At a Microsite Scale, Native Vegetation Determines Spatial Patterns and Survival of Pinus contorta Invasion in Patagonia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species
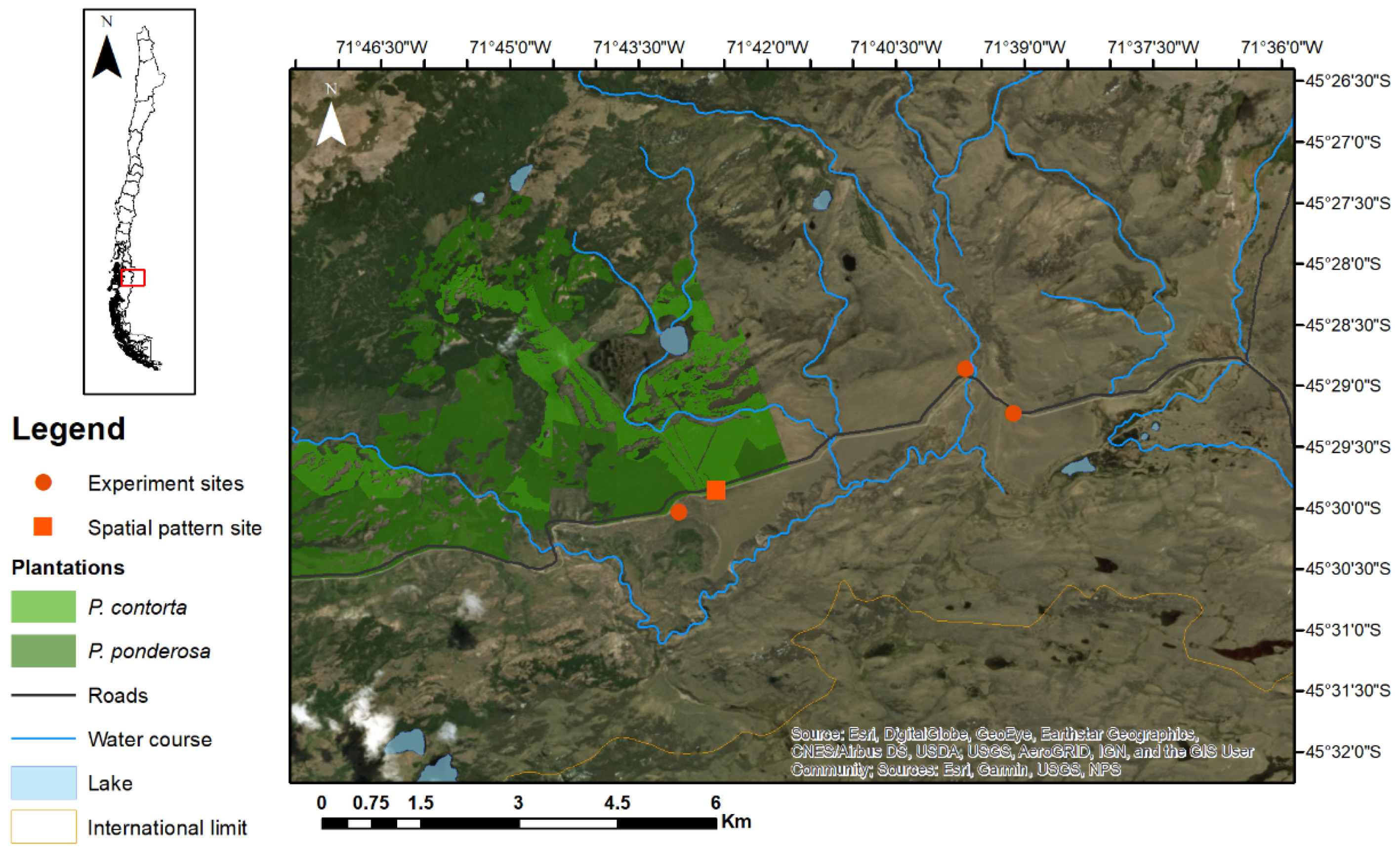
2.2. Study Area
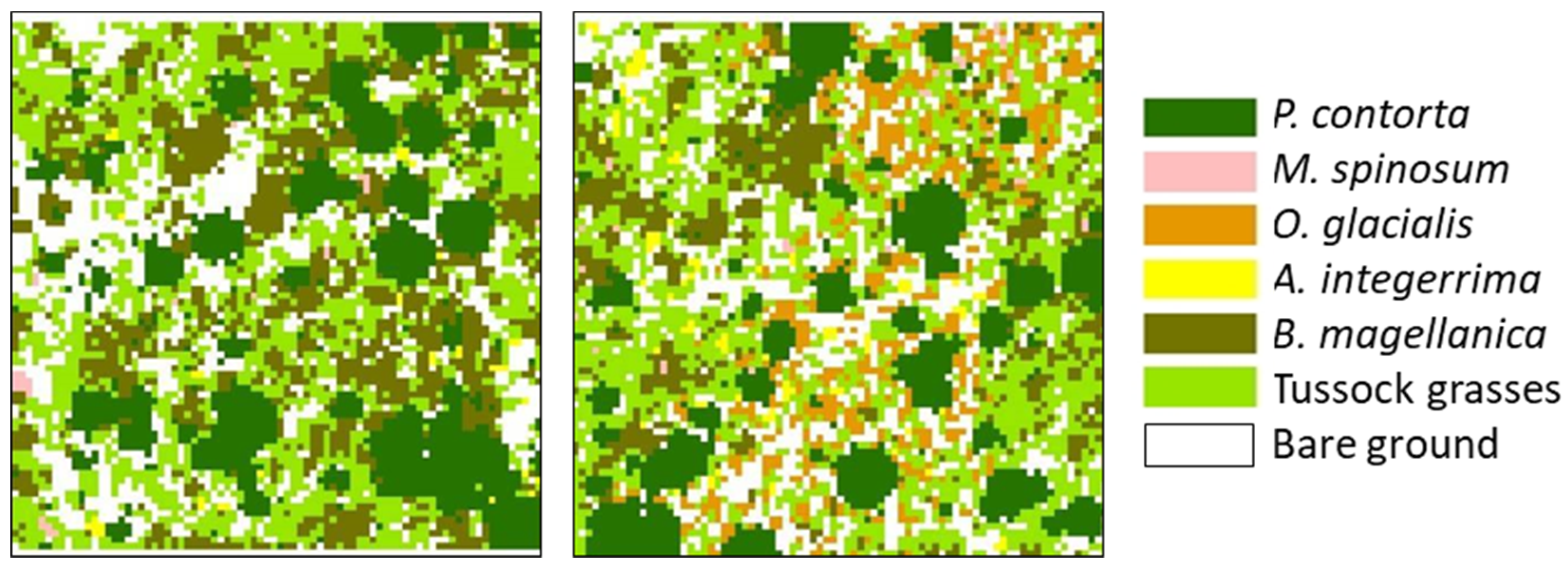
2.3. Spatial Associations with Native Vegetation
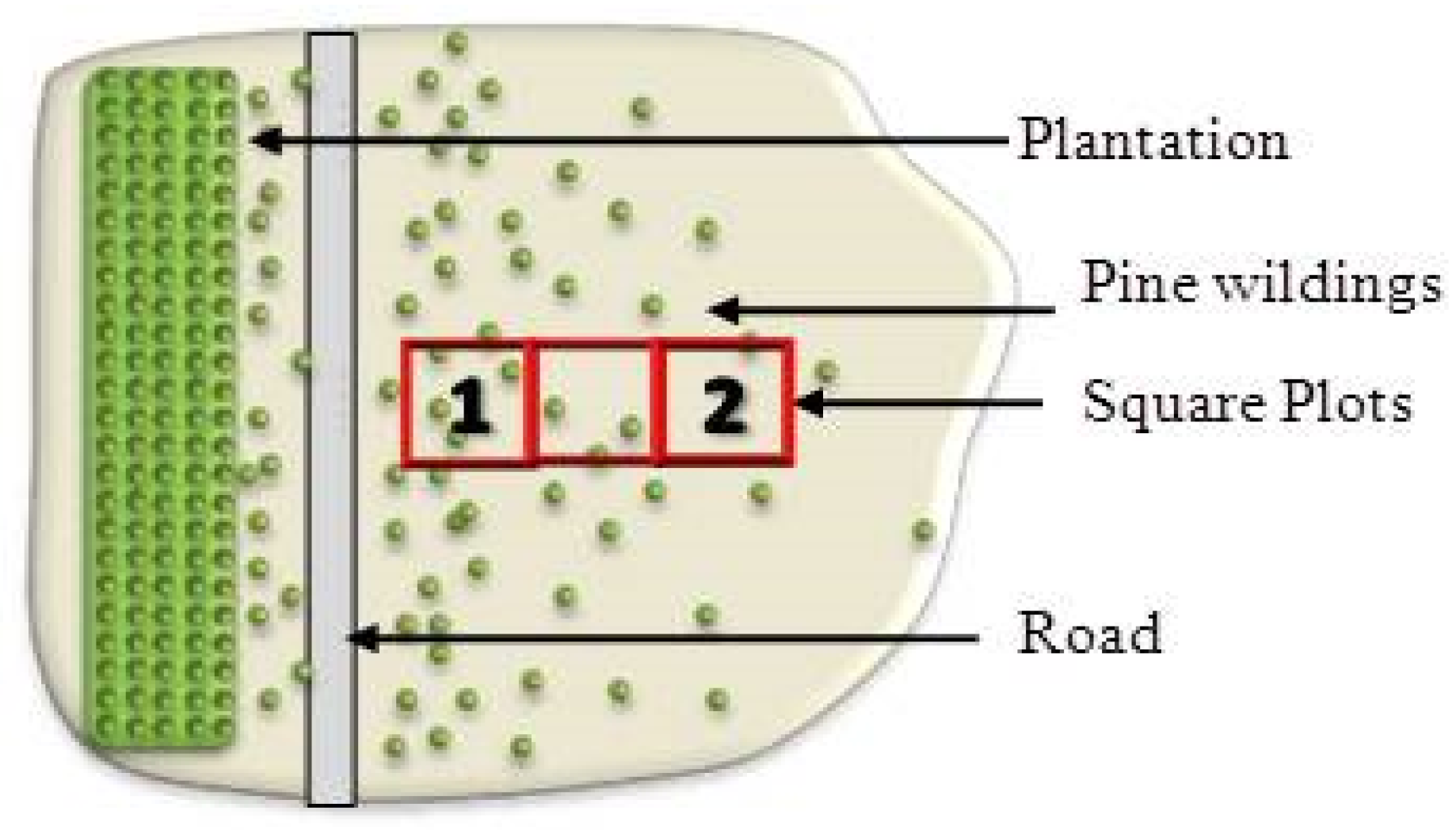
2.4. Pinus contorta Establishment and Survival
3. Results
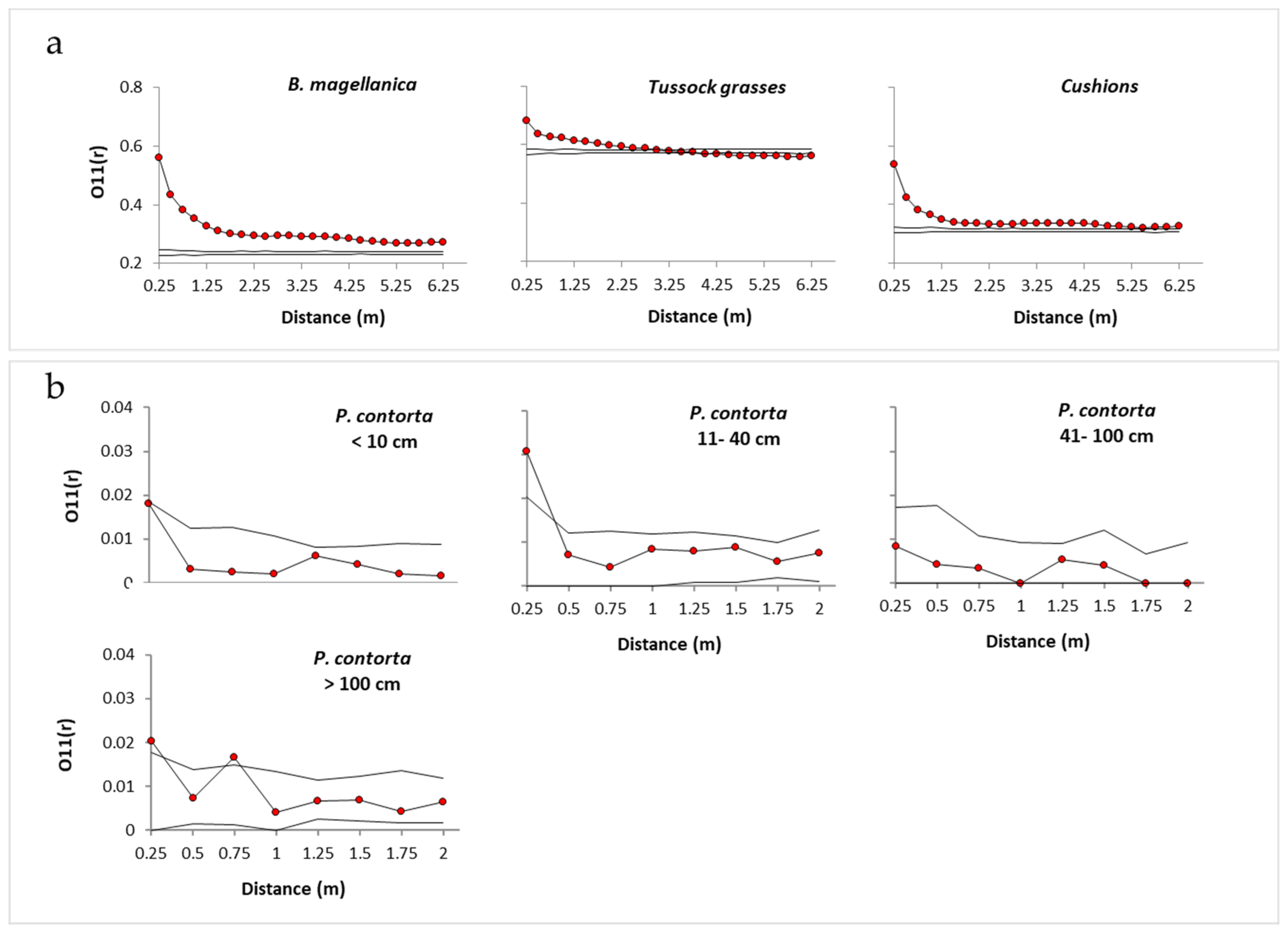
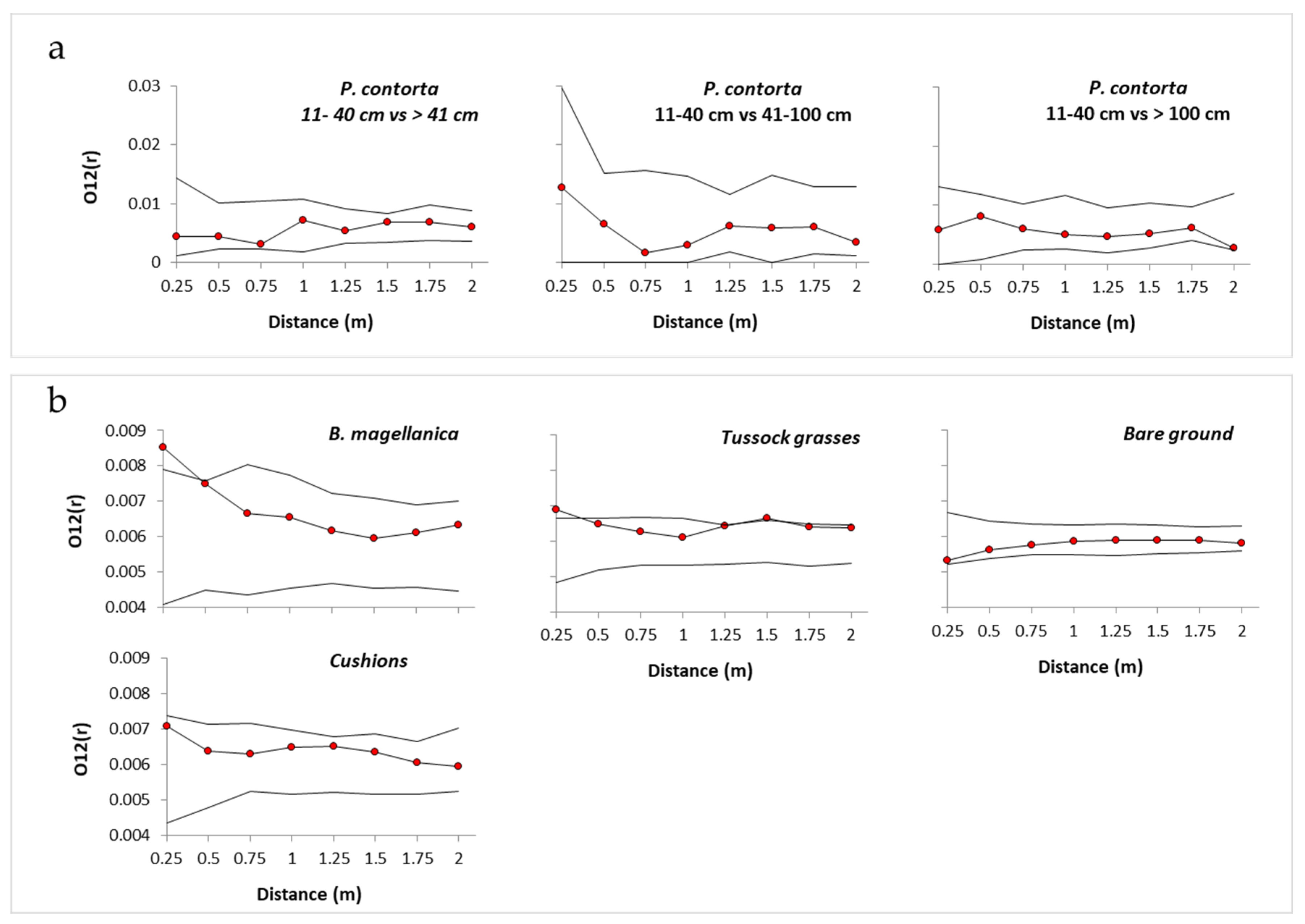
3.1. Spatial Associations with Native Vegetation
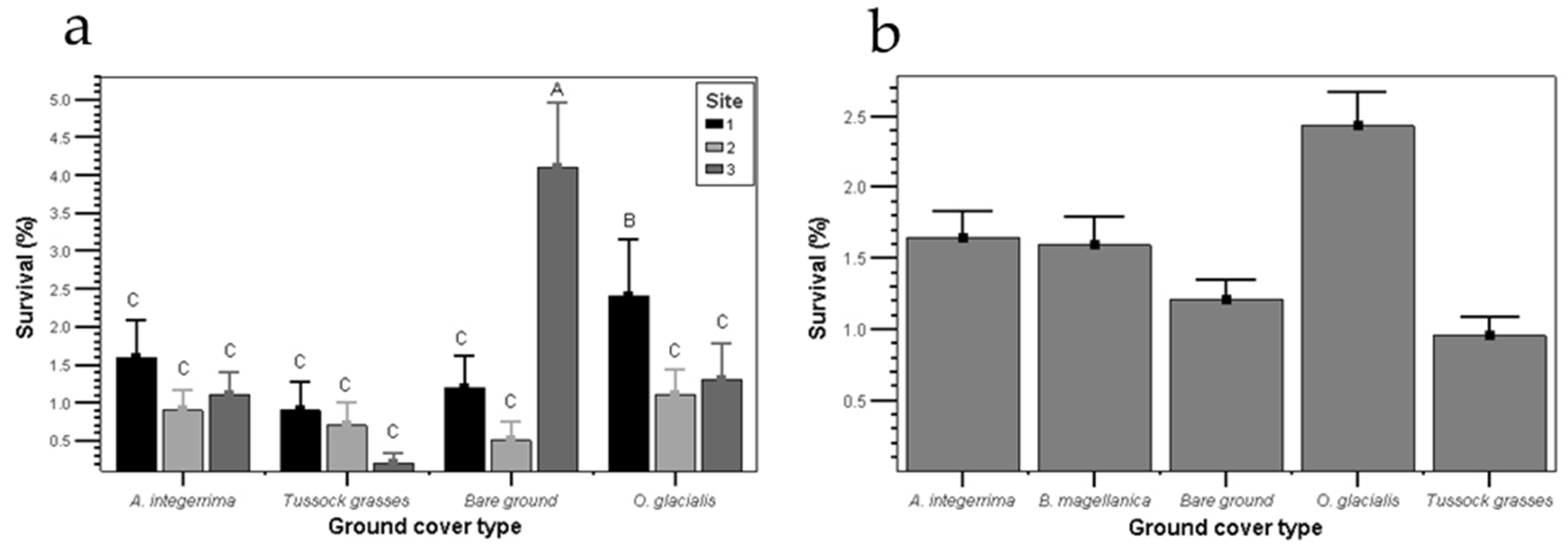
3.2. Pinus contorta Establishment and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vila, M.; Weiner, J. Are Invasive Plant Species Better Competitors than Native Plant Species? Evidence from Pair-Wise Experiments. Oikos 2004, 105, 229–238. [Google Scholar] [CrossRef]
- Tilman, D. Niche Tradeoffs, Neutrality, and Community Structure: A Stochastic Theory of Resource Competition, Invasion, and Community Assembly. Proc. Natl. Acad. Sci. USA 2004, 101, 10854–10861. [Google Scholar] [CrossRef] [PubMed]
- Alpert, P.; Bone, E.; Holzapfel, C. Invasiveness, Invasibility and the Role of Environmental Stress in the Spread of Non-Native Plants. Perspect. Plant Ecol. Evol. Syst. 2000, 3, 52–66. [Google Scholar] [CrossRef]
- Pauchard, A.; Shea, K. Integrating the Study of Non-Native Plant Invasions across Spatial Scales. Biol. Invasions 2006, 8, 399–413. [Google Scholar] [CrossRef]
- Pauchard, A. Conservation Research in Invasions as Spatially Explicit Processes. Front. Ecol. Environ. 2007, 5, 123–124. [Google Scholar]
- D’Antonio, C.; Mahall, B. Root profiles and competition between the invasive, exotic perennial, carpobrotus edulis, and two native shrub species in california coastal scrub. Am. J. Bot. 1991, 78, 885–894. [Google Scholar] [CrossRef]
- D’Antonio, C.M. Mechanisms Controlling Invasion of Coastal Plant Communities by the Alien Succulent Carpobrotus Edulis. Ecology 1993, 74, 83–95. [Google Scholar] [CrossRef]
- Richardson, D.M.; Ek, P.P.Y.S.; Rejmánek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Callaway, R. Positive Interactions among Plants (Review). Bot. Rev. Posit. Interact. Plants 1995, 61, 306–349. [Google Scholar]
- Bruno, J.F.; Stachowicz, J.J.; Bertness, M.D.; Bertness Mark, D.; Bruno John, F.; Stachowicz John, J. Inclusion of Facilitation into Ecological Theory. Trends Ecol. Evol. 2003, 18, 119–125. [Google Scholar] [CrossRef]
- Fajardo, A.; Quiroz, C.L.; Cavieres, L.A. Spatial Patterns in Cushion-Dominated Plant Communities of the High Andes of Central Chile: How Frequent Are Positive Associations? J. Veg. Sci. 2008, 19, 87–96. [Google Scholar] [CrossRef]
- Cavieres, L.A.; Quiroz, C.L.; Molina-Montenegro, M.A.; Muñoz, A.A.; Pauchard, A. Nurse Effect of the Native Cushion Plant Azorella Monantha on the Invasive Non-Native Taraxacum Officinale in the High-Andes of Central Chile. Perspect. Plant Ecol. Evol. Syst. 2005, 7, 217–226. [Google Scholar] [CrossRef]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J.M.J.; Anthelme, F.; et al. Facilitation in Plant Communities: The Past, the Present, and the Future. J. Ecol. 2008, 96, 18–34. [Google Scholar] [CrossRef]
- Franco, A.; Noble, P. Interactions Between Seedlings of Agave Deserti and the Nurse Plant Hilaria Rigida. Ecology 1988, 69, 1731–1740. [Google Scholar] [CrossRef]
- Franco, A.; Nobel, P. Effect of Nurse Plants on the Microhabitat and Growth of Cacti. J. Ecol. 1989, 77, 870–886. [Google Scholar] [CrossRef]
- Journal, S.; Aug, N.; Mary, T.K. Nurse Effect of Bolax Gummifera Cushion Plants in the Alpine Vegetation of the Chilean Patagonian Andes. J. Veg. Sci. 2016, 13, 547–554. [Google Scholar]
- Cavieres, L.A.; Badano, E.I.; Sierra-Almeida, A.; Molina-Montenegro, M.A. Microclimatic Modifications of Cushion Plants and Their Consequences for Seedling Survival of Native and Non-Native Herbaceous Species in the High Andes of Central Chile. Arct. Antarct. Alp. Res. 2007, 39, 229–236. [Google Scholar] [CrossRef]
- Nuñez, C.I.; Aizen, M.A.; Ezcurra, C. Species Associations and Nurse Plant Effects in Patches of High-Andean Vegetation. J. Veg. Sci. 2006, 10, 357–364. [Google Scholar] [CrossRef]
- Bertness, M.D.; Callaway, R. Positive Interactions in Communities. Trends Ecol. Evol. 1994, 9, 191–193. [Google Scholar] [CrossRef]
- Brooker, R.W.; Callaghan, T.V. The Balance between Positive and Negative Plant Interactions and Its Relationship to Environmental Gradients: A Model. Oikos 1998, 81, 196–207. [Google Scholar] [CrossRef]
- Cavieres, L.A.; Fajardo, A. Browsing by Guanaco (Lama Guanicoe) on Nothofagus Pumilio Forest Gaps in Tierra Del Fuego, Chile. For. Ecol. Manag. 2005, 204, 237–248. [Google Scholar] [CrossRef]
- Badano, E.; Villarroel, E.; Bustamante, R.O.; Marquet, P.A.; Cavieres, L.A. Ecosystem Engineering Facilitates Invasions by Exotic Plants in High-Andean Ecosystems. J. Ecol. 2007, 95, 682–688. [Google Scholar] [CrossRef]
- Aguiar, M.R.; Sala, O.E. Patch Structure, Dynamics and Implications for the Functioning of Arid Ecosystems. Trends Ecol. Evol. 1999, 14, 273–277. [Google Scholar] [CrossRef]
- Soriano, A.; Sala, O.; Perelman, S. Patch Structure and Dynamics in a Patagonian Arid Steppe. Vegetatio 1994, 111, 127–135. [Google Scholar] [CrossRef]
- Aguiar, M.; Sala, O. Interactions among Grasses, Shrubs and Herbivores Patagonia. Ecol. Austral 1998, 8, 201–210. [Google Scholar]
- Armas, C.; Pugnaire, F.I.; Sala, O.E. Patch Structure Dynamics and Mechanisms of Cyclical Succession in a Patagonian Steppe (Argentina). J. Arid Environ. 2008, 72, 1552–1561. [Google Scholar] [CrossRef]
- Tirado, R.; Pugnaire, F.I. Shrub Spatial Aggregation and Consequences for Reproductive Success. Oecologia 2003, 136, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Rodríguez, F.; Bautista, S.; Cortina, J.; Bellot, J. Spatial Associations and Patterns of Perennial Vegetation Ina Semi-Arid Steppe: A Multivariate Geostatistics Approach. Plant Ecol. 2005, 179, 133–147. [Google Scholar] [CrossRef]
- Halpern, C.B.; Antos, J.A.; Rice, J.M.; Haugo, R.D.; Lang, L. Tree Invasion of a Montane Meadow Complex: Temporal Trends, Spatial Patterns, and Biotic Interactions. J. Veg. Sci. 2010, 21, 717–732. [Google Scholar] [CrossRef]
- Kikvidze, Z.; Pugnaire, F.I.; Brooker, R.W.; Choler, P.; Lortie, C.J.; Michalet, R.; Callaway, R.M. Linking Patterns and Processes in Alpine Plant Communities: A Global Study. Ecology 2005, 86, 1395–1400. [Google Scholar] [CrossRef]
- Pugnaire, F.; Haase, P.; Puigdefabregas, J.; Cueto, M.; Clarck, S.; Incoll, L. Facilitation and Succession under the Canopy of a Leguminous Shrub, Retama Sphaerocarpa, in a Semi-Arid Environment in South-East Spain. Oikos 1996, 76, 455–464. [Google Scholar] [CrossRef]
- Benecke, U. The Invasive Potential of Lodgepole Pine. Tussock Grassl. Mt. Lands Inst. Rev. 1967, 13, 36–43. [Google Scholar]
- Ledgard, N. The Spread of Lodgepole Pine (Pinus contorta, Dougl.) in New Zealand. For. Ecol. Manag. 2001, 141, 43–57. [Google Scholar] [CrossRef]
- Richardson, D.M.; Bond, W.J. Determinants of Plant Distribution: Evidence from Pine Invasions. Am. Nat. 1991, 137, 639–668. [Google Scholar] [CrossRef]
- Richardson, D.; Higgins, S. Pines as Invaders in the Southern Hemisphere. In Ecology and Biogeography of Pinus; Richardson, D., Ed.; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Despain, D.G. Dispersal Ecology of Lodgepole Pine (Pinus contorta Dougl.) in Its Native Environment as Related to Swedish Forestry. For. Ecol. Manag. 2001, 141, 59–68. [Google Scholar] [CrossRef]
- Richardson, D.M.; Rejmánek, M.; Rejmanek, M. Conifers as Invasive Aliens: A Global Survey and Predictive Framework. Divers. Distrib. 2004, 10, 321–331. [Google Scholar] [CrossRef]
- Rejmanek, M. Invasive Trees and Shrubs: Where Do They Come from and What We Should Expect in the Future? Biol. Invasions 2014, 16, 483–498. [Google Scholar] [CrossRef]
- Gundale, M.J.; Pauchard, A.; Langdon, B.; Peltzer, D.A.; Maxwell, B.D.; Nuñez, M.A. Can Model Species Be Used to Advance the Field of Invasion Ecology? Biol. Invasions 2014, 16, 591–607. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Global Forest Resources Assessment 2000 (FRA 2000); FAO Forestry Paper No 140; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010. [Google Scholar]
- Richardson, D.M.; Petit, R.J. Pines as Invasive Aliens: Outlook on Transgenic Pine Plantations in the Southern Hemisphere. In Landscapes, Genomics and Transgenic Conifers. Managing Forest Ecosystems; Springer: Dordrecht, The Netherlands, 2006; pp. 169–188. [Google Scholar] [CrossRef]
- Peña, E.; Hidalgo, M.; Langdon, B.; Pauchard, A. Patterns of Spread of Pinus contorta Dougl. Ex Loud. Invasion in a Natural Reserve in Southern South America. For. Ecol. Manag. 2008, 256, 1049–1054. [Google Scholar] [CrossRef]
- Engelmark, O.; Sjöberg, K.; Andersson, B.; Rosvall, O.; Ågren, G.I.; Baker, W.L.; Barklund, P.; Björkman, C.; Despain, D.G.; Elfving, B.; et al. Ecological Effects and Management Aspects of an Exotic Tree Species: The Case of Lodgepole Pine in Sweden. For. Ecol. Manag. 2001, 141, 3–13. [Google Scholar] [CrossRef]
- Hepp, C. Praderas En La Zona Austral: XI Región (Aysén). In Praderas para Chile; Instituto de Investigaciones Agropecuarias (INIA): Santiago, Chile, 1996. [Google Scholar]
- Ganderats, S. Antecedentes Sobre La Producción de Praderas En Aysen. In Boletín INIA-Instituto de Investigaciones Agropecuarias 69; INIA: Madrid, Spain, 2001. [Google Scholar]
- Bravo-Monasterio, P.; Pauchard, A.; Fajardo, A. Pinus contorta Invasion into Treeless Steppe Reduces Species Richness and Alters Species Traits of the Local Community. Biol. Invasions 2016, 18, 1883–1894. [Google Scholar] [CrossRef]
- Franzese, J.; Urrutia, J.; García, R.A.; Taylor, K.; Pauchard, A. Pine Invasion Impacts on Plant Diversity in Patagonia: Invader Size and Invaded Habitat Matter. Biol. Invasions 2017, 19, 1015–1027. [Google Scholar] [CrossRef]
- Taylor, K.T.; Maxwell, B.D.; Pauchard, A.; Nuñez, M.A.; Rew, L.J. Native versus Non-Native Invasions: Similarities and Differences in the Biodiversity Impacts of Pinus contorta in Introduced and Native Ranges. Divers. Distrib. 2016, 22, 578–588. [Google Scholar] [CrossRef]
- Taylor, K.T.; Maxwell, B.D.; McWethy, D.B.; Pauchard, A.; Nuñez, M.A.; Whitlock, C. Pinus contorta Invasions Increase Wildfire Fuel Loads and May Create a Positive Feedback with Fire. Ecology 2017, 98, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.T.; Maxwell, B.D.; Caplat, P.; Pauchard, A.; Nuñez, M.A. Simulation Model Suggests That Fire Promotes Lodgepole Pine (Pinus contorta) Invasion in Patagonia. Biol. Invasions 2019, 1. [Google Scholar] [CrossRef]
- Langdon, B.; Pauchard, A.; Aguayo, M. Pinus contorta Invasion in the Chilean Patagonia: Local Patterns in a Global Context. Biol. Invasions 2010, 12, 3961–3971. [Google Scholar] [CrossRef]
- Pauchard, A.; Escudero, A.; García, R.A.; de la Cruz, M.; Langdon, B.; Cavieres, L.A.; Esquivel, J. Pine Invasions in Treeless Environments: Dispersal Overruns Microsite Heterogeneity. Ecol. Evol. 2016, 447–459. [Google Scholar] [CrossRef]
- Richardson, D.M. Plant Invasions. Encycl. Biodivers. 2001. [Google Scholar] [CrossRef]
- Lotan, J.E.; Critchfield, W.B. Lodgepole Pine. In Silvics of North America; Burns, R.M., Honkala, B.H., Eds.; USDA Forest Service and Agriculture Hanbook N°13; USDA: Washington, DC, USA, 1990; Volume 1, pp. 302–315. [Google Scholar] [CrossRef]
- Pablo, U.; Aceituno, P.; Fuenzalida, H.; Rutllant, J.; Santibañez, F. Caracterizacion Climatica. In Perséctivas de Desarrollo de los Recursos de La Region Aisen del GEneral Carlos Ibañez del Campo; Intendencia Regional de Aysén: Coyhaique, Chile, 1979. [Google Scholar]
- Gascón, A. Situación Forestal y Biodiversidad En La Región De Aysén; Informe técnico “Proyecto Fortalecimiento ciudadano en la Región de Aysen”; Ecosistemas y Fundacion Manfred Hermsen: Santiago-Coyhaique, Chile, 2005. [Google Scholar]
- Schlesinger, W.H.; Whitford, W.G.; Virginia, R.A.; Huenneke, L.F.; Reynolds, J.F.; Jarrell, W.M.; Cunningham, G.L. Biological Feedbacks in Global Desertification. Science 2006, 247, 1043–1048. [Google Scholar] [CrossRef]
- Maestre, F.T.; Valladares, F.; Reynolds, J.F. Is the Change of Plant-Plant Interactions with Abiotic Stress Predictable? A Meta-Analysis of Field Results in Arid Environments. J. Ecol. 2005, 93, 748–757. [Google Scholar] [CrossRef]
- Schleicher, J.; Wiegand, K.; Ward, D. Changes of Woody Plant Interaction and Spatial Distribution between Rocky and Sandy Soil Areas in a Semi-Arid Savanna, South Africa. J. Arid Environ. 2011, 75, 270–278. [Google Scholar] [CrossRef]
- López, R.P.; Larrea-Alcázar, D.; Zenteno-Ruiz, F. Spatial Pattern Analysis of Dominant Species in the Prepuna: Gaining Insight into Community Dynamics in the Semi-Arid, Subtropical Andes. J. Arid Environ. 2010, 74, 1534–1539. [Google Scholar] [CrossRef]
- Ramsay, P.M.; Fotherby, R.M. Implications of the Spatial Pattern of Vigur’s Eyebright (Euphrasia Vigursii) for Heathland Management. Basic Appl. Ecol. 2007, 8, 242–251. [Google Scholar] [CrossRef]
- Wiegand, T.; Kissling, W.D.; Cipriotti, P.A.; Aguiar, M.R. Extending Point Pattern Analysis for Objects of Finite Size and Irregular Shape. J. Ecol. 2006, 94, 825–837. [Google Scholar] [CrossRef]
- Wiegand, T.; Moloney, K.A. Rings, Circles, and Null-Models for Point Pattern Analysis in Ecology. Oikos 2004, 104, 209–229. [Google Scholar] [CrossRef]
- Wiegand, T.; Moloney, K.A. Handbook of Spatial Point-Pattern Analysis in Ecology, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2013. [Google Scholar]
- Haase, P. Spatial Pattern Analysis in Ecology Based on Ripley’s K-Function: Introduction and Methods of Edge Correction. J. Veg. Sci. 2006, 6, 575–582. [Google Scholar] [CrossRef]
- Callaway, R. Positive Interactions and Interdependence in Plant Communities; Springer International Publishing: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Ledgard, N.J. Wilding Conifers—New Zealand History and Research Background. In Managing Wilding Conifers in New Zealand—Present and Future; New Zealand Plant Protection Society: Christchurch, New Zealand, 2004; pp. 1–25. [Google Scholar]
- Cattaneo, M. Effects of Microsite Characteristics, Competition and Grazing on Pinus contorta Dougl. and Pseudotsuga menziesii (Mirb.) Franco Seedling Establishment. Master’s Thesis, University of Canterbury, Christchurch, New Zealand, 2002; pp. 1–306. [Google Scholar]
- Allen, R.B.; Lee, W.G. Seedling Establishment Microsites of Exotic Conifers in Chionochloa Rigida Tussock Grassland, Otago, New Zealand. N. Z. J. Bot. 1989, 27, 491–498. [Google Scholar] [CrossRef]
- Bullock, J.M. A Long-Term Study of the Roles of Competition and Facilitation in the Establishment of an Invasive Pine Following Heathland Fires. J. Ecol. 2009, 97, 646–656. [Google Scholar] [CrossRef]
- Miriti, M.N. Twenty Years of Changes in Spatial Association and Community Structure among Desert Perennials. Ecology 2007, 88, 1177–1190. [Google Scholar] [CrossRef]
- Callaway, R.M.; Walker, L.R. Competition and Facilitation: A Synthetic Approach to Interactions in Plant Communities. Ecology 1997, 78, 1958–1965. [Google Scholar] [CrossRef]
- Davis, M.R. Establishment of Conifer Plantations in the South Island High Country by Direct drilling. N. Z. For. 1989, 34, 21–24. [Google Scholar]



| Height Class | n | Height (cm) | DCH * (mm) | Crown Diameter (cm) |
|---|---|---|---|---|
| <10 cm | 44 | 7.49 | 6.625 | 10.86 |
| 11–40 cm | 77 | 20.46 | 10.53 | 18.25 |
| 41–100 cm | 30 | 70.30 | 21.88 | 48.54 |
| >100 cm | 85 | 229.60 | 65.80 | 138.98 |
| >41 cm | 115 | 188.16 | 54.33 | 115.44 |
| Total | 236 ** | 103.20 *** | 26.21 *** | 54.16 *** |
| DF | F-Value | Pr > F | |
|---|---|---|---|
| Site | 2 | 4.41 | 0.0133 |
| Substrate | 3 | 6.28 | 0.0004 |
| Site × Substrate interaction | 6 | 6.12 | <0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langdon, B.; Cavieres, L.A.; Pauchard, A. At a Microsite Scale, Native Vegetation Determines Spatial Patterns and Survival of Pinus contorta Invasion in Patagonia. Forests 2019, 10, 654. https://doi.org/10.3390/f10080654
Langdon B, Cavieres LA, Pauchard A. At a Microsite Scale, Native Vegetation Determines Spatial Patterns and Survival of Pinus contorta Invasion in Patagonia. Forests. 2019; 10(8):654. https://doi.org/10.3390/f10080654
Chicago/Turabian StyleLangdon, Bárbara, Lohengrin A. Cavieres, and Aníbal Pauchard. 2019. "At a Microsite Scale, Native Vegetation Determines Spatial Patterns and Survival of Pinus contorta Invasion in Patagonia" Forests 10, no. 8: 654. https://doi.org/10.3390/f10080654
APA StyleLangdon, B., Cavieres, L. A., & Pauchard, A. (2019). At a Microsite Scale, Native Vegetation Determines Spatial Patterns and Survival of Pinus contorta Invasion in Patagonia. Forests, 10(8), 654. https://doi.org/10.3390/f10080654





