Patterns of Biomass, Carbon, and Soil Properties in Masson pine (Pinus massoniana Lamb) Plantations with Different Stand Ages and Management Practices
Abstract
1. Introduction
2. Materials and Methods
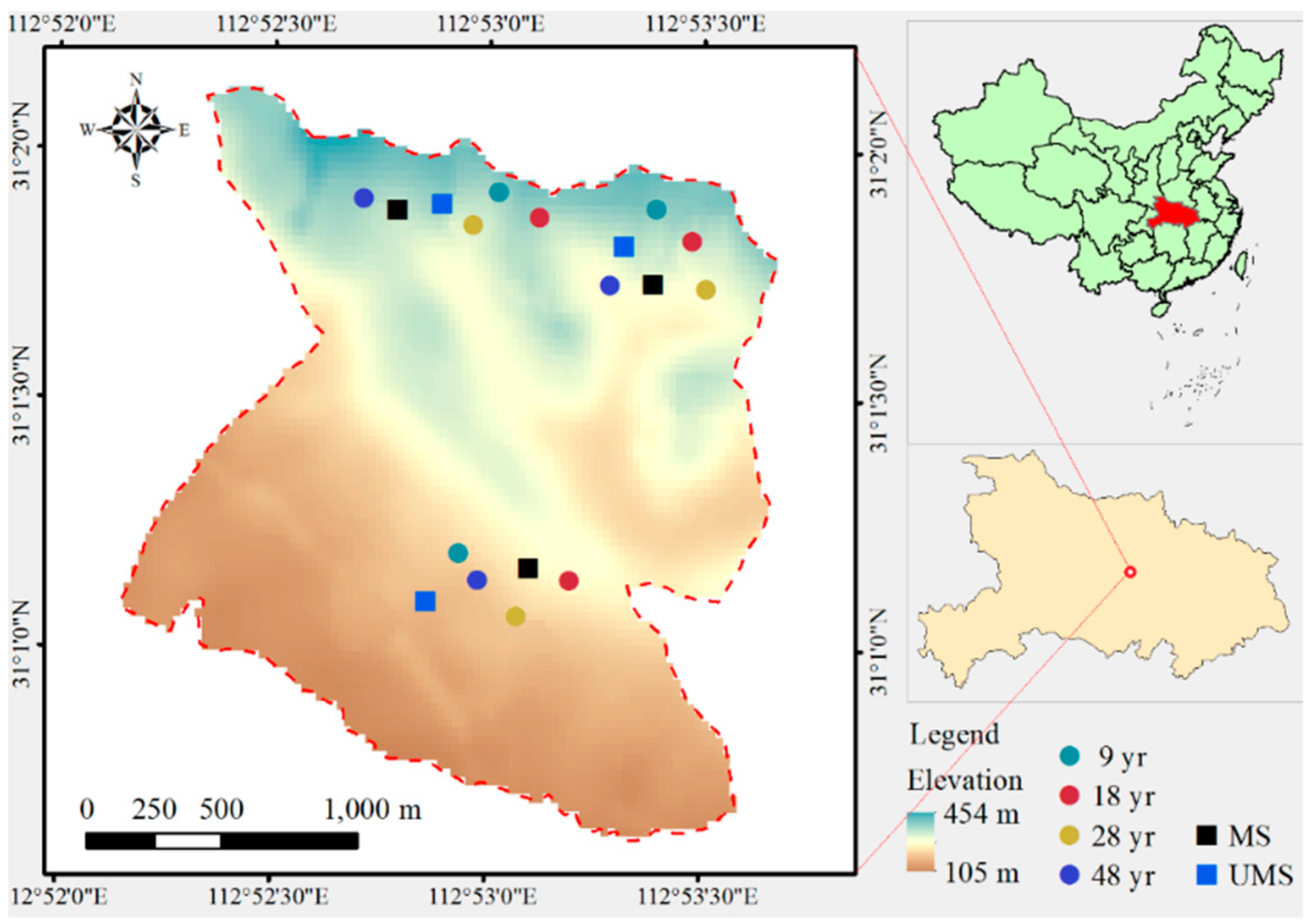
2.1. Study Site
2.2. Field Sampling and Data Collection
2.3. Soil Properties
2.4. Data Analysis
3. Results
3.1. Biomass of P. massoniana Stands of Different Age and under Management
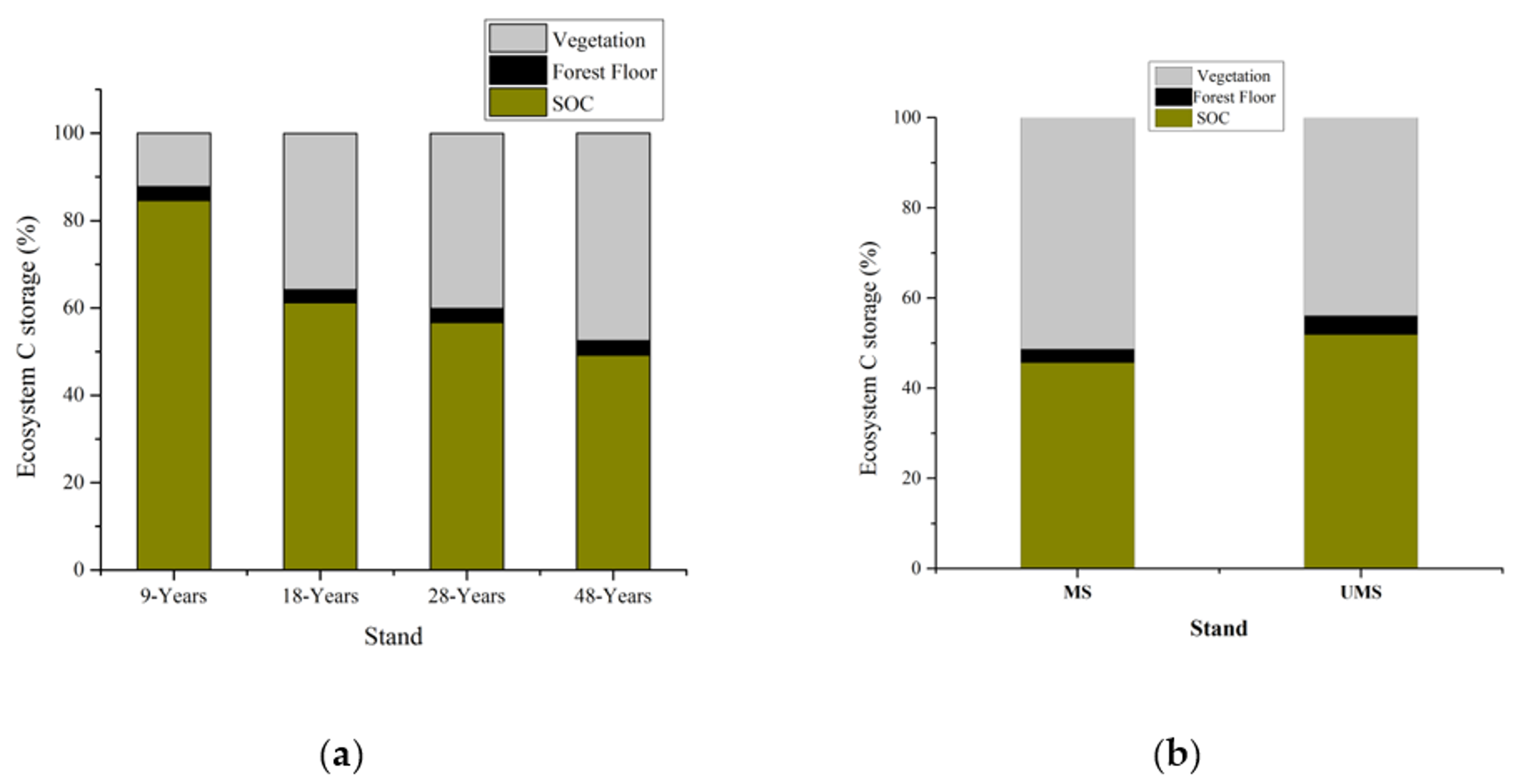
3.2. Vegetation Carbon and Soil Carbon
3.3. Effect of Age and Management on Soil Physicochemical Properties
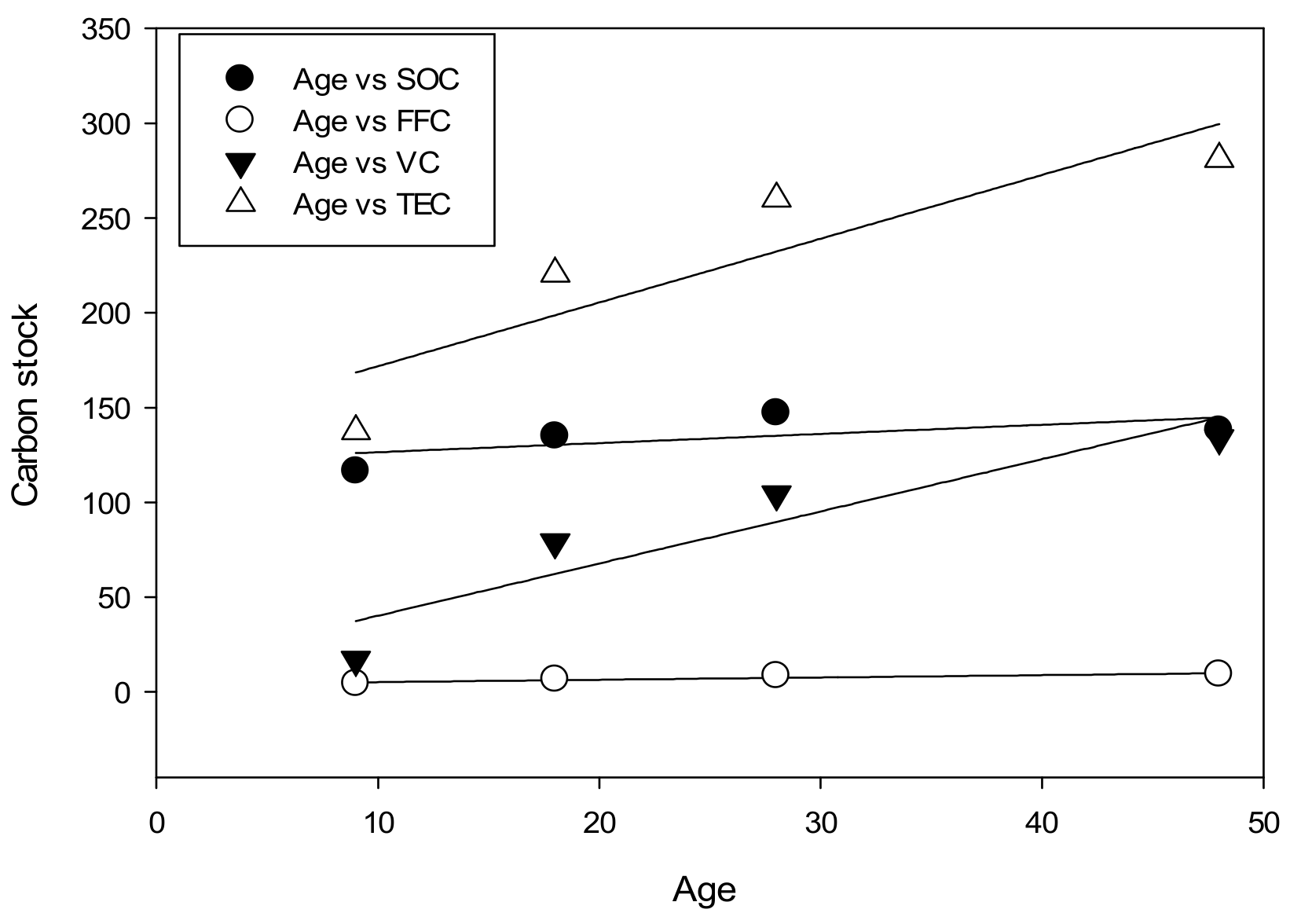
3.4. Relationship of Soil, Vegetation Carbon, and Soil Properties to Stand Age and Management
4. Discussion
4.1. Biomass and Carbon of P. massoniana Stands at Different Ages and under Management Schemes
4.2. P. massoniana Plantations and SOC
4.3. Ecosystem Carbon Storage and P. massoniana Stands
4.4. Soil Physicochemical Properties under Contrasting Stand Management Schemes and Stand Ages
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, W.; Gong, P.; Gao, L. A Review of Carbon Forest Development in China. Forests 2017, 8, 295. [Google Scholar] [CrossRef]
- Dangal, S.P.; Das, A.K.; Paudel, S.K. Effectiveness of management interventions on forest carbon stock in planted forests in Nepal. J. Environ. Manag. 2017, 196, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Terakunpisut, J.; Gajaseni, N.; Ruankawe, N. Carbon sequestration potential in aboveground biomass of Thong Pha Phun National Forest, Thailand. Appl. Ecol. Environ. Res. 2007, 5, 93–102. [Google Scholar] [CrossRef]
- Chen, L.C.; Liang, M.J.; Wang, S.L. Carbon stock density in planted versus natural Pinus massoniana forests in sub-tropical China. Ann. For. Sci. 2016. [Google Scholar] [CrossRef]
- Hyvönen, R.; Ågren, G.I.; Linder, S.; Persson, T.; Cotrufo, F.; Ekblad, A.; Freeman, M.; Grelle, A.; Ivan, A.; Jarvis, P.G.; et al. The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: A literature review. New Phytol. 2006, 173, 463–480. [Google Scholar] [CrossRef]
- Ahmad, A.; Moazzam, N.S.; Marwat, K.B.; Muhammad, J. Annual accumulation of carbon in the coniferous forest of Dir Kohistan: An inventory based estimate. Pak. J. Bot. 2016, 47, 115–118. [Google Scholar]
- Barford, C.C.; Wofsy, S.C.; Goulden, M.L.; Munger, J.W.; Pyle, E.H.; Urbanski, S.P.; Hutyra, L.; Saleska, S.R.; Fitzjarrald, D.; Moore, K. Factors Controlling Long- and Short-Term Sequestration of Atmospheric CO2 in a Mid-latitude Forest. Science 2001, 294, 1688–1692. [Google Scholar] [CrossRef]
- Vafaei, S.; Soosani, J.; Adeli, K.; Fadaei, H.; Naghavi, H.; Pham, T.D.; Bui, D.T. Improving accuracy estimation of Forest Aboveground Biomass based on incorporation of ALOS-2 PALSAR-2 and Sentinel-2A imagery and machine learning: A case study of the Hyrcanian forest area (Iran). Remote Sens. 2018, 10, 172. [Google Scholar] [CrossRef]
- Pham, T.D.; Yokoya, N.; Bui, D.T.; Yoshino, K. Remote Sensing Approaches for Monitoring Mangrove Species, Structure, and Biomass: Opportunities and Challenges. Remote Sens. 2019, 11, 230. [Google Scholar] [CrossRef]
- Malmsheimer, R.W.; Heffernan, P.; Brink, S.; Crandall, D.; Deneke, F.; Galik, C.; Gee, E.; Helms, J.A.; Mcclure, N.; Mortimer, M.; et al. Forest Management Solutions for Mitigating Climate Change in the United States. J. For. 2008, 106, 115–173. [Google Scholar]
- The Food and Agriculture Organization (FAO). Global Forest Resources Assessment 2015 Desk Reference; FAO: Rome, Italy, 2015. [Google Scholar]
- Saeed, S.; Yujun, S.; Beckline, M.; Chen, L.; Zhang, B. Forest edge effect on biomass carbon along altitudinal gradients in Chinese Forest edge effect on biomass carbon along altitudinal gradients in Chinese Fir (Cunninghamia lanceolata): A study from Southeastern China. Carbon Manag. 2019, 11–22. [Google Scholar] [CrossRef]
- Ahmad, A.; Liu, Q.; Nizami, S.M.; Mannan, A.; Saeed, S. Carbon emission from deforestation, forest degradation and wood harvest in the temperate region of Hindukush Himalaya, Pakistan between 1994 and 2016. Land Use Policy 2018, 78, 781–790. [Google Scholar] [CrossRef]
- Berninger, F.; Nikinmaa, E. Implications of varying pipe model relationships on Scots Pine growth in different climates. Funct. Ecol. 1997, 11, 146–156. [Google Scholar] [CrossRef]
- Schulze, E.D.; Aas, G.; Grimm, G.W.; Gossner, M.M.; Walentowski, H.; Ammer, C.; Kühn, I.; Bouriaud, O.; von Gadow, K. A review on plant diversity and forest management of European beech forests. Eur. J. For. Res. 2016, 135. [Google Scholar] [CrossRef]
- Kang, B.; Liu, S.R.; Zhang, G.J.; Chang, J.G.; Wen, Y.G.; Ma, J.M.; Hao, W.F. Carbon accumulation and distribution in Pinus massoniana and Cunninghamia lanceolata mixed forest ecosystem in Daqingshan, Guangxi of China. Acta Ecol. Sin. 2006, 26, 1320–1329. [Google Scholar] [CrossRef]
- Zianis, D.; Mencuccini, M. On simplifying allometric analyses of forest biomass. For. Ecol. Manag. 2004, 187, 311–332. [Google Scholar] [CrossRef]
- Sonogyi, Z.; Cienciala, E.; Makipaa, R.; Muukkonen, P.; Lehtonen, A.; Weiss, P. Indirect methods of large-scale forest biomass estimation. Eur. J. For. Res. 2007. [Google Scholar] [CrossRef]
- Wang, C. Biomass allometric equations for 10 co-occurring tree species in Chinese temperate forests. For. Ecol. Manag. 2006, 222, 9–16. [Google Scholar] [CrossRef]
- Dixon, R.K.; Brown, S.; Houghton, R.A.; Solomon, A.M.; Trexler, M.C.; Wispniewski, J. Carbon Pools and Flux of Global Forest Ecosystems. Science 1991, 63, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Payn, T.; Carnus, J.M.; Freer-Smith, P.; Kimberley, M.; Kollert, W.; Liu, S.; Orazio, C.; Rodriguez, L.; Silva, L.N.; Wingfield, M.J. Changes in planted forests and future global implications. For. Ecol. Manag. 2015, 352, 57–67. [Google Scholar] [CrossRef]
- Justine, M.F.; Yang, W.; Wu, F.; Tan, B.; Khan, M.N.; Zhao, Y. Biomass stock and carbon sequestration in a chronosequence of Pinus massoniana plantations in the upper reaches of the Yangtze River. Forests 2015, 6, 3665–3682. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, S.; Liu, S.; Wang, X.; Zhang, Y.; Liu, T.; Zhou, L.; Zhang, W.; Fu, S. Reforestation makes a minor contribution to soil carbon accumulation in the short term: Evidence from four subtropical plantations. For. Ecol. Manag. 2017, 384, 400–405. [Google Scholar] [CrossRef]
- Karu, H.; Szava-Kovats, R.; Pensa, M.; Kull, O. Carbon sequestration in a chronosequence of Scots pine stands in a reclaimed opencast oil shale mine. Can. J. For. Res. 2009. [Google Scholar] [CrossRef]
- Houghton Revised estimates of the annual net flux of carbon to the atmosphere from changes in land use and land management 1850—2000. Tellus 2003, 558, 378–390.
- Malins, C. Indirect Land Use Change in Europe—Considering the Policy Options; The International Council on Clean Transportation: Washington, DC, USA, 2011. [Google Scholar]
- Atauri, J.A.; De Pablo, C.L.; De Agar, P.M.; Schmitz, M.F.; Pineda, F.D. Effects of management on understory diversity in the forest ecosystems of northern Spain. Environ. Manag. 2004. [Google Scholar] [CrossRef] [PubMed]
- Duguid, M.C.; Ashton, M.S. A meta-analysis of the effect of forest management for timber on understory plant species diversity in temperate forests. For. Ecol. Manag. 2013, 303, 81–90. [Google Scholar] [CrossRef]
- Pendleton, L.; Donato, D.C.; Murray, B.C.; Crooks, S.; Jenkins, W.A.; Megonigal, P.; Pidgeon, E.; Herr, D.; Gordon, D.; Baldera, A. Estimating Global “Blue Carbon” Emissions from Conversion and Degradation of Vegetated Coastal Ecosystems. PLoS ONE 2012, 7, e43542. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Jactel, H.; Parrotta, J.A.; Quine, C.P.; Sayer, J. Plantation forests and biodiversity: Oxymoron or opportunity? Biodivers. Conserv. 2008, 17, 925–951. [Google Scholar] [CrossRef]
- Cao, S.; Chen, L.; Shankman, D.; Wang, C.; Wang, X.; Zhang, H. Excessive reliance on afforestation in China’s arid and semi-arid regions: Lessons in ecological restoration. Earth Sci. Rev. 2011, 104, 240–245. [Google Scholar] [CrossRef]
- Xue, L.; Li, Q.; Chen, H. Effects of a wildfire on selected physical, chemical and biochemical soil properties in a Pinus massoniana forest in South China. Forests 2014, 5, 2947–2966. [Google Scholar] [CrossRef]
- Wang, F.; Xu, X.; Zou, B.; Guo, Z.; Li, Z.; Zhu, W. Biomass Accumulation and Carbon Sequestration in Four Different Aged Casuarina equisetifolia Coastal Shelterbelt Plantations in South China. PLoS ONE 2013, 8, e77449. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.J.; Wang, P.C.; Zhang, Y.; Teng, M.J.; Zhou, Z.X. Effect of gap disturbances on soil properties and understory plant diversity in a pinus massoniana plantation in hubei, central China. J. Anim. Plant Sci. 2016, 26, 988–1001. [Google Scholar]
- Chen, D.; Ali, A.; Yong, X.H.; Lin, C.G.; Niu, X.H.; Cai, A.M.; Dong, B.C.; Zhou, Z.X.; Wang, Y.J.; Yu, F.H. A multi-species comparison of selective placement patterns of ramets in invasive alien and native clonal plants to light, soil nutrient and water heterogeneity. Sci. Total Environ. 2019. [Google Scholar] [CrossRef] [PubMed]
- Xiuhong, J.; Yi, Z.; Zhixiang, Z.; Daxiong, G.; Jingrong, X.; Guozhi, G. Hydrological characteristics of forest litter and soil in different successional stages in Hill region of central Hubei. J. Soil Water Conserv. 2013, 27, 125–129. [Google Scholar]
- Wang, Y.; Müller-Schärer, H.; van Kleunen, M.; Cai, A.; Zhang, P.; Yan, R.; Dong, B.; Yu, F. Invasive alien plants benefit more from clonal integration in heterogeneous environments than natives. New Phytol. 2017, 216, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Sakai, T.; Takahashi, K.; Sato, T.; Tanouchi, H.; Sugita, H.; Tanaka, H. Effects of management, environment and landscape conditions on establishment of hardwood seedlings and saplings in central Japanese coniferous plantations. For. Ecol. Manag. 2011, 262, 1280–1288. [Google Scholar] [CrossRef]
- State Forestry Administration (SFA). Tree Biomass Methods and Related Parameters of Carbon Accumulating in Pinus massonina; SFA: Beijing, China, 2014. [Google Scholar]
- Zhang, H.; Song, T.; Wang, K.; Du, H.; Yue, Y. Biomass and carbon storage in an age-sequence of Cyclobalanopsis glauca plantations in southwest China. Ecol. Eng. 2014, 73, 184–191. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, M.; Wang, G.G. Effects of Thinning on Soil Organic Carbon Fractions and Soil Properties in Cunninghamia lanceolata Stands in Eastern China. Forests 2017, 8, 198. [Google Scholar] [CrossRef]
- Lal, R.; Shukla, M.K. Principles of Soil Physics; Marcel Dekker, Inc.: New York, NY, USA, 2004; ISBN 0-203-02123-1. [Google Scholar]
- Chen, Y.; Cao, Y. Response of tree regeneration and understory plant species diversity to stand density in mature Pinus tabulaeformis plantations in the hilly area of the Loess Plateau, China. Ecol. Eng. 2014, 73, 238–245. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Y. Ecosystem C: N: P stoichiometry and carbon storage in plantations and a secondary forest on the Loess Plateau, China. Ecol. Eng. 2017, 105, 125–132. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods of Soil Agrochemistry; China Agricultural Science and Technology Publishing House: Beijing, China, 1999; pp. 18–99. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Justine, M.F.; Yang, W.; Wu, F.; Khan, M.N. Dynamics of biomass and carbon sequestration across a chronosequence of masson pine plantations. J. Geophys. Res. Biogeosci. 2017, 122, 578–591. [Google Scholar] [CrossRef]
- Ali, A.; Ma, W.J.; Yang, X.D.; Sun, B.W.; Shi, Q.R.; Xu, M.S. Biomass and carbon stocks in Schima superba dominated subtropical forests of eastern China. J. For. Sci. 2014, 60, 198–207. [Google Scholar] [CrossRef]
- Yadav, D.; Wang, J. Modelling carbon dioxide emissions from agricultural soils in Canada. Environ. Pollut. 2017, 230, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zeng, W.; Zhang, H.; Wang, G.; Lei, Y.; Tang, S. Generic linear mixed-effects individual-tree biomass models for Pinus massoniana in southern China. South. For. J. For. Sci. 2014, 76, 47–56. [Google Scholar] [CrossRef]
- Yu, Y.F.; Song, T.Q.; Zeng, F.P.; Peng, W.X.; Wen, Y.G.; Huang, C.B.; Wu, Q.B.; Zeng, Z.X.; Yu, Y. Dynamic changes of biomass and its allocation in Cunninghamia lanceolata plantations of different stand ages. Chin. J. Ecol. 2013, 32, 1660–1666. [Google Scholar]
- Turner, J.; Long, J.N. Accumulation of organic matter in a series of Douglas fir stands. Can. J. For. Res. 1975, 5, 681–690. [Google Scholar] [CrossRef]
- Li, X.; Yi, M.J.; Son, Y. Biomass and Carbon Storage in an Age-Sequence of Korean Pine (Pinus koraiensis) Plantation Forests in Central Korea. J. Plant Biol. 2011, 54, 33–42. [Google Scholar] [CrossRef]
- Noormets, A.; Epron, D.; Domec, J.C.; McNulty, S.G.; Fox, T.; Sun, G.; King, J.S. Effects of forest management on productivity and carbon sequestration: A review and hypothesis. For. Ecol. Manag. 2015, 355, 124–140. [Google Scholar] [CrossRef]
- Farley, K.A.; Kelly, E.F.; Hofstede, R.G.M. Soil Organic Carbon and Water Retention after Conversion of Grasslands to Pine Plantations in the Ecuadorian Andes. Ecosystems 2004, 7, 729–739. [Google Scholar] [CrossRef]
- Pan, Y. A large and persistent carbon sink in the world’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, I.; Fried, Y.; Yakir, D. Biogeochemical factors contributing to enhanced carbon storage following afforestation of a semi-arid shrubland. Biogeosciences 2007, 4, 891–904. [Google Scholar]
- Laganiere, J.; Angers, D.A.; Pare, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Glob. Chang. Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Han, X.; Gao, G.; Chang, R.; Li, Z.; Ma, Y.; Wang, S.; Wang, C.; Lü, Y.; Fu, B. Changes in soil organic and inorganic carbon stocks in deep pro fi les following cropland abandonment along a precipitation gradient across the Loess Plateau of China. Agric. Ecosyst. Environ. 2018, 258, 1–13. [Google Scholar] [CrossRef]
- Sartori, F.; Lal, R.; Ebinger, M.H.; Eaton, J.A. Changes in soil carbon and nutrient pools along a chronosequence of poplar plantations in the Columbia Plateau, Oregon, USA. Agric. Ecosyst. Environ. 2007, 122, 325–339. [Google Scholar] [CrossRef]
- Brown, S.; Pare, D. Dynamics of carbon and nitrogen mineralization in relation to stand type, stand age and soil texture in the boreal mixedwood. Soil Biol. Biochem. 2000, 32, 1079–1090. [Google Scholar]
- Sun, O.J.; Campbell, J.; Law, B.E.; Wolf, V. Dynamics of carbon stocks in soils and detritus across chronosequences of different forest types in the Pacific Northwest, USA. Glob. Chang. Biol. 2004, 10, 1470–1481. [Google Scholar] [CrossRef]
- Fonseca, W.; María, J.; Benayas, R.; Alice, F.E. Carbon accumulation in the biomass and soil of different aged secondary forests in the humid tropics of Costa Rica. For. Ecol. Manag. 2011, 262, 1400–1408. [Google Scholar] [CrossRef]
- Mao, R.; Zeng, D.-H.; Hu, Y.-L.; Li, L.-J.; Yang, D. Soil organic carbon and nitrogen stocks in an age-sequence of poplar stands planted on marginal agricultural land in Northeast China. Plant Soil 2010, 332, 277–287. [Google Scholar] [CrossRef]
- Prescott, C.E. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 2016, 101, 133–149. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, W.; Ren, H.; Chen, H.; Wang, J. Impact of atmospheric nitrogen deposition on soil properties and herb-layer diversity in remnant forests along an urban—Rural gradient in Guangzhou, southern. Plant Ecol. 2012, 1187–1202. [Google Scholar] [CrossRef]
- Piao, S.; Fang, J.; Ciais, P.; Peylin, P.; Huang, Y.; Sitch, S.; Wang, T. The carbon balance of terrestrial ecosystems in China. Nature 2009, 458, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Berthrong, S.T.; Jobbagy, E.G.; Jackson, R.B. A global meta-analysis of soil exchangeable cations, pH, carbon, and nitrogen with afforestation. Ecol. Appl. 2009, 19, 2228–2241. [Google Scholar] [CrossRef] [PubMed]
- Pibumrung, P.; Gajaseni, N.; Popan, A. Profiles of carbon stocks in forest, reforestation and agricultural land. J. For. Res. 2008, 19, 11–18. [Google Scholar] [CrossRef]
- Stinson, G.; Kurz, W.A.; Smyth, C.E.; Neilson, E.T.; Dymond, C.C.; Metsaranta, J.M.; Boisvenue, C.; Rampley, G.J.; Li, Q.; White, T.M.; et al. An inventory-based analysis of Canada’ s managed forest carbon dynamics, 1990 to 2008. Glob. Chang. Biol. 2011, 2227–2244. [Google Scholar] [CrossRef]
- Alifragis, D.; Smiris, P.; Maris, F.; Kavvadias, V.; Konstantinidou, E.; Stamou, N. The effect of stand age on the accumulation of nutrients in the aboveground components of an Aleppo pine ecosystem. For. Ecol. Manag. 2001. [Google Scholar] [CrossRef]



| Variables | Biomass Components | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stand Age (Years) | Stem | Bark | Branches | Leaves | Aboveground | Belowground | Total Tree | Shrubs | Herbs | TB |
| 9 | 18.3 ± 5.7d | 3.1 ± 0.9c | 9.6 ± 4.5c | 6.2 ± 2.5b | 37.3 ± 10.2d | 8.7 ± 2.4d | 46.1 ± 12.1 | 6.1 ± 4.2a | 2.7 ± 0.8a | 54.8 ± 10.1d |
| 18 | 98.5 ± 8.3c | 8.7 ± 1.4b | 20.5 ± 5.1b | 8.6 ± 1.2b | 136.3 ± 15.6c | 21.7 ± 5.4c | 158.0 ± 21.6 | 8.6 ± 6.1a | 5.5 ± 3.5a | 172.1 ± 41.5c |
| 28 | 142.5 ± 13.3b | 11.2 ± 2.7ab | 13.7 ± 66.2b | 10.0 ± 1.7a | 177.5 ± 19.3b | 31.3 ± 8.2b | 208.8 ± 18.7 | 10.3 ± 4.2a | 6.3 ± 5.2a | 225.4 ± 56.1b |
| 48 | 149.3 ± 15.4a | 12.9 ± 3.4a | 38.6 ± 6.2a | 12.1 ± 3.1a | 212.8 ± 21.1a | 53.2 ± 5.3a | 266.0 ± 22.4 | 10.7 ± 7.3a | 7.7 ± 3.2a | 284.4 ± 44.7a |
| Management Scheme | ||||||||||
| MS | 134 ± 15.3a | 12.0 ± 2.2b | 28.6 ± 5.8a | 14.1 ± 2.5a | 188.7 ± 13.2a | 42.6 ± 7.2a | 231.3 ± 17.5 | 8.3 ± 5.3b | 5.7 ± 5.5b | 245.4 ± 51.4b |
| UMS | 114.9 ± 14.2a | 10.9 ± 2.4a | 26.3 ± 5.1a | 11.2 ± 1.2a | 163.3 ± 16.4b | 32.1 ± 8.3b | 195.4 ± 14.2 | 10.4 ± 9.7a | 7.1 ± 6.8a | 212.9 ± 36.1a |
| p | Tree Components | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stand Age (Years) | Stem | Bark | Branch | Leaves | Aboveground | Belowground | Total tree | Shrubs | Herbaceous | TC |
| 9 | 9.2 ± 2.7d | 1.5 ± 0.5c | 4.8 ± 2.2c | 3.1 ± 1.3b | 18.6 ± 5.6d | 4.4 ± 1.2d | 23.0 ± 6.5 | 3.0 ± 2.2a | 1.4 ± 0.4a | 27.4 ± 9.2d |
| 18 | 49.2 ± 4.2c | 4.4 ± 0.7b | 10.2 ± 2.5b | 4.3 ± 0.6b | 68.1 ± 7.8c | 10.9 ± 2.3c | 79.0 ± 10.3 | 4.3 ± 3.1a | 2.7 ± 1.7a | 86.0 ± 22.1c |
| 28 | 71.3 ± 6.6b | 5.6 ± 1.4ab | 6.9 ± 3.1b | 5.0 ± 0.8a | 88.7 ± 9.6b | 15.7 ± 4.1b | 104.4 ± 9.3 | 5.2 ± 1.1a | 3.2 ± 2.6a | 112.7 ± 28.4b |
| 48 | 74.6 ± 7.9a | 6.5 ± 1.8a | 19.3 ± 3.1a | 6.0 ± 1.5a | 106.4 ± 10.2a | 26.6 ± 2.6a | 133.0 ± 11.2 | 5.4 ± 3.6a | 3.9 ± 1.8a | 142.2 ± 21.4a |
| Management Schemes | ||||||||||
| MS | 66.9 ± 7.6a | 6.0 ± 1.1b | 14.3 ± 3.1a | 7.1 ± 1.2a | 94.3 ± 6.6a | 21.8 ± 3.6a | 116 ± 11.4 | 4.2 ± 2.1b | 2.7 ± 1.3b | 122.6 ± 25.7a |
| UMS | 57.5 ± 7.1a | 5.5 ± 1.2a | 13.2 ± 2.5a | 5.5 ± 0.6a | 81.6 ± 8.2b | 16.0 ± 4.1a | 97.7 ± 10.7 | 5.2 ± 4.7a | 3.6 ± 2.4a | 106.5 ± 17.2a |
| Variables Stand Age (Years) | SOC | Forest Floor | Total Tree | Ecosystem |
|---|---|---|---|---|
| 9 | 116.4 ± 12.5ab | 4.3 ± 1.6a | 23.0 ± 6.5a | 143.7 ± 24.2b |
| 18 | 135.0 ± 10.5ab | 6.4 ± 2.2b | 79.0 ± 10.3ab | 220.4 ± 21.7b |
| 28 | 147.4 ± 17.1a | 8.3 ± 2.5b | 104.4 ± 9.3b | 260.1 ± 33.5a |
| 48 | 138.1 ± 14.4a | 9.2 ± 2.8ab | 133.0 ± 11.2b | 280.3 ± 49.1a |
| Mean | 134.2 ± 13.1ab | 7.1 ± 3.3ab | 84.8 ± 15.4ab | 226.1 ± 26.7ab |
| Management Schemes | ||||
| MS | 104.9 ± 15.6a | 6.4 ± 2.1a | 116 ± 11.4a | 227.3 ± 21.5a |
| UMS | 115.4 ± 18.2b | 9.3 ± 3.4a | 97.7 ± 10.7b | 222.4 ± 18.7b |
| Mean | 110.1 ± 15.1ab | 7.9 ± 3.3ab | 112.1 ± 13.5ab | 225.0 ± 14.5ab |
| Soil Physical Properties | |||||||
|---|---|---|---|---|---|---|---|
| TPo (%) | CP (%) | CTWHC (g kg−1) | MNWHC (g kg−1) | MXWHC (g kg−1) | NCP (%) | SD (g cm−3) | |
| Management Schemes | |||||||
| MS | 5.7a | 20.6b | 168.6b | 135.4a | 212.9a | 5.1a | 1.2a |
| UMS | 6.2a | 21.7a | 180.2a | 140.3a | 217.6a | 4.4b | 1.2a |
| Age | |||||||
| 9 years | 25.3a | 20.7b | 159.2b | 117.1b | 195.5b | 4.5b | 1.3a |
| 18 years | 26.2a | 20.5b | 182.3a | 148.9a | 233.9a | 5.6a | 1.1c |
| 28 years | 26.1a | 23.2a | 183.5a | 145.6a | 207.6b | 2.9c | 1.2a |
| 48 years | 26.3a | 20.4b | 172.7a | 139.7a | 223.8a | 5.8a | 1.2b |
| Soil Depth | |||||||
| 1st layer | 26.4a | 20.8a | 183.5a | 145.3a | 235.4a | 5.6a | 1.1b |
| 2nd layer | 26.4a | 21.2a | 170.5b | 133.6a | 212.8b | 5.2a | 1.2a |
| 3rd layer | 25.1b | 21.6a | 169.2b | 134.6a | 197.3c | 3.5b | 1.2a |
| Interactions | |||||||
| A*M | Ns | NS | NS | NS | NS | NS | NS |
| A*SD | ** | ** | * | NS | * | NS | NS |
| M*SD | NS | NS | NS | NS | NS | NS | NS |
| M*A*SD | ** | NS | NS | NS | NS | NS | NS |
| Soil Chemical Properties | ||||||
|---|---|---|---|---|---|---|
| TP (g kg−1) | AP (g kg−1) | AN (mg kg−1) | OM (%) | TN (g kg−1) | pH | |
| Management Schemes | ||||||
| UMS | 3.7b | 4.1a | 113.7a | 3.9a | 0.7a | 5.5a |
| MS | 4.8a | 2.7b | 130.5a | 3.8a | 0.7a | 5.5a |
| Age | ||||||
| 9 years | 3.7b | 3.8a | 118.8ab | 3.5a | 0.7a | 5.6a |
| 18 years | 5.4a | 3.4a | 107.6b | 4.1a | 0.8a | 5.6a |
| 28 years | 2.6c | 3.3a | 119.0ab | 3.8a | 0.7a | 5.5a |
| 48 years | 5.4a | 2.9a | 142.9a | 4.1a | 0.8a | 5.3b |
| Soil Depth | ||||||
| soil Layer | 5.4a | 4.5a | 197.1a | 6.5a | 1.3a | 5.2c |
| (0–10 cm) | ||||||
| 3.9b | 3.1b | 100.5b | 3.1b | 0.6b | 5.5b | |
| (10–20 cm) | ||||||
| 3.6b | 2.4b | 68.7c | 2.1c | 0.4c | 5.8a | |
| (20–40 cm) | ||||||
| Interactions | ||||||
| A*M | *** | NS | *** | NS | NS | ** |
| A*SD | * | NS | ** | ** | ** | * |
| M*SD | NS | NS | NS | NS | NS | NS |
| A*M*SD | NS | NS | NS | NS | NS | NS |
| Correlation | R2 | RMSE |
|---|---|---|
| Age and Soil organic carbon | 0.39 | 8.66 |
| Age and Forest Floor carbon | 0.87 | 0.88 |
| Age and Vegetation carbon | 0.86 | 16.10 |
| Age and Total Ecosystem carbon | 0.78 | 25.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Ahmad, A.; Akhtar, K.; Teng, M.; Zeng, W.; Yan, Z.; Zhou, Z. Patterns of Biomass, Carbon, and Soil Properties in Masson pine (Pinus massoniana Lamb) Plantations with Different Stand Ages and Management Practices. Forests 2019, 10, 645. https://doi.org/10.3390/f10080645
Ali A, Ahmad A, Akhtar K, Teng M, Zeng W, Yan Z, Zhou Z. Patterns of Biomass, Carbon, and Soil Properties in Masson pine (Pinus massoniana Lamb) Plantations with Different Stand Ages and Management Practices. Forests. 2019; 10(8):645. https://doi.org/10.3390/f10080645
Chicago/Turabian StyleAli, Ashfaq, Adnan Ahmad, Kashif Akhtar, Mingjun Teng, Weisheng Zeng, Zhaogui Yan, and Zhixiang Zhou. 2019. "Patterns of Biomass, Carbon, and Soil Properties in Masson pine (Pinus massoniana Lamb) Plantations with Different Stand Ages and Management Practices" Forests 10, no. 8: 645. https://doi.org/10.3390/f10080645
APA StyleAli, A., Ahmad, A., Akhtar, K., Teng, M., Zeng, W., Yan, Z., & Zhou, Z. (2019). Patterns of Biomass, Carbon, and Soil Properties in Masson pine (Pinus massoniana Lamb) Plantations with Different Stand Ages and Management Practices. Forests, 10(8), 645. https://doi.org/10.3390/f10080645






