Seasonal Variation in Phenolic Compounds and Antioxidant Activity in Leaves of Cyclocarya paliurus (Batal.) Iljinskaja
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sample Collection
2.3. Chemical Reagents and Reference
2.4. Sample and Standard Solutions Preparation
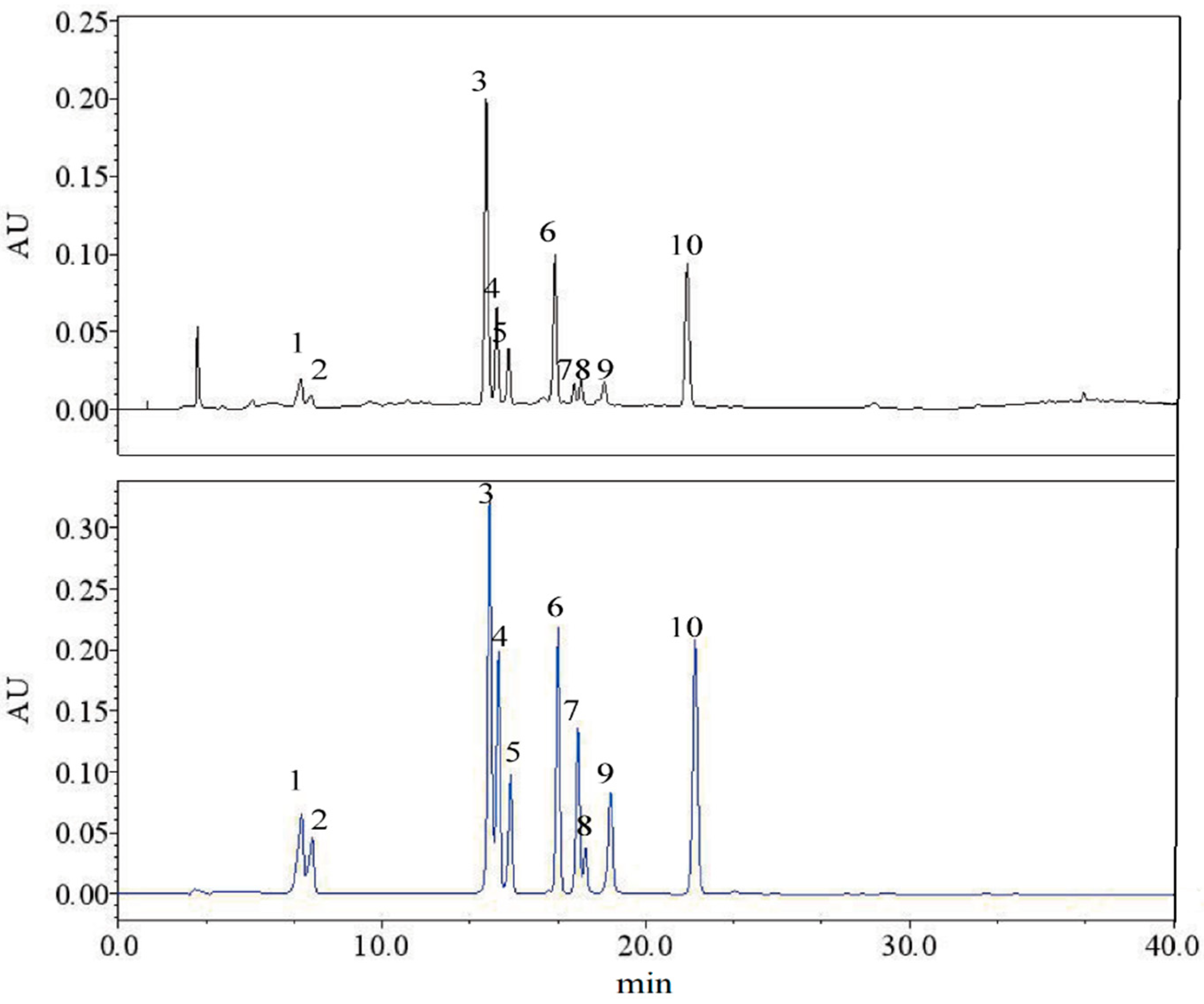
2.5. HPLC Determination of Phenolic Compounds
2.6. Measurement of Antioxidant Activities
2.6.1. DPPH Radical Scavenging Activity
2.6.2. Ferric Reducing Antioxidant Power (FRAP)
2.6.3. ABTS Radical Cation Scavenging Activity
2.7. Data Analysis
3. Results
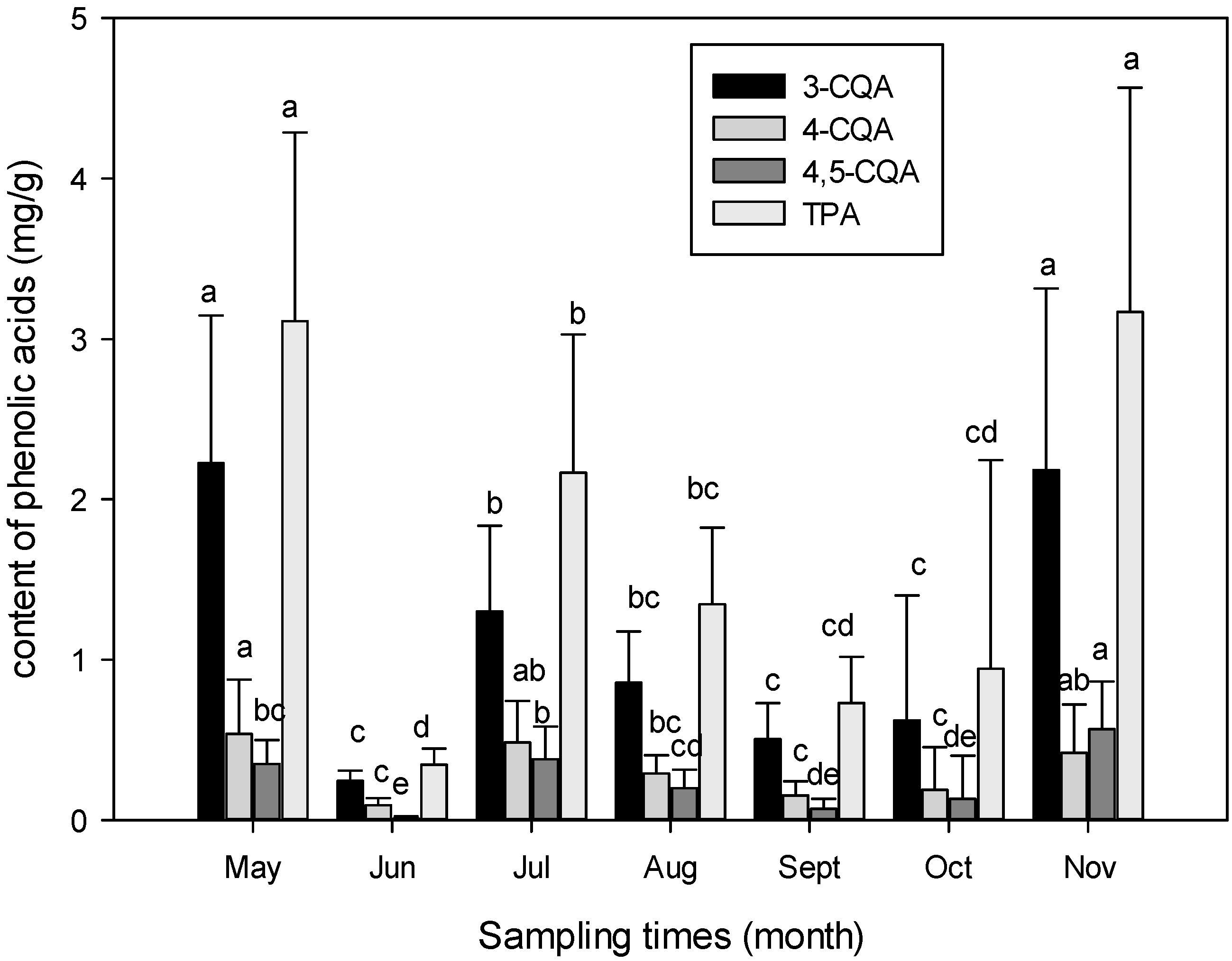
3.1. Seasonal Variation of Phenolic Acids Contents
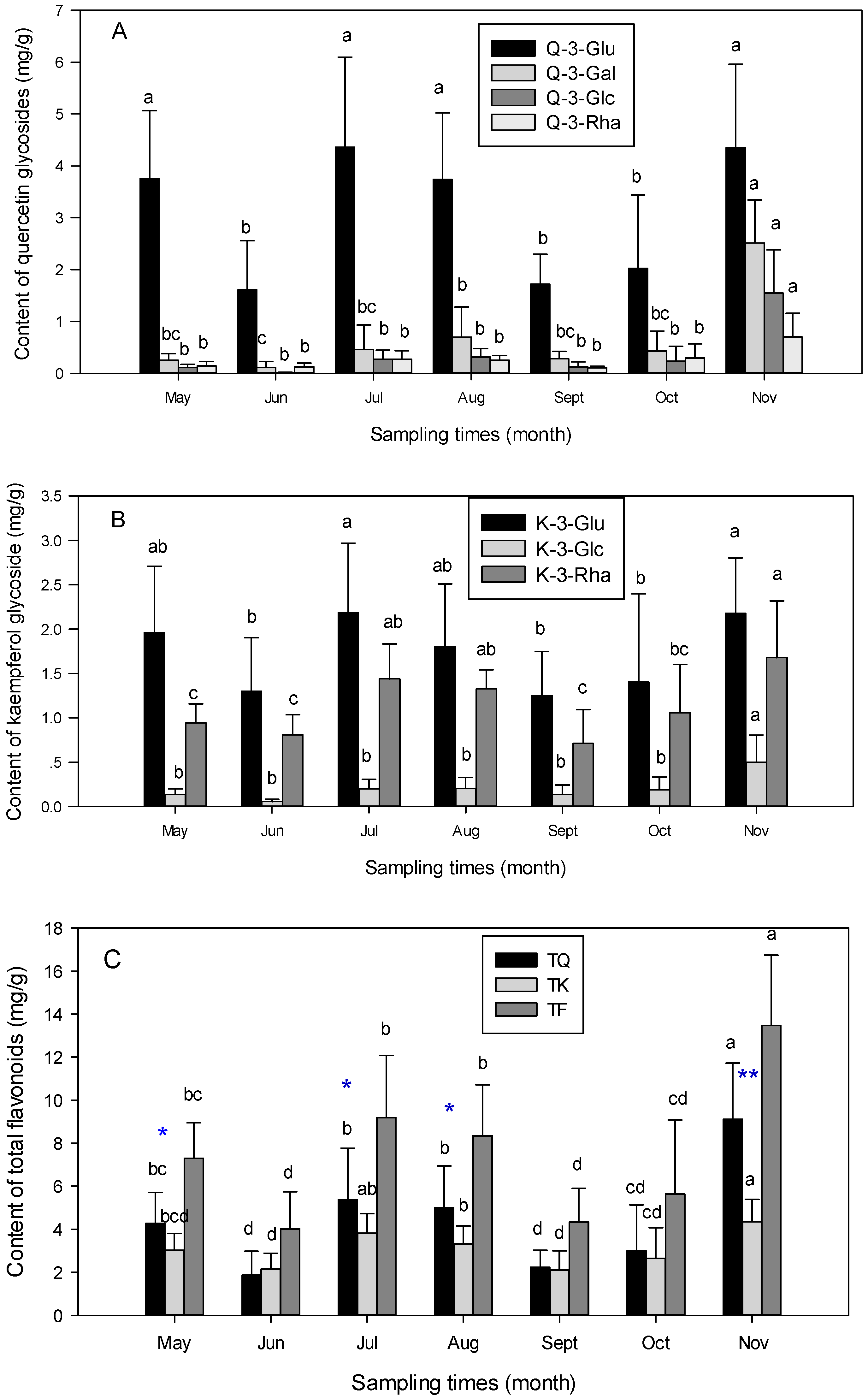
3.2. Seasonal Variation of Flavonoids Content
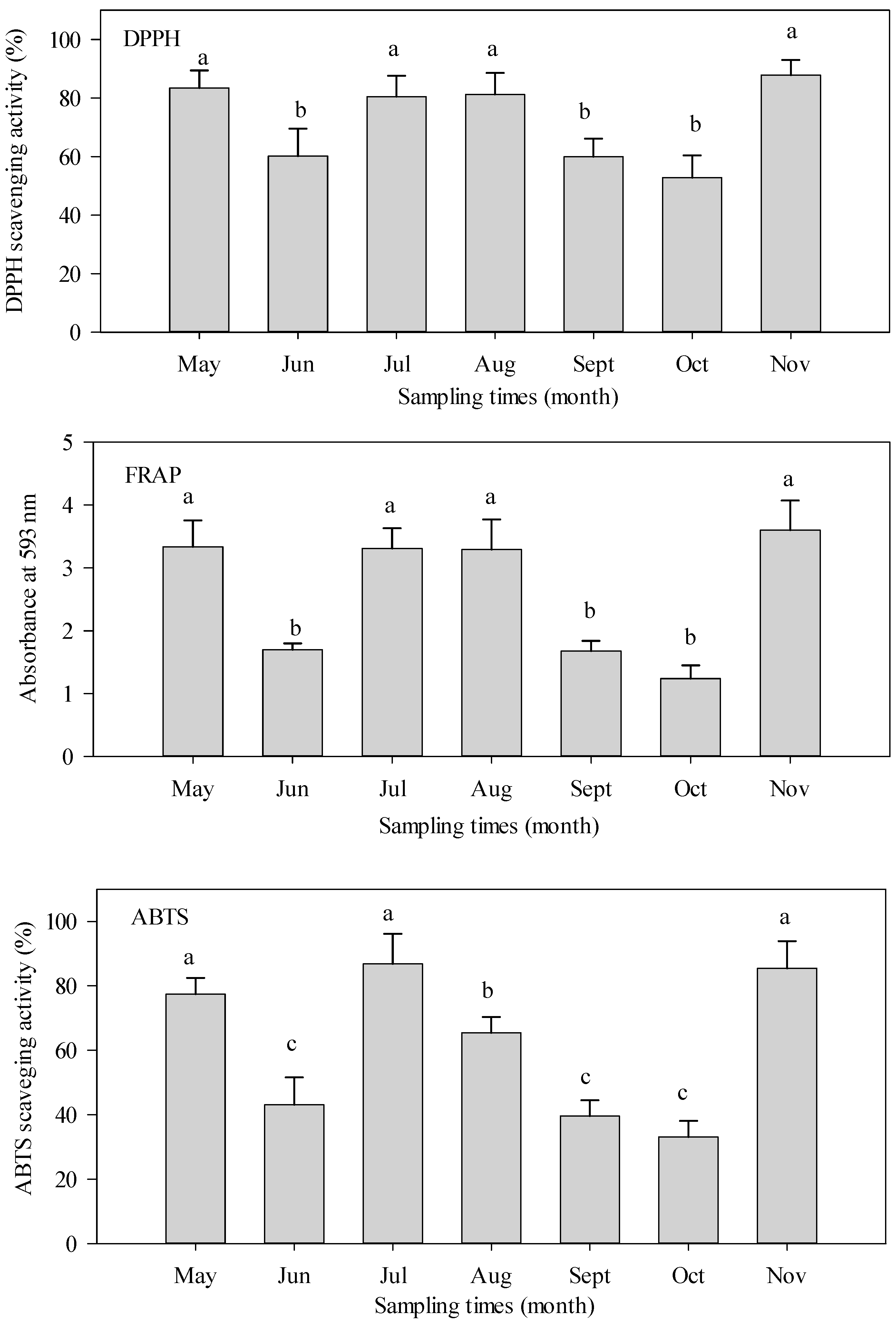
3.3. Seasonal Variation of Antioxidant Activities
3.4. Correlation between Contents of Phenolic Compounds and Antioxidant Activity
3.5. Effects of Individual Phenolic Compounds on Antioxidant Capacity
4. Discussion
4.1. Effects of Environment and Development Phase on Leaf Phenolic Accumulation
4.2. Relationship between Phenolic Composition and Antioxidant Capacity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.D.; Bertoldi, M.C.; Krenek, K.; Talcott, S.T.; Stringheta, P.C.; Mertens-Talcott, S.U. Anticarcinogenic effects of polyphenolics from mango (Mangifera indica) varieties. J. Agric. Food Chem. 2010, 58, 4104–4112. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Chang, Y.Y.; Hsu, C.L.; Liu, C.W.; Lin, Y.L.; Lin, Y.H.; Liu, K.C.; Chen, Y.C. Antiobesity and hypolipidemic effects of polyphenol-rich longan (Dimocarpus longans Lour.) flower water extract in hypercaloric-dietary rats. J. Agric. Food Chem. 2010, 58, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Dillard, C.J.; German, J.B. Phytochemicals: nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Su, L.; Yin, J.J.; Charles, D.; Zhou, K.; Moore, J.; Yu, L. Total phenolic contents, chelating capacities, and radical-scavenging properties of black peppercorn, nutmeg, rosehip, cinnamon and oregano leaf. Food Chem. 2007, 100, 990–997. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From chemistry to biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Ghosh, S.; Padilla-González, G.F.; Rangan, L. Alpinia nigra seeds: A potential source of free radical scavenger and antibacterial agent. Ind. Crops Prod. 2013, 49, 348–356. [Google Scholar] [CrossRef]
- Stanojević, L.; Stanković, M.; Nikolić, V.; Nikolić, L.; Ristić, D.; Čanadanovic-Brunet, J.; Tumbas, V. Antioxidant activity and total phenolic and flavonoid contents of Hieracium pilosella L. extracts. Sensors 2009, 9, 5702–5714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shen, Q.; Lu, J.C.; Li, J.Y.; Liu, W.Y.; Yang, J.J.; Li, J.; Xiao, K. Phenolic compounds from the leaves of Cyclocarya paliurus (Batal.) Ijinskaja and their inhibitory activity against PTP1B. Food Chem. 2010, 119, 1491–1496. [Google Scholar] [CrossRef]
- Treutter, D. Biosynthesis of phenolic compounds and its regulation in apple. Plant Growth Regul. 2001, 34, 71–89. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defense mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Amaral, J.S.; Seabra, R.M.; Andrade, P.B.; Valentão, P.; Pereira, J.A.; Ferreres, F. Phenolic profile in the quality control of walnut (Juglans regia L.) leaves. Food Chem. 2004, 88, 373–379. [Google Scholar] [CrossRef]
- Radix, P.; Bastien, C.; Jay-Allemand, C.; Charlot, G.; Seigle-Murandi, F. The influence of soil nature on polyphenols in walnut tissues. A possible explanation of differences in the expression of walnut blight. Agronomie 1998, 18, 627–637. [Google Scholar] [CrossRef]
- Fang, S.Z.; Wang, J.Y.; Wei, Z.Y.; Zhu, Z.X. Methods to break seed dormancy in Cyclocarya paliurus (Batal.) Iljinskaja. Sci. Hortic. 2006, 110, 305–309. [Google Scholar] [CrossRef]
- Birari, R.B.; Bhutani, K.K. Pancreatic lipase inhibitors from natural source: unexplored potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef]
- Hou, X.L.; Liu, X.X.; Wang, S.; Zhou, X.L.; Wei, F.; Miao, J.H. Effect of the flavonoids from Cyclocarya paliurus on spontaneous hypertension rats. Pharm. Clin. Chin. Mater. Med. 2014, 30, 62–69. [Google Scholar]
- Kurihara, H.; Fukami, H.; Kusumoto, A.; Toyoda, Y.; Shibata, H.; Matsui, Y. Hypoglycemic action of Cyclocarya paliurus (Batal.) Iljinskaja in normal and diabetic mice. Biosci. Biotechnol. Biochem. 2003, 67, 877–880. [Google Scholar] [CrossRef]
- Wu, Z.F.; Gao, T.H.; Zhong, R.L.; Lin, Z.; Jiang, C.H.; Ouyang, S.; Zhao, M.; Che, C.T.; Zhang, J.; Yin, Z.Q. Antihyperlipidaemic effect of triterpenic acid-enriched fraction from Cyclocarya paliurus leaves in hyperlipidaemic rats. Pharm. Biol. 2017, 55, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, J.H.; Jia, S.; Huang, L.X.; Wang, Z.J.; Li, C.; Xie, M.Y. Immunomodulatory effects of an acetylated Cyclocarya paliurus polysaccharide on murine macrophages raw264.7. Int. J. Biol. Macromol. 2017, 98, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Liu, X.; Shen, M.Y.; Nie, S.P.; Zhang, H.; Li, C.; Gong, D.M.; Xie, M.Y. Purification, physicochemical characterization and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chem. 2013, 136, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Shen, M.Y.; Xie, M.Y.; Nie, S.P.; Chen, Y.; Li, C.; Huang, D.F.; Wang, Y.X. Ultrasonic-assisted extraction, antimicrobial and antioxidant activities of Cyclocarya paliurus (Batal.) Iljinskaja polysaccharides. Carbohydr. Polym. 2012, 89, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.N.; Fang, S.Z.; Yin, Z.Q.; Fu, X.X.; Shang, X.L.; Yang, W.X.; Yang, H.M. Chemical fingerprint and multicomponent quantitative analysis for the quality evaluation of Cyclocarya paliurus leaves by HPLC–Q–TOF–MS. Molecules 2017, 22, 1927. [Google Scholar] [CrossRef] [PubMed]
- Shu, R.G.; Xu, C.R.; Li, L.N.; Yu, Z.L. Cyclocariosides II and III: two secodammarane triterpenoid saponins from Cyclocarya paliurus. Planta Med. 1995, 61, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, E.J.; Cai, L.; Long, L.; Shamon, L.; Zaw, K.; Zhou, B.N.; Pezzuto, J.M.; Kinghorn, A.D. Novel highly sweet secodammarane glycosides from Pterocarya paliurus. J. Agric. Food Chem. 1995, 43, 2602–2607. [Google Scholar] [CrossRef]
- Xie, J.H.; Xie, M.Y.; Nie, S.P.; Shen, M.Y.; Wang, Y.X.; Li, C. Isolation, chemical composition and antioxidant activities of a watersoluble polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chem. 2010, 119, 1626–1632. [Google Scholar] [CrossRef]
- Deng, B.; Fang, S.Z.; Shang, X.L.; Fu, X.X.; Li, Y. Influence of provenance and shade on biomass production and triterpenoid accumulation in Cyclocarya paliurus. Agrofor. Syst. 2019, 93, 483–492. [Google Scholar] [CrossRef]
- Cao, Y.N.; Deng, B.; Fang, S.Z.; Shang, X.L.; Fu, X.X.; Yang, W.X. Genotypic variation in tree growth and selected flavonoids in leaves of Cyclocarya paliurus. South. For. 2018, 80, 67–74. [Google Scholar] [CrossRef]
- Liu, Z.; Carpenter, S.B.; Bourgeois, W.J.; Yu, Y.; Constantin, R.J.; Falcon, M.J.; Adams, J.C. Variations in the secondary metabolite camptothecin in relation to tissue age and season in Camptotheca acuminata. Tree Physiol. 1998, 18, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.T.; Cui, X.M.; Song, Z.H.; Zhao, K.J.; Ji, Z.N.; Lo, C.K.; Tsim, K.W. Chemical assessment of roots of Panax notoginseng in China: regional and seasonal variations in its active constituents. J. Agric. Food Chem. 2003, 51, 4617–4623. [Google Scholar] [CrossRef] [PubMed]
- González-Molina, E.; Moreno, D.A.; García-Viguera, C. Genotype and harvest time influence the phytochemical quality of Fino lemon juice (Citrus limon (L.) Burm. F.) for industrial use. J. Agric. Food Chem. 2008, 56, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.X.; Zhou, X.D.; Deng, B.; Shang, X.L.; Fang, S.Z. Seasonal and genotypic variation of water-soluble polysaccharide content in leaves of Cyclocarya paliurus. South. For. 2015, 77, 231–236. [Google Scholar] [CrossRef]
- Fang, S.Z.; Yang, W.X.; Chu, X.L.; Shang, X.L.; She, C.Q.; Fu, X.X. Provenance and temporal variations in selected flavonoids in leaves of Cyclocarya paliurus. Food Chem. 2011, 124, 1382–1386. [Google Scholar] [CrossRef]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: vaccinium, rubus, and ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Ryan, D.A.; Duy, J.C.; Prior, R.L.; Ehlenfeldt, M.K.; Kloet, S.P. Interspecific variation in anthocyanins, phenolics, and antioxidant capacity among genotypes of highbush and lowbush blueberries (Vaccinium section cyanococcus spp.). J. Agric. Food Chem. 2001, 49, 4761–4767. [Google Scholar] [CrossRef] [PubMed]
- Rühmann, S.; Leser, C.; Bannert, M.; Treutter, D. Relationship between growth, secondary metabolism, and resistance of apple. Plant Biol. 2002, 4, 137–143. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Lindroth, R.L.; Peterson, S.S. Effects of plant phenols of performance of southern armyworm larvae. Oecologia 1988, 75, 185–189. [Google Scholar] [CrossRef]
- Deng, B.; Shang, X.L.; Fang, S.Z.; Li, Q.Q.; Fu, X.X.; Su, J. Integrated effects of light intensity and fertilization on growth and flavonoid accumulation in Cyclocarya paliurus. J. Agric. Food Chem. 2012, 60, 6286–6292. [Google Scholar] [CrossRef] [PubMed]
- Tsormpatsidis, E.; Henbest, R.G.C.; Davis, F.J.; Battey, N.H.; Hadley, P.; Wagstaffe, A. UV irradiance as a major influence on growth, development and secondary products of commercial importance in Lollo Rosso lettuce ‘Revolution’ grown under polyethylene films. Environ. Exp. Bot. 2008, 63, 232–239. [Google Scholar] [CrossRef]
- Edreva, A. The importance of non-photosynthetic pigments and cinnamic acid derivatives in the photoprotection. Agric. Ecosyst. Environ. 2005, 106, 135–146. [Google Scholar] [CrossRef]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Aggati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef]
- Wang, L.J.; Su, S.; Wu, J.; Du, H.; Li, S.S.; Huo, J.W.; Zhang, Y.; Wang, L.S. Variation of anthocyanins and flavonols in Vaccinium uliginosum berry in lesser khingan mountains and its antioxidant activity. Food Chem. 2014, 160, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L.; Määttä-Riihinen, K.; Kärenlampi, S.; Hohtola, A. Activation of flavonoid biosynthesis by solar radiation in bilberry (Vaccinium myrtillus L.) leaves. Planta 2004, 218, 721–728. [Google Scholar] [PubMed]
- Reyes, L.F.; Miller, J.C.; Cisneros-Zevallos, L. Environmental conditions influence the content and yield of anthocyanins and total phenolics in purple- and red-flesh potatoes during tuber development. Am. J. Potato Res. 2004, 81, 187–193. [Google Scholar] [CrossRef]
- Klimov, S.V.; Burakhanova, E.A.; Dubinina, I.M.; Alieva, G.P.; Sal’nikova, E.B.; Olenichenko, N.A.; Zagoskina, N.V.; Trunova, T.I. Suppression of the source activity affects carbon distribution and frost hardiness of vegetating winter wheat plants. Russ. J. Plant Physiol. 2008, 55, 308–314. [Google Scholar] [CrossRef]
- Christie, P.J.; Alfenito, M.R.; Walbot, V. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: Enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 1994, 194, 541–549. [Google Scholar] [CrossRef]
- Leyva, A.; Jarillo, J.A.; Salinas, J.; Martinez-Zapater, J.M. Low-temperature induces the accumulation of Phenylalanine Ammonia-Lyase and Chalcone Synthase m-RNAs of Arabidopsis thaliana in a light-dependent manner. Plant Physiol. 1995, 108, 39–46. [Google Scholar] [CrossRef]
- Vogt, T.; Gul, P.G. Accumulation of flavonoids during leaf development in Citrus laurifolius. Phytochemistry 1994, 36, 591–597. [Google Scholar] [CrossRef]
- Crespo-Sempere, A.; Selma-Lázaro, C.; Palumbo, J.D.; González-Candelas, L.; Martínez-Culebras, P.V. Effect of oxidant stressors and phenolic antioxidants on the ochratoxigenic fungus Aspergillus carbonarius. J. Sci. Food Agric. 2016, 96, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Zhou, G.M.; Xu, S.Y.; Wu, J.S.; Yin, E.Q. Provenance variation in camptothecin concentrations of Camptotheca acuminata grown in China. New For. 2002, 24, 215–224. [Google Scholar] [CrossRef]
- Margna, U. Control at the level of substrate supply-an alternative in the regulation of phenylpropanoid accumulation in plant cells. Phytochemistry 1977, 16, 419–426. [Google Scholar] [CrossRef]
- Margna, U.; Margna, E.; VainjÄrv, T. Influence of nitrogen nutrition on the utilization of L-phenylalanine for building flavonoids in buckwheat seedling tissues. J. Plant Physiol. 1989, 134, 697–702. [Google Scholar] [CrossRef]
- Phillips, R.; Henshaw, G.G. The regulation of synthesis of phenolics in stationary phase cell cultures of Acer pseudoplatanus L. J. Exp. Bot. 1977, 28, 785–794. [Google Scholar] [CrossRef]
- Ma, M.; Hong, C.L.; An, S.Q.; Li, B. Seasonal, spatial, and interspecific variation in quercetin in Apocynum venetum and Poacynum hendersonii, Chinese traditional herbal teas. J. Agric. Food Chem. 2003, 51, 2390–2393. [Google Scholar] [CrossRef]
- Hofmann, R.W.; Swinny, E.E.; Bloor, S.J.; Markham, K.R.; Ryan, K.G.; Campbell, B.D.; Jordan, B.R.; Fountain, D.W. Responses of nine Trifolium repens L. populations to ultraviolet-B radiation: Differential flavonol glycoside accumulation and biomass production. Ann. Bot. 2000, 86, 527–537. [Google Scholar] [CrossRef]
- Schmidt, S.; Zietz, M.; Schreiner, M.; Rohn, S.; Kroh, L.W.; Krumbein, A. Genotypic and climatic influence on the concentration and composition of flavonoids in kales (Brassica oleracea var. sabellica). Food Chem. 2010, 119, 1293–1299. [Google Scholar] [CrossRef]
- André, C.M.; Oufir, M.; Hoffmann, L.; Hausman, J.F.; Rogez, H.; Larondelle, Y.; Evers, D. Influence of environment and genotype on polyphenol compounds and vitro antioxidant capacity of native Andean potatoes (Solanum tuberosum L.). J. Food Compos. Anal. 2009, 22, 517–524. [Google Scholar] [CrossRef]
- Zhang, W.N.; Zhao, X.Y.; Sun, C.D.; Li, X.; Chen, K.S. Phenolic composition from different loquat, (Eriobotrya japonica Lindl.) cultivars grown in China, and their antioxidant properties. Molecules 2015, 20, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Goodner, K.L.; Park, J.D.; Choi, J.; Talcott, S.T. Changes in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentation. Food Chem. 2011, 129, 1331–1342. [Google Scholar] [CrossRef]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Cao, G.H.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure–activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef]
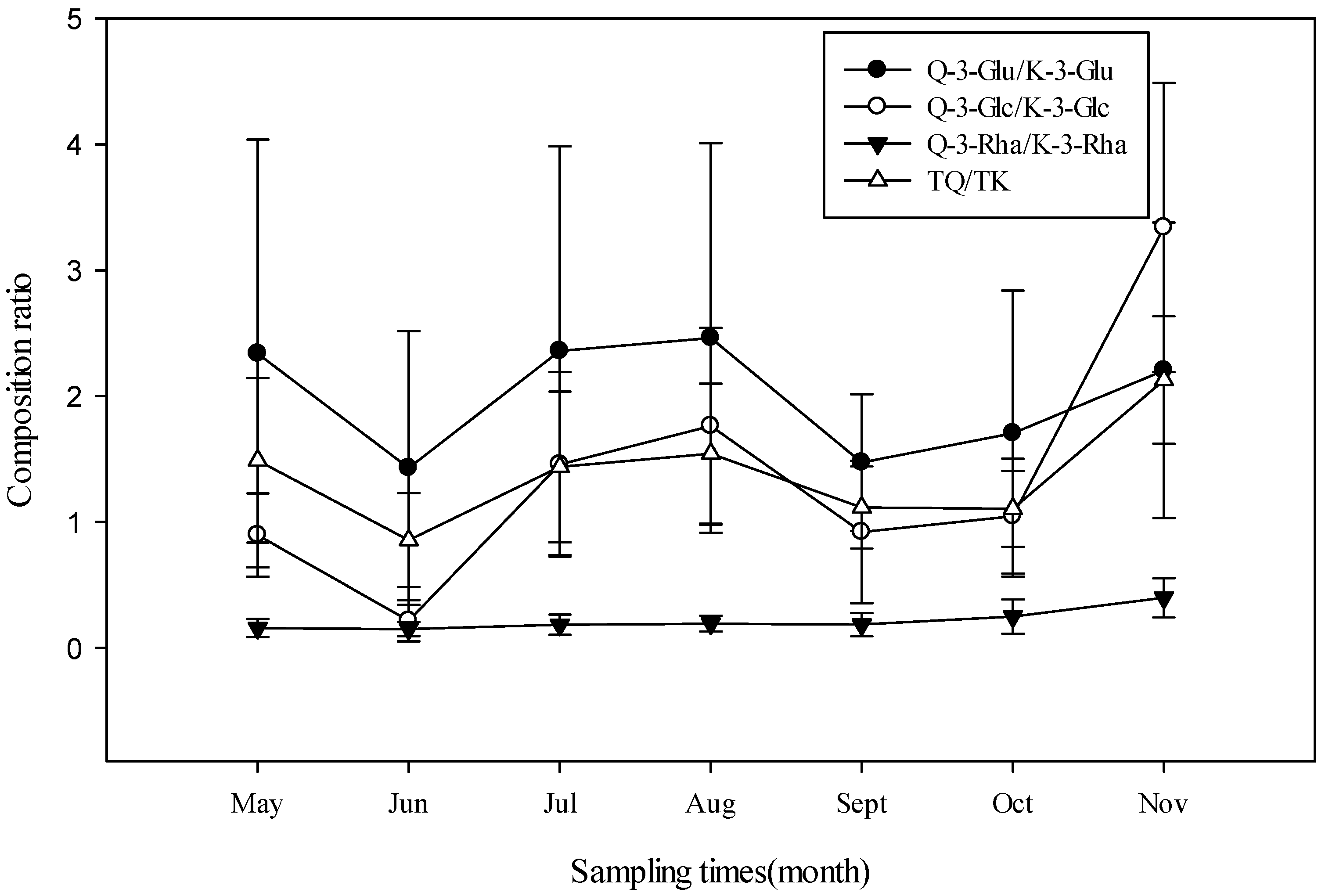
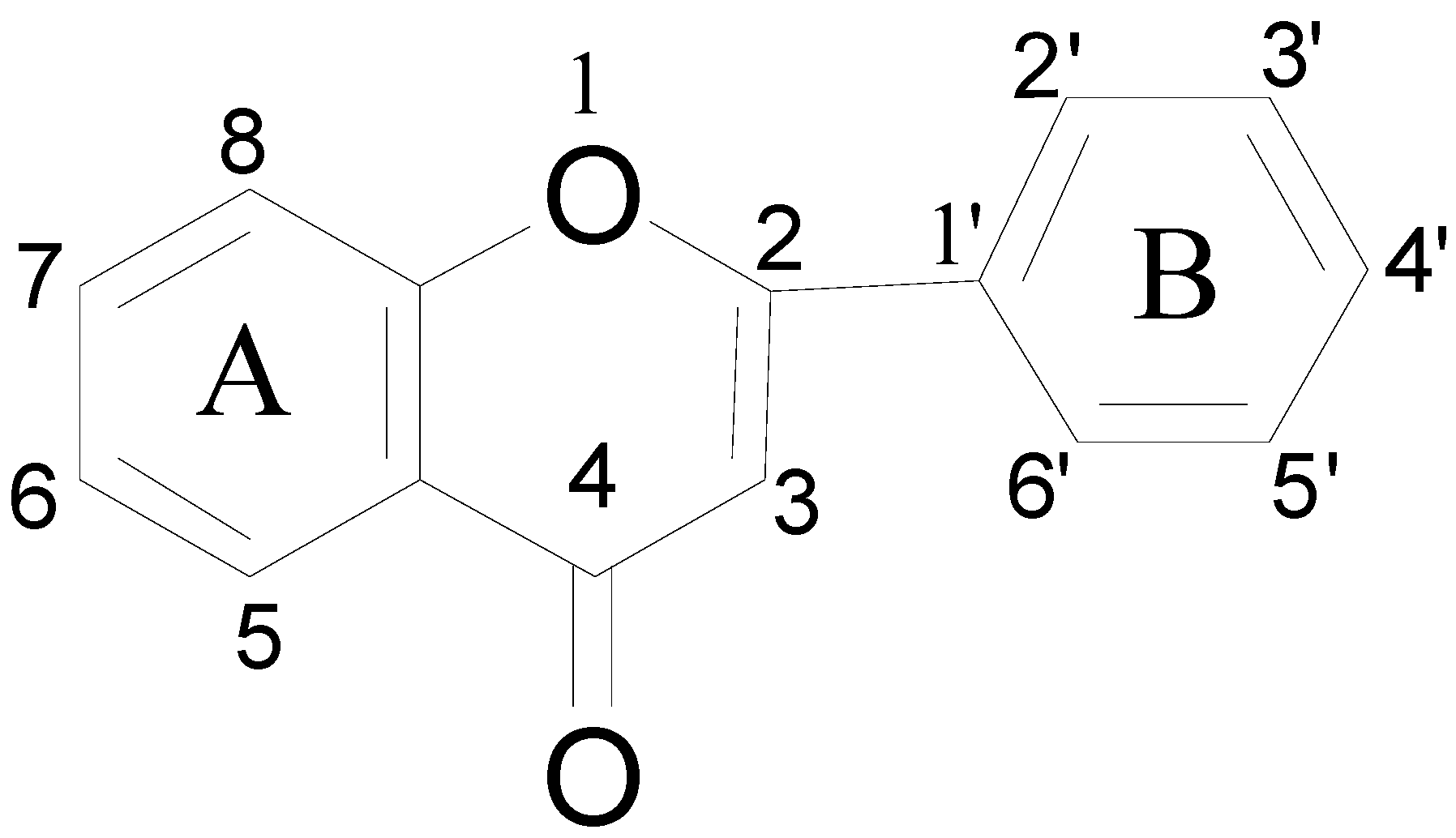
| Compounds | DPPH | FRAP | ABTS |
|---|---|---|---|
| Total phenolic aids | 0.71 * | 0.80 * | 0.85 ** |
| Total quercetin glycosides | 0.73 * | 0.86 ** | 0.91 ** |
| Total kaempferol glycosides | 0.6 | 0.76 * | 0.74 * |
| Total flavonoids | 0.7 | 0.85 ** | 0.90 ** |
| Compounds | DPPH (IC50 mg/mL) | FRAP (mmol TEAC/g) | ABTS (mmol TEAC/g) |
|---|---|---|---|
| 3-O-caffeoylquinic acid | 0.24 ± 0.003 | 3.51 ± 0.02 | 4.58 ± 0.03 |
| 4-O-caffeoylquinic acid | 0.17 ± 0.001 | 3.38 ± 0.06 | 4.91 ± 0.07 |
| 4,5-di-O-caffeoylquinic acid | 0.24 ± 0.002 | 4.70 ± 0.04 | 5.13 ± 0.03 |
| Quercetin-3-O-glucuronide | 0. 12 ± 0.000 | 3.77 ± 0.02 | 4.44 ± 0.04 |
| Quercetin-3-O-galactoside | 0.13 ± 0.002 | 3.33 ± 0.01 | 4.41 ± 0.01 |
| Quercetin-3-O-glucoside | 0.13 ± 0.002 | 2.80 ± 0.03 | 4.27 ± 0.06 |
| Quercetin-3-O-rhamnoside | 0.13 ± 0.000 | 2.65 ± 0.02 | 4.39 ± 0.06 |
| Kaempferol-3-O-glucuronide | inactive | 0.22 ± 0.00 | 0.55 ± 0.01 |
| Kaempferol-3-O-glucoside | inactive | 0.30 ± 0.0 | 0.57 ± 0.00 |
| Kaempferol-3-O-rhamnoside | inactive | 0.30 ± 0.03 | 0.68 ± 0.04 |
| Quercetin | 0.008 ± 0.000 | 11.25 ± 0.24 | 6.37 ± 0.21 |
| Kaempferol | 0. 26 ± 0.002 | 3.36 ± 0.05 | 3.67 ± 0.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Fang, S.; Fu, X.; Shang, X.; Yang, W. Seasonal Variation in Phenolic Compounds and Antioxidant Activity in Leaves of Cyclocarya paliurus (Batal.) Iljinskaja. Forests 2019, 10, 624. https://doi.org/10.3390/f10080624
Cao Y, Fang S, Fu X, Shang X, Yang W. Seasonal Variation in Phenolic Compounds and Antioxidant Activity in Leaves of Cyclocarya paliurus (Batal.) Iljinskaja. Forests. 2019; 10(8):624. https://doi.org/10.3390/f10080624
Chicago/Turabian StyleCao, Yanni, Shengzuo Fang, Xiangxiang Fu, Xulan Shang, and Wanxia Yang. 2019. "Seasonal Variation in Phenolic Compounds and Antioxidant Activity in Leaves of Cyclocarya paliurus (Batal.) Iljinskaja" Forests 10, no. 8: 624. https://doi.org/10.3390/f10080624
APA StyleCao, Y., Fang, S., Fu, X., Shang, X., & Yang, W. (2019). Seasonal Variation in Phenolic Compounds and Antioxidant Activity in Leaves of Cyclocarya paliurus (Batal.) Iljinskaja. Forests, 10(8), 624. https://doi.org/10.3390/f10080624






