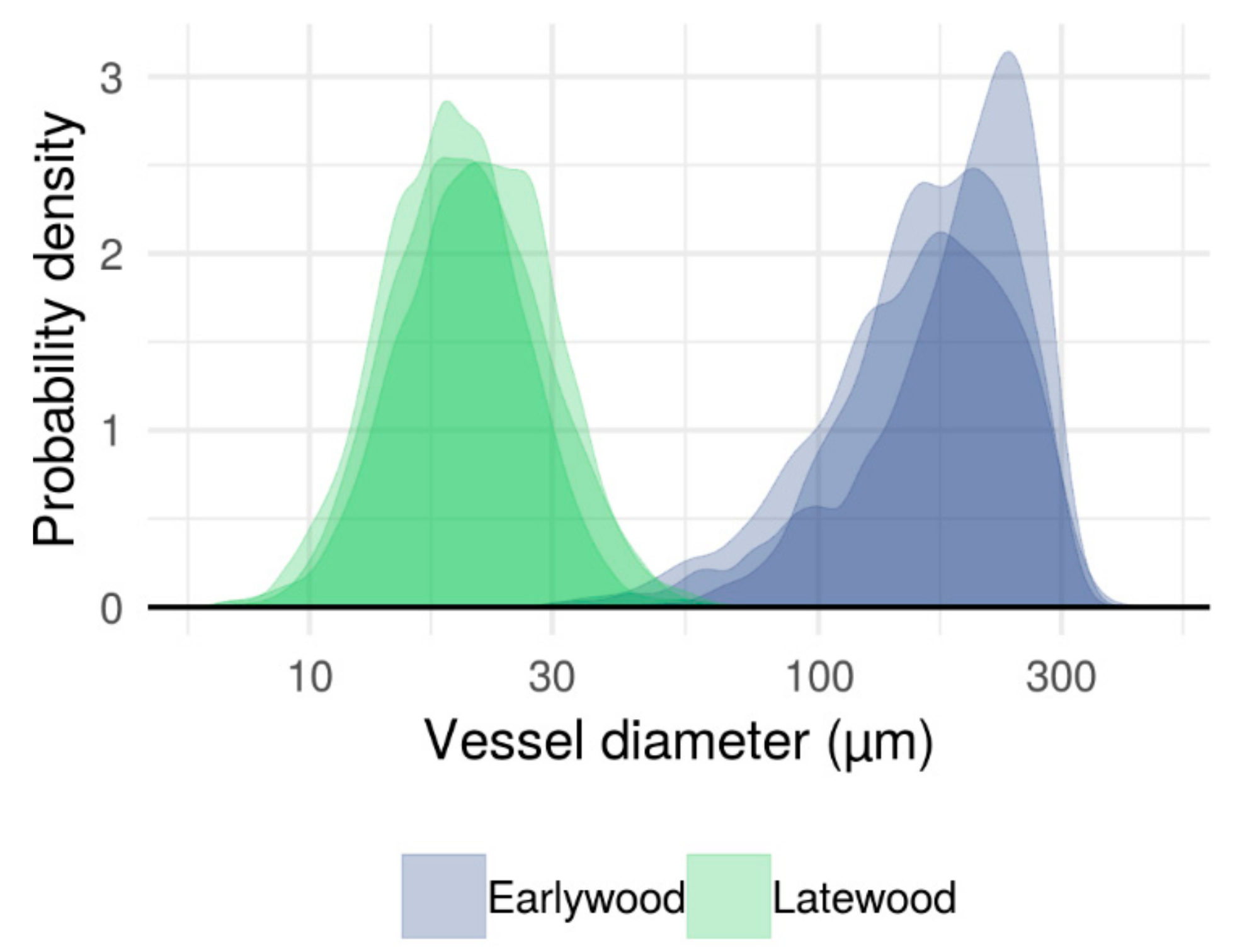
3.1. Typical Xylem Features of Ring-Porosity
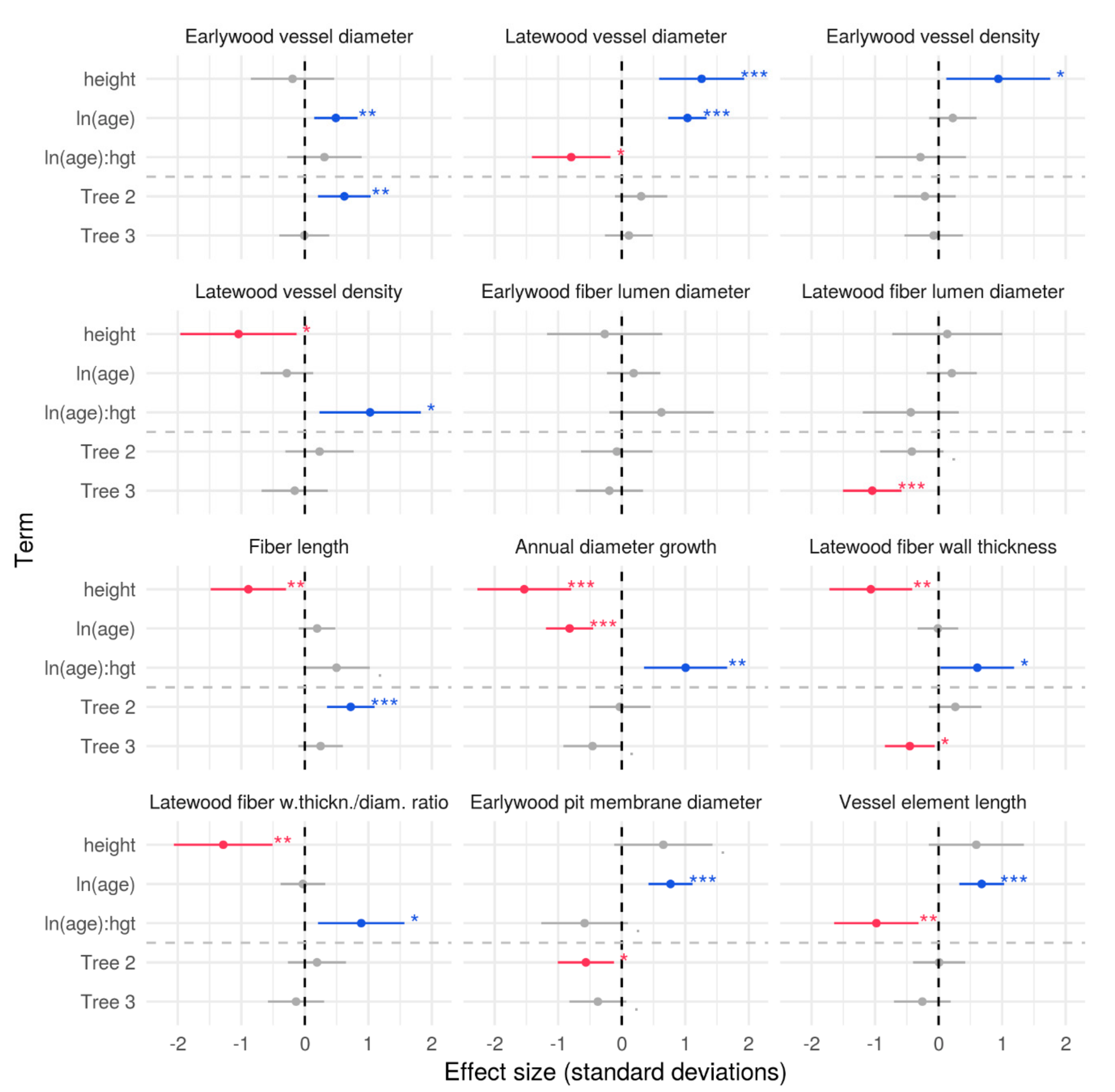
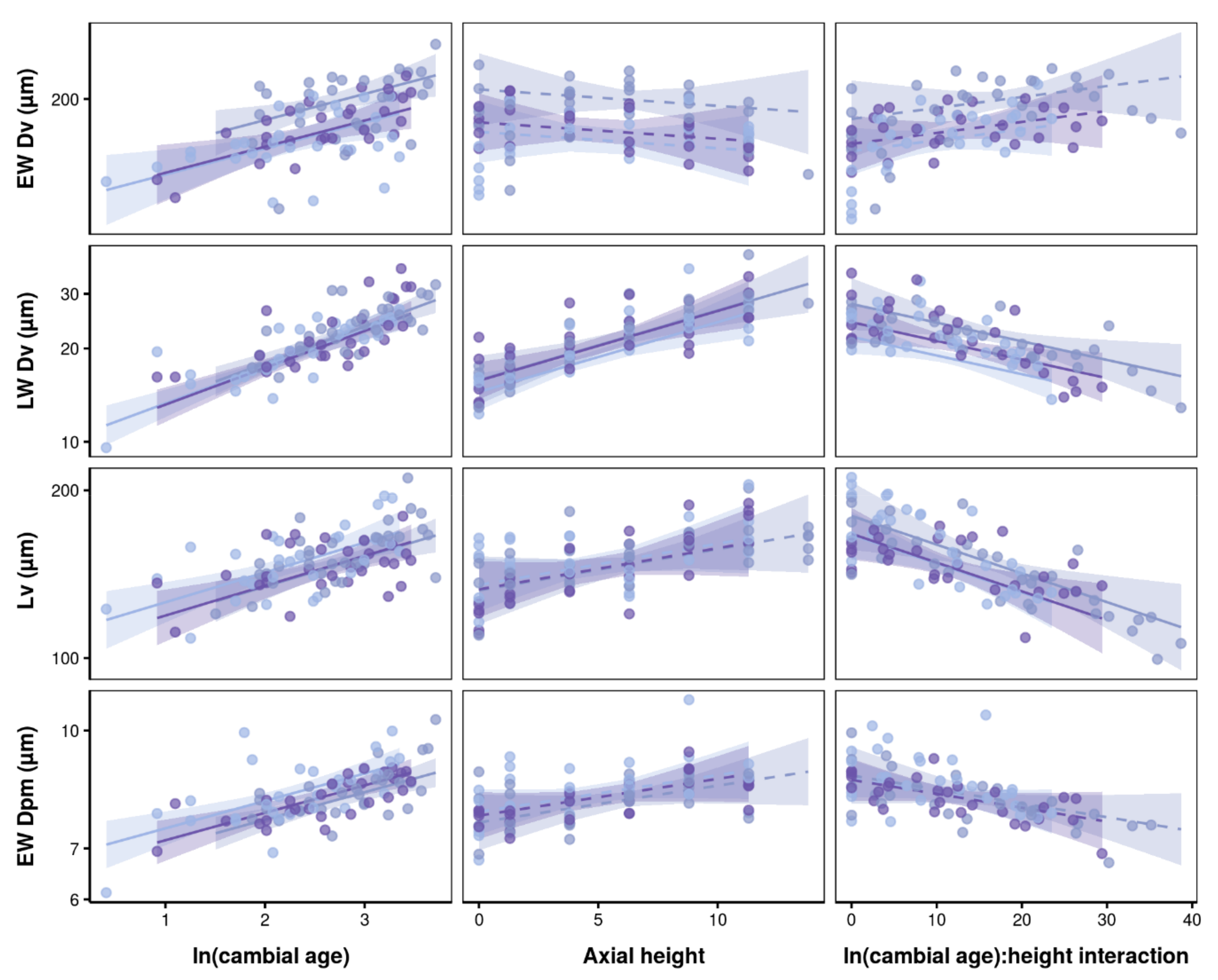
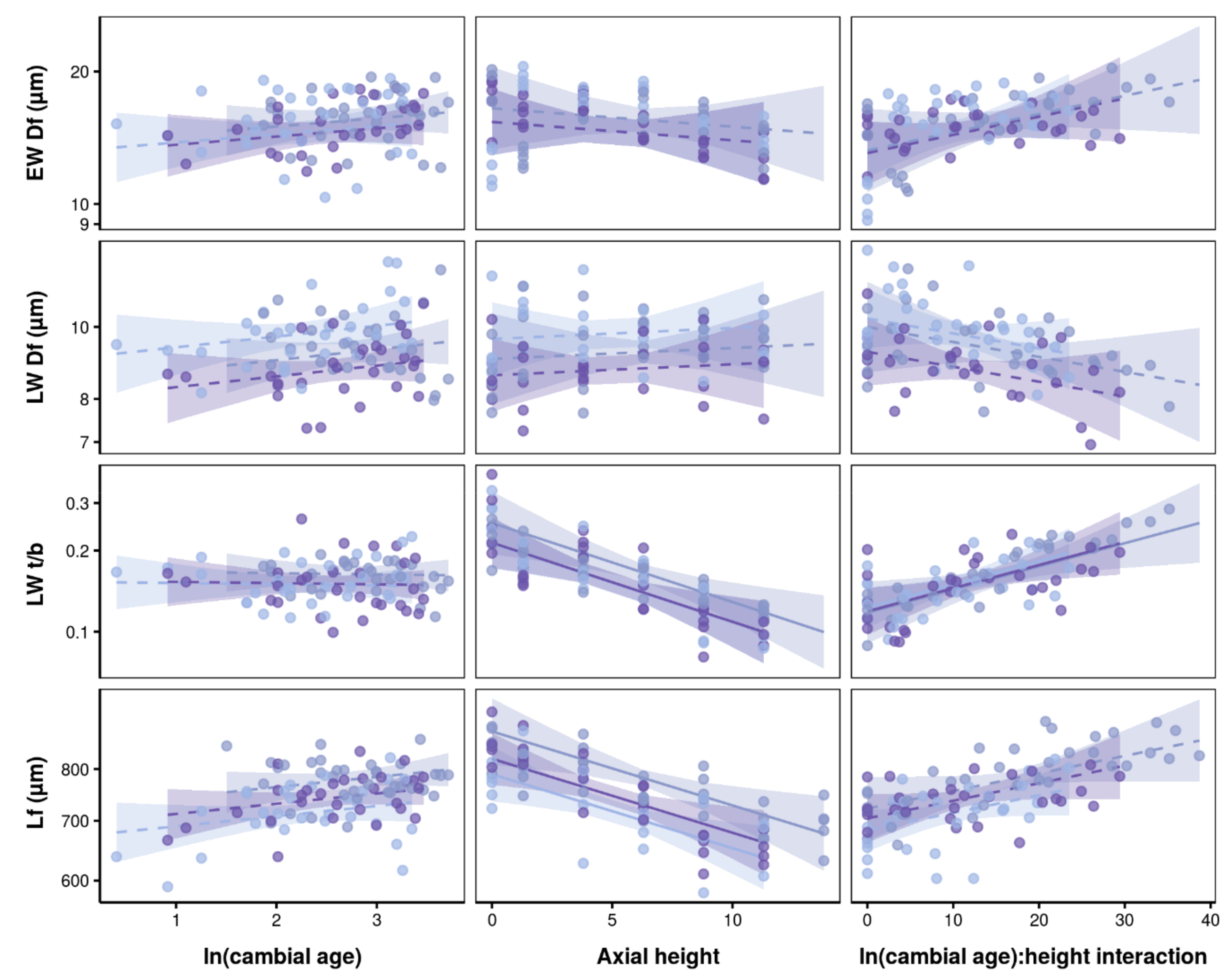
3.2. Effects of Axial Height and Cambial Age on Xylem Traits
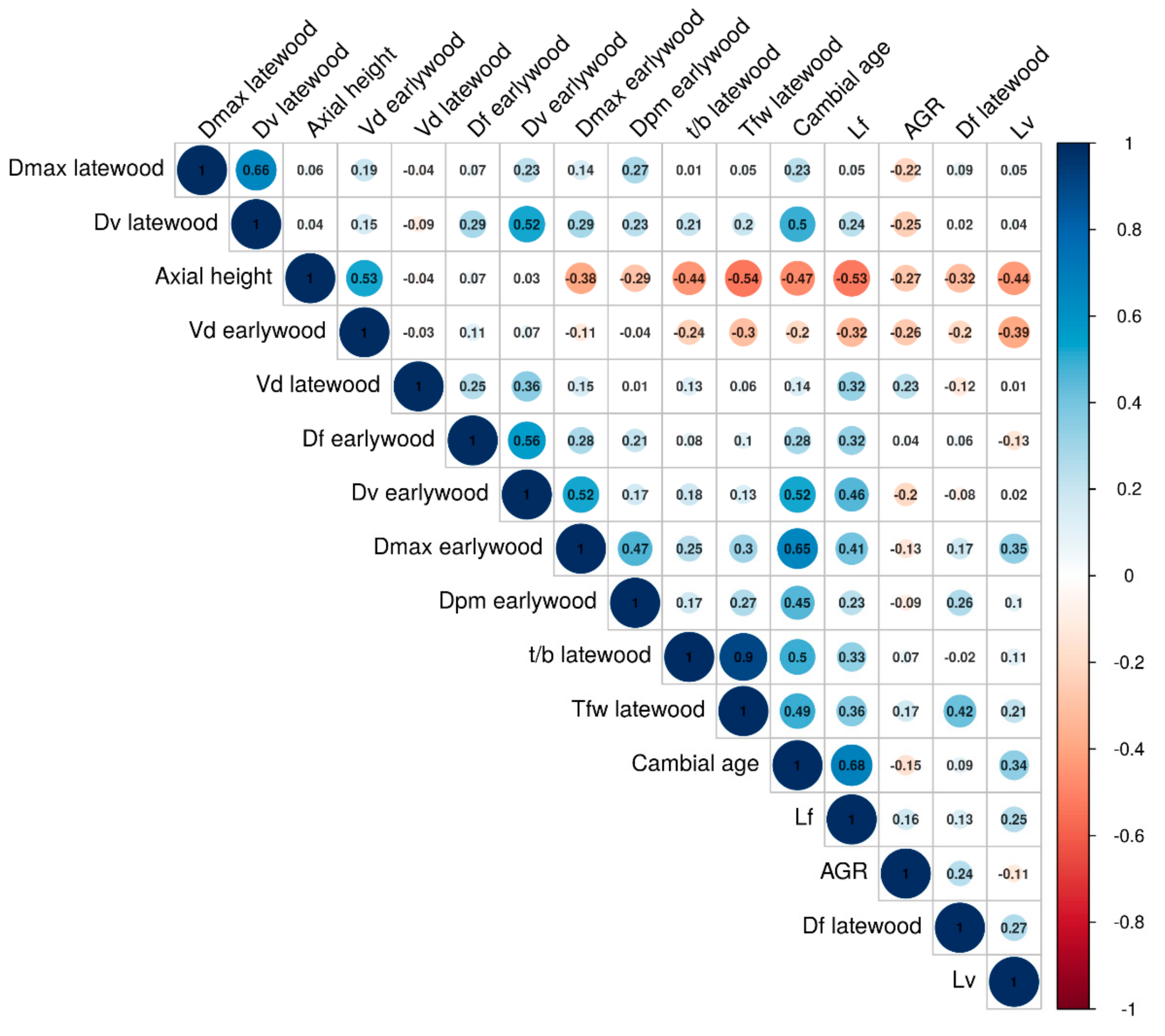
3.3. Interrelationships between Wood Anatomical Traits
4. Discussion
4.1. Wood Anatomical Features of C. bungei
4.2. Vertical and Radial Patterns of Wood Anatomical Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zheng, J.; Zhao, X.; Morris, H.; Jansen, S. Phylogeny best explains latitudinal patterns of xylem tissue fractions for woody angiosperm species across China. Front. Plant Sci. 2019, 10, 556. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Zimmerman, M.H. Xylem Structure and the Ascent of Sap; Springer: Berlin, Germany, 2002; pp. 17–21. [Google Scholar]
- Cai, J.; Hacke, U.; Zhang, S.; Tyree, M.T. What happens when stems are embolized in a centrifuge? Testing the cavitron theory. Physiol. Plant. 2010, 140, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Anfodillo, T.; Carraro, V.; Carrer, M.; Fior, C.; Rossi, S. Convergent tapering of xylem conduits in different woody species. New Phytol. 2006, 169, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Petit, G.; Anfodillo, T.; Mencuccini, M. Tapering of xylem conduits and hydraulic limitations in sycamore (Acer pseudoplatanus) trees. New Phytol. 2007, 177, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Tomlinson, P. Analysis of complex vascular systems in plants: Optical shuttle method. Science 1966, 152, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Kitin, P.; Fujii, T.; Abe, H.; Funada, R. Anatomy of the vessel network within and between tree rings of Fraxinus lanuginosa(Oleaceae). Am. J. Bot. 2004, 91, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, C.R.; Lee, E.F.; Choat, B.; Jansen, S.; Phillips, R.J.; Shackel, K.A.; Mcelrone, A.J.; Matthews, M.A. Automated analysis of three-dimensional xylem networks using high-resolution computed tomography. New Phytol. 2011, 191, 1168–1179. [Google Scholar] [CrossRef]
- Cai, J.; Tyree, M.T. Measuring vessel length in vascular plants: Can we divine the truth? History, theory, methods, and contrasting models. Trees 2014, 28, 643–655. [Google Scholar] [CrossRef]
- Cai, J.; Tyree, M.T. The impact of vessel size on vulnerability curves: Data and models for within-species variability in saplings of aspen, Populus tremuloides Michx. Plant Cell Environ. 2010, 33, 1059–1069. [Google Scholar] [CrossRef]
- Morris, H.; Gillingham, M.A.F.; Plavcová, L.; Gleason, S.M.; Olson, M.E.; Coomes, D.A.; Fichtler, E.; Klepsch, M.M.; Martínez-Cabrera, H.I.; McGlinn, D.J.; et al. Vessel diameter is related to amount and spatial arrangement of axial parenchyma in woody angiosperms. Plant Cell Environ. 2017, 41, 245–260. [Google Scholar] [CrossRef]
- Leal, S.; Sousa, V.B.; Pereira, H. Radial variation of vessel size and distribution in cork oak wood (Quercus suber L.). Wood Sci. Technol. 2007, 41, 339–350. [Google Scholar] [CrossRef]
- Rita, A.; Cherubini, P.; Leonardi, S.; Todaro, L.; Borghetti, M. Functional adjustments of xylem anatomy to climatic variability: Insights from long-term Ilex aquifolium tree-ring series. Tree Physiol. 2015, 35, 817. [Google Scholar] [CrossRef] [PubMed]
- Cochard, H.; Tyree, T. Xylem dysfunction in Quercus: Vessel sizes, tyloses, cavitation and seasonal changes in embolism. Tree Physiol. 1990, 6, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.E.; Rosell, J.A. Vessel diameter-stem diameter scaling across woody angiosperms and the ecological causes of xylem vessel diameter variation. New Phytol. 2013, 197, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.E.; Anfodillo, T.; Rosell, J.A.; Petit, G.; Crivellaro, A.; Isnard, S.; León-Gómez, C.; Alvarado-Cárdenas, L.O.; Castorena, M. Universal hydraulics of the flowering plants: Vessel diameter scales with stem length across angiosperm lineages, habits and climates. Ecol. Lett. 2014, 17, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Rosell, J.A.; Olson, M.E.; Anfodillo, T. Scaling of xylem vessel diameter with plant size: Causes, predictions, and outstanding questions. Curr. For. Rep. 2017, 3, 46–59. [Google Scholar] [CrossRef]
- Rodriguez-Zaccaro, F.D.; Valdovinos-Ayala, J.; Percolla, M.I.; Venturas, M.D.; Pratt, R.B.; Jacobsen, A.L. Wood structure and function change with maturity: Age of the vascular cambium is associated with xylem changes in current year growth. Plant Cell Environ. 2019, 42, 1816–1831. [Google Scholar] [CrossRef] [PubMed]
- Dória, L.C.; Podadera, D.S.; Lima, R.S.; Lens, F. Axial sampling height outperforms site as predictor of wood trait variation. IAWA J. 2019, 40, 191–214. [Google Scholar] [CrossRef]
- Gartner, B.L.; Lei, H.; Milota, M.R. Variation in the anatomy and specific gravity of wood within and between trees of red alder (Alnus rubra Bong.). Wood Fibre Sci. 1997, 29, 10–20. [Google Scholar]
- Fan, Z.; Cao, K.; Shou-Qing, Z. Axial and radial changes in xylem anatomical characteristics in six evergreen broadleaved tree species in ailao mountain, yunnan. Acta Phytoecol. Sin. 2005, 29, 968–975. [Google Scholar]
- Fan, Z.; Cao, K.; Becker, P. Axial and radial variations in xylem anatomy of angiosperm and conifer trees in Yunnan, China. IAWA J. 2009, 30, 1–13. [Google Scholar] [CrossRef]
- Cardoso, S.; Sousa, V.B.; Quilho, T.; Pereira, H. Anatomical variation of teakwood from unmanaged mature plantations in East Timor. J. Wood Sci. 2015, 61, 326–333. [Google Scholar] [CrossRef]
- Spicer, R.; Gartner, B.L. The effects of cambial age and position within the stem on specific conductivity in Douglas-fir (Pseudotsuga menziesii) sapwood. Trees 2001, 15, 222–229. [Google Scholar] [CrossRef]
- Zobel, B.J.; van Buijtenen, J.P. Wood Variation: Its Causes and Control; Springer: Berlin, Germany, 1989; pp. 100–119. [Google Scholar]
- James, S.A.; Meinzer, F.C.; Goldstein, G.; Woodruff, D.; Jones, T.; Restom, T.; Mejia, M. Axial and radial water transport and internal water storage in tropical forest canopy trees. Oecologia 2003, 134, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yin, Y.; Urakami, H. Variation within tree of wood anatomical properties and basic density of I-214 poplar in Beijing area and their relationship modelling equations. Sci. Silvae Sin. 2003, 39, 115–121. [Google Scholar]
- Zhao, X. Effects of cambial age and flow path-length on vessel characteristics in birch. J. Res. 2015, 20, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Domec, J.C.; Gartner, B.L. Age-and position-related changes in hydraulic versus mechanical dysfunction of xylem: Inferring the design criteria for Douglas-fir wood structure. Tree Physiol. 2002, 22, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.D. Natural Variation in Specific Gravity, Fibre Length, and Interlocked Grain of Sweetgum (Liquidambar styraciflua) in the South Atlantic States. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 1964. [Google Scholar]
- Saucier, J.R.; Thras, M.A. Specific gravity and fiber length variation within annual height increments crements of red maple. For. Prod. J. 1966, 16, 33–36. [Google Scholar]
- Taylor, F.W. Fibre length variation within growth rings of certain angiosperms. Wood Fibre Sci. 1975, 8, 116–119. [Google Scholar]
- Wheeler, J.K.; Sperry, J.S.; Hacke, U.G.; Hong, N. Inter-vessel pitting and cavitation in woody Rosaceae and other vesselled plants: A basis for a safety versus efficiency trade-off in xylem transport. Plant Cell Environ. 2005, 28, 800–812. [Google Scholar] [CrossRef]
- Choat, B.; Cobb, A.R.; Jansen, S. Structure and function of bordered pits: New discoveries and impacts on whole-plant hydraulic function. New Phytol. 2008, 177, 608–626. [Google Scholar] [CrossRef] [PubMed]
- Plomion, C.; Stokes, A. Update on wood formation in trees. Plant Physiol. 2015, 127, 1513–1523. [Google Scholar] [CrossRef]
- Ziegler, H. Biological aspects of the Heartwood formation. Holz Roh Werkst. 1968, 26, 61–68. [Google Scholar] [CrossRef]
- IAWA Committee. IAWA list of microscopic features for hardwood identification. IAWA Bull. 1989, 10, 219–332. [Google Scholar]
- Bonsen, K.J.M.; Kucera, L.J. Vessel occlusions in plants: Morphological, functional and evolutionary aspects. IAWA Bull. 1990, 11, 393–399. [Google Scholar] [CrossRef]
- Saitoh, T.; Ohtani, J.; Fukazawa, K. The occurrence and morphology of tyloses and gums in the vessels of Japanese hardwoods. IAWA J. 1993, 14, 359–371. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, L.; Zhou, Y. A study of tyloses and gums in the vessels of Chinese hardwood. Sci. Silvae Sin. 1995, 31, 155–159. [Google Scholar]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Funtions, and Development, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 297–298. [Google Scholar]
- De Micco, V.; Balzano, A.; Wheeler, E.A.; Baas, P. Tyloses and gums: A review of structure, function and occurrence of vessel occlusions. IAWA J. 2016, 37, 196–205. [Google Scholar] [CrossRef]
- Kitin, P.; Funada, R. Earlywood vessels in ring-porous trees become functional for water transport after bud burst and before the maturation of the current-year leaves. IAWA J. 2016, 37, 315–331. [Google Scholar] [CrossRef]
- Jacobsen, A.L.; Valdovinos-ayala, J.; Pratt, R.B. Functional lifespans of xylem vessels: Development, hydraulic function, and post--function of vessels in several species of woody plants. Am. J. Bot. 2018, 105, 142–150. [Google Scholar] [CrossRef] [PubMed]
- National Forestry and Grassland Administration. Available online: http://www.forestry.gov.cn/main/4818/content-1045202.html (accessed on 5 July 2019).
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Schreiber, S.G.; Hacke, U.G.; Hamann, A. Variation of xylem vessel diameters across a climate gradient: Insight from a reciprocal transplant experiment with a widespread boreal tree. Funct. Ecol. 2015, 29, 1392–1401. [Google Scholar] [CrossRef]
- Hacke, U.G.; Spicer, R.; Schreiber, S.G.; Plavcová, L. An ecophysiological and developmental perspective on variation in vessel diameter. Plant Cell Environ. 2016, 40, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de-lis, G.; Rozas, V.; Vázquez-ruiz, R.A.; García-gonzález, I. Do ring-porous oaks prioritize earlywood vessel efficiency over safety? Environmental effects on vessel lumen diameter and tyloses formation. Agric. For. Meteorol. 2018, 248, 205–214. [Google Scholar] [CrossRef]
- Mishra, G.; Collings, D.A.; Altaner, C.M. Physiological changes during heartwood formation in young Eucalyptus bosistoana trees. IAWA J. 2018, 39, 1–13. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, J.; Rossler, R.; Kerp, H.; Wei, H.B. Complete tylosis formation in a latest Permianconifer stem. Ann. Bot. 2013, 6, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Chattaway, M.M. The development of tyloses and secretion of gum in heartwood formation. Aust. J. Biol. Sci. 1949, 2, 227–240. [Google Scholar] [CrossRef]
- Wheeler, E.A. Inside Wood—A web resource for hardwood anatomy. IAWA J. 2011, 32, 199–211. [Google Scholar] [CrossRef]
- Parke, J.L.; Oh, E.; Voelker, S.; Hansen, E.M.; Buckles, G.; Lachenbruch, B. Phytophthora ramorum colonizes tanoak xylem and is associated with reduced stem water transport. Phytopathology 2007, 97, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.R.; Parke, J.L.; Lachenbruch, B.; Hansen, E.M. The effects of Phytophthora ramorum infection on the hydraulic conductivity and tylosis formation in tanoak sapwood. Can. J. For. Res. 2009, 39, 1766–1776. [Google Scholar] [CrossRef]
- Khan, M.A.; Bera, M.; Spicer, R.A.; Spicer, T.E.V.; Bera, S. Evidence of simultaneous occurrence of tylosis formation and fungal interaction in a late cenozoic angiosperm from the eastern himalaya. Rev. Palaeobot. Palynol. 2018, 259, 171–184. [Google Scholar] [CrossRef]
- McElrone, A.J.; Grant, J.A.; Kluepfel, D.A. The role of tyloses in crown hydraulic failure of mature walnut trees afflicted by apoplexy disorder. Tree Physiol. 2010, 30, 761–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohonen, M.M. Engineered wettability in tree capillaries. Langmuir 2006, 22, 3148–3153. [Google Scholar] [CrossRef] [PubMed]
- Kohonen, M.M.; Helland, A. On the function of wall sculpturing in xylem Conduits. J. Bionics Eng. 2009, 6, 324–329. [Google Scholar] [CrossRef]
- Anfodillo, T.; Petit, G.; Crivellaro, A. Axial conduit widening in woody species: A still neglected anatomical pattern. IAWA J. 2013, 34, 352–364. [Google Scholar] [CrossRef]
- Aloni, R.; Zimmermann, M.H. The control of vessel size and density along the plant axis: A new hypothesis. Differentiation 1983, 24, 203–208. [Google Scholar] [CrossRef]
- Aloni, R. Ecophysiological implications of vascular differentiation and plant evolution. Trees 2014, 29, 1–15. [Google Scholar] [CrossRef]
- Kirfel, K.; Leuschner, C.; Hertel, D.; Schuldt, B. Influence of root diameter and soil depth on the xylem anatomy of fine- to medium-sized roots of mature beech trees in the top- and subsoil. Front. Plant Sci. 2017, 8, 1194. [Google Scholar] [CrossRef] [PubMed]
- HelinÄska-Raczkowska, L.; Fabisiak, E. Radial variation of earlywood vessel lumen diameter as an indicator of the juvenile growth period in ash (Fraxinus excelsior L.). Holz Roh Werkst. 1999, 57, 283–286. [Google Scholar] [CrossRef]
- Lazzarin, M.; Crivellaro, A.; Williams, C.B.; Dawson, T. Tracheid and pit anatomy vary in tandem in a tall Sequoiadendron giganteum tree. IAWA J. 2016, 37, 172–185. [Google Scholar] [CrossRef]
- Losso, A.; Anfodillo, T.; Ganthaler, A.; Ko, W.; Markl, Y.; Nardini, A.; Oberhuber, W.; Purin, G.; Mayr, S. Robustness of xylem properties in conifers: Analyses of tracheid and pit dimensions along elevational transects. Tree Physiol. 2018, 38, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Sseremba, O.E.; Mugabi, P.; Banana, A.Y. Within-tree and tree-age variation of selected anatomical properties of the wood of Ugandan-Grown Eucalyptus grandis. For. Prod. J. 2016, 66, 433–442. [Google Scholar]
- Lockhart, J.A. An analysis of irreversible plant cell elongation. J. Theor. Biol. 1965, 8, 264–275. [Google Scholar] [CrossRef]
- Hsiao, T.C.; Acevedo, E.; Fereres, E.; Henderson, D.W. Water stress, growth, and osmotic adjustment. Philos. Trans. R. Soc. Lond. B 1976, 273, 479–500. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Q.; Tyree, M.T. Factors controlling plasticity of leaf morphology in Robinia pseudoacacia L.I: Height-associated variation in leaf structure. Ann. For. Sci. 2012, 69, 29–37. [Google Scholar] [CrossRef]
- Woodruff, D.R.; Bond, B.J.; Meinzer, F.C. Does turgor limit growth in tall trees? Plant Cell Environ. 2004, 27, 229–236. [Google Scholar] [CrossRef]
- Vidaurre, G.B.; Vital, B.R.; de Cássia Oliveira, A.; da Silva Oliveira, J.T.; Moulin, J.C.; da Silva, J.G.M.; Soranso, D.R. Physical and mechanical properties of juvenile Schizolobium amazonicum wood. Rev. Árvore 2018, 42, e420101. [Google Scholar] [CrossRef]
- Rungwattana, K.; Vienna, L.S.; Phumichai, T.; Rattanawong, R.; Hietz, P.; Vienna, L.S. Trait evolution in tropical rubber (Hevea brasiliensis) trees is related to dry season intensity. Funct. Ecol. 2018, 32, 2638–2651. [Google Scholar] [CrossRef]
- Schuldt, B.; Leuschner, C.; Brock, N.; Horna, V. Changes in wood density, wood anatomy and hydraulic properties of the xylem along the root-to-shoot flow path in tropical rainforest trees. Tree Physiol. 2013, 33, 161–174. [Google Scholar] [CrossRef] [Green Version]
- Pfautsch, S.; Harbusch, M.; Wesolowski, A.; Smith, R.; Macfarlane, C.; Tjoelker, M.G.; Reich, P.B.; Adams, M.A. Climate determines vascular traits in the ecologically diverse genus Eucalyptus. Ecol. Lett. 2016, 19, 240–248. [Google Scholar] [CrossRef]
| Variable | Unit | Wood Type | Magnification | n | Mean | SD |
|---|---|---|---|---|---|---|
| DV | µm | earlywood | 40× | 100 vessels | 180.7 | 28.2 |
| latewood | 100× | 100 vessels | 21.6 | 3.9 | ||
| DMAX | µm | earlywood | 40× | 100 vessels | 297.4 | 41.6 |
| latewood | 100× | 100 vessels | 44.7 | 11.2 | ||
| LV | µm | both | 40× | 50 vessels | 150.8 | 19.3 |
| VD | n mm−2 | earlywood | 40× | 5 measurements | 11.0 | 2.6 |
| latewood | 40× | 5 measurements | 378.2 | 126.9 | ||
| TFW | µm | latewood | 400× | 50 fibres | 1.5 | 0.4 |
| Df | µm | earlywood | 200× | 100 fibres | 15.5 | 2.3 |
| latewood | 400× | 100 fibres | 9.4 | 1.1 | ||
| Lf | µm | both | 40× | 50 fibres | 752.0 | 67.1 |
| t/b | - | latewood | - | 0.2 | 0.0 | |
| Dpm | µm | earlywood | 400× | 50 pits | 8.2 | 0.6 |
| AGR | mm yr−1 | both | 10× | 1–7 year rings | 6.8 | 3.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, X.; Link, R.; Li, R.; Deng, L.; Schuldt, B.; Jiang, X.; Zhao, R.; Zheng, J.; Li, S.; et al. Influence of Cambial Age and Axial Height on the Spatial Patterns of Xylem Traits in Catalpa bungei, a Ring-Porous Tree Species Native to China. Forests 2019, 10, 662. https://doi.org/10.3390/f10080662
Li S, Li X, Link R, Li R, Deng L, Schuldt B, Jiang X, Zhao R, Zheng J, Li S, et al. Influence of Cambial Age and Axial Height on the Spatial Patterns of Xylem Traits in Catalpa bungei, a Ring-Porous Tree Species Native to China. Forests. 2019; 10(8):662. https://doi.org/10.3390/f10080662
Chicago/Turabian StyleLi, Shan, Xin Li, Roman Link, Ren Li, Liping Deng, Bernhard Schuldt, Xiaomei Jiang, Rongjun Zhao, Jingming Zheng, Shuang Li, and et al. 2019. "Influence of Cambial Age and Axial Height on the Spatial Patterns of Xylem Traits in Catalpa bungei, a Ring-Porous Tree Species Native to China" Forests 10, no. 8: 662. https://doi.org/10.3390/f10080662
APA StyleLi, S., Li, X., Link, R., Li, R., Deng, L., Schuldt, B., Jiang, X., Zhao, R., Zheng, J., Li, S., & Yin, Y. (2019). Influence of Cambial Age and Axial Height on the Spatial Patterns of Xylem Traits in Catalpa bungei, a Ring-Porous Tree Species Native to China. Forests, 10(8), 662. https://doi.org/10.3390/f10080662





