Linking Dendrometry and Dendrochronology in the Dominant Azorean Tree Laurus azorica (Seub.) Franco
Abstract
1. Introduction
2. Materials and Methods
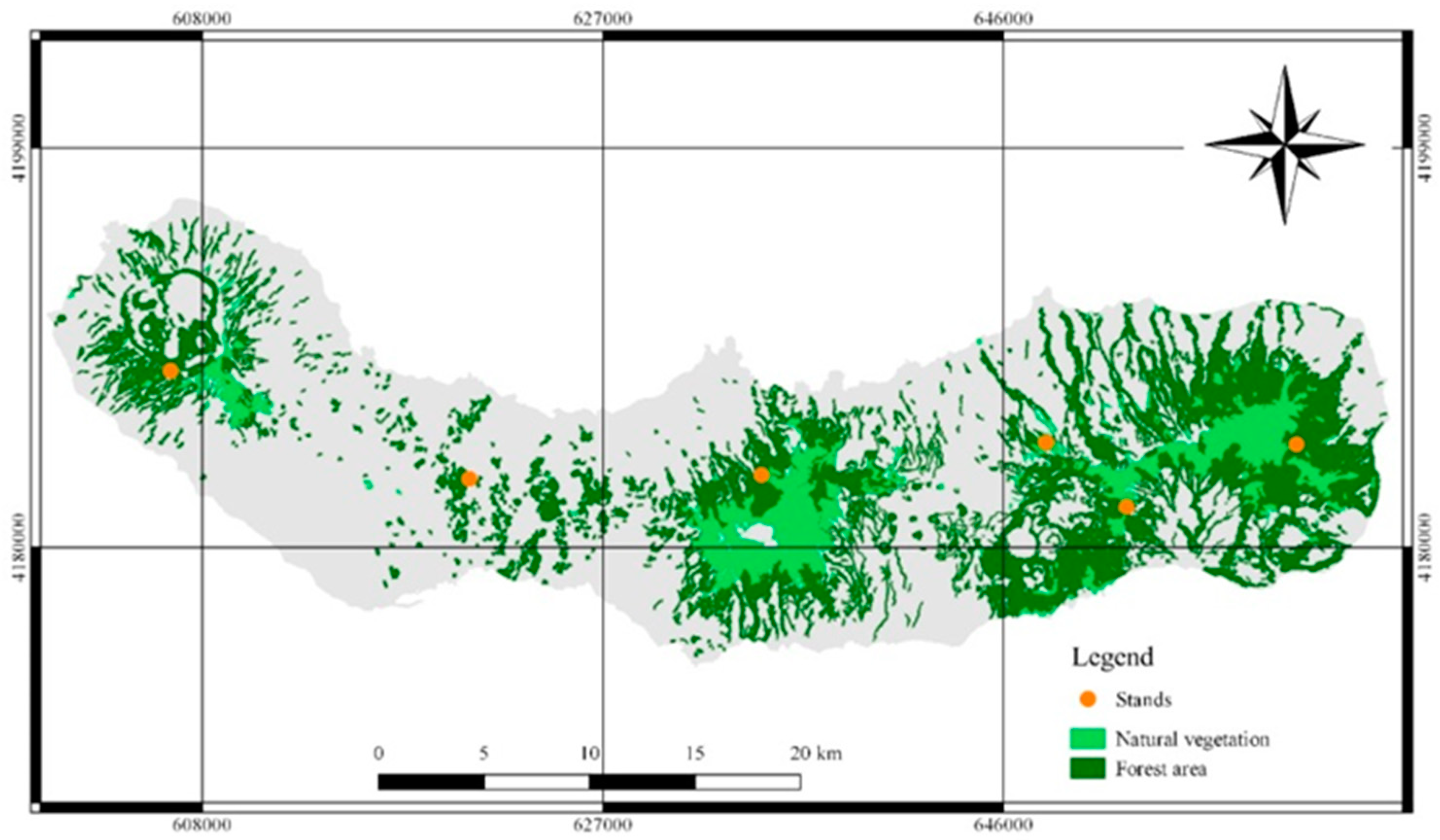
2.1. Study Area
2.2. Target Species
2.3. Stand Characterization
2.4. Field Sampling
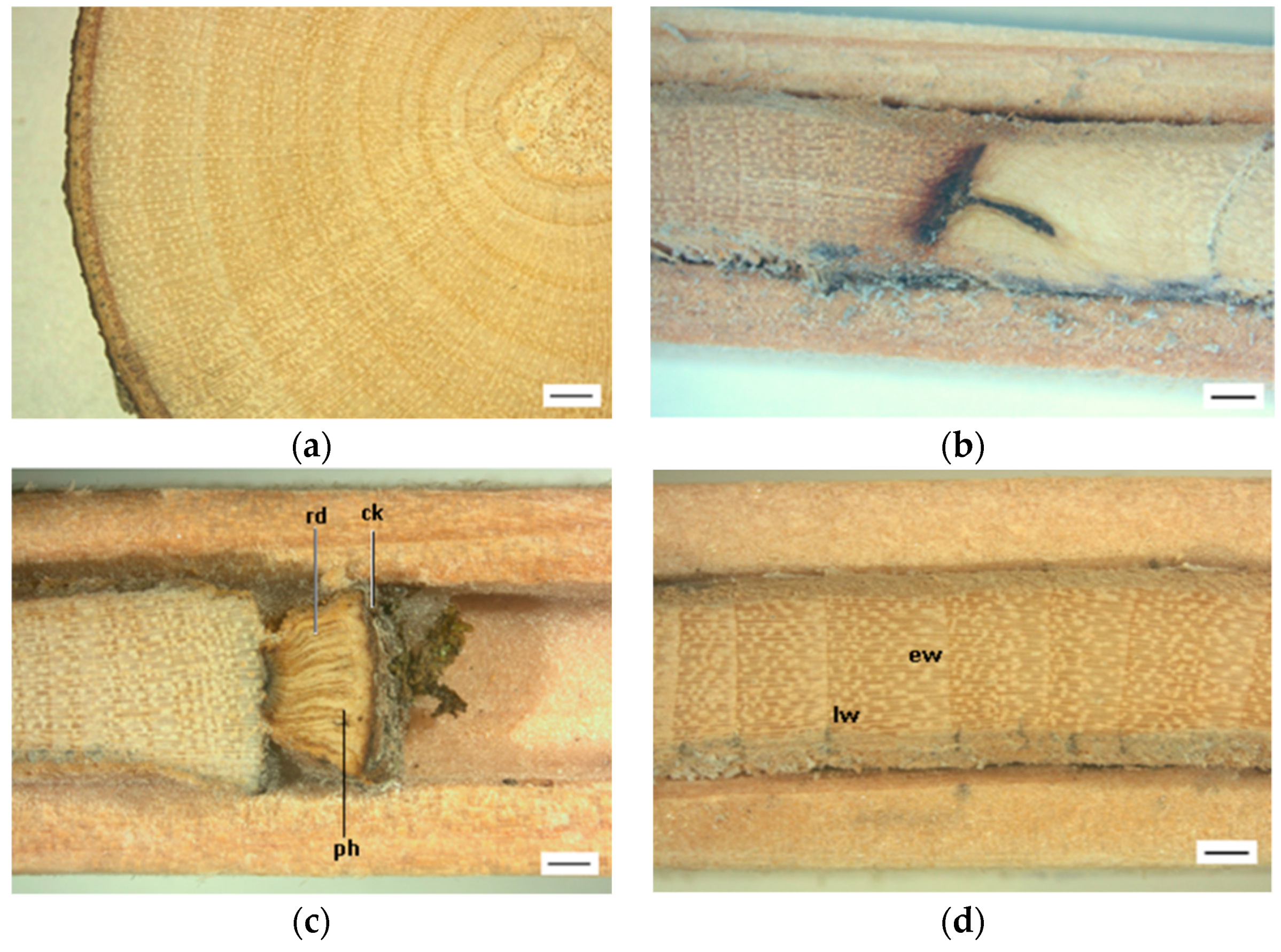
2.5. Wood Sample Preparation for Macroscopic Analysis
2.6. Wood Sample Preparation for Microscopic Analysis
2.7. Statistical Analyses
2.7.1. Dendrometric Traits
2.7.2. Annual Increment
2.7.3. Relationship between Tree Age and Dendrometric Traits
3. Results
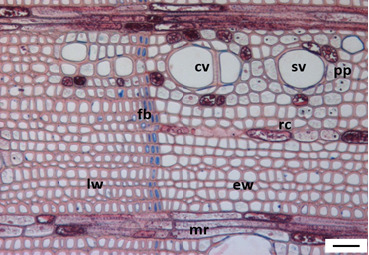
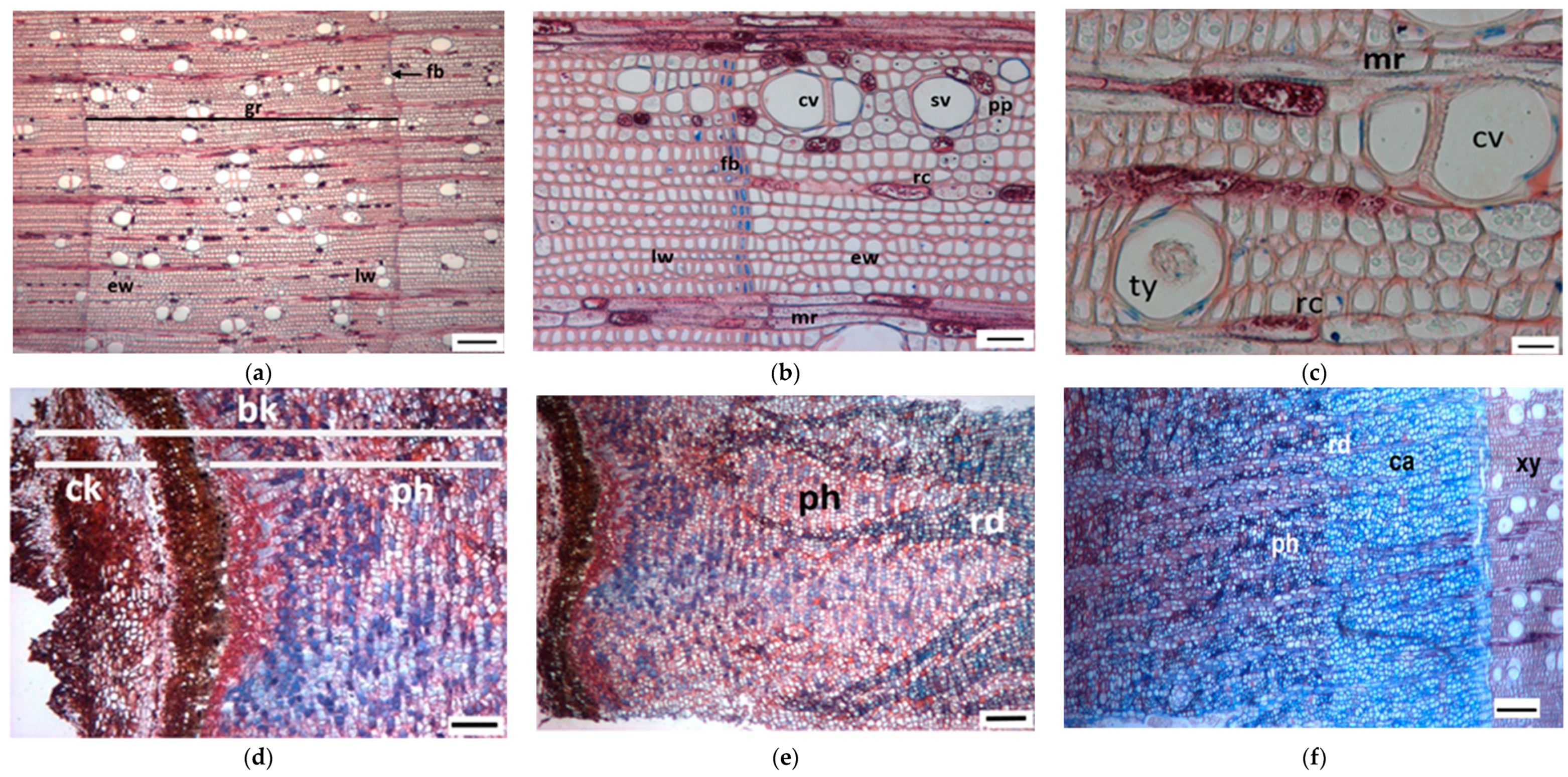
3.1. Tree Ring Structure
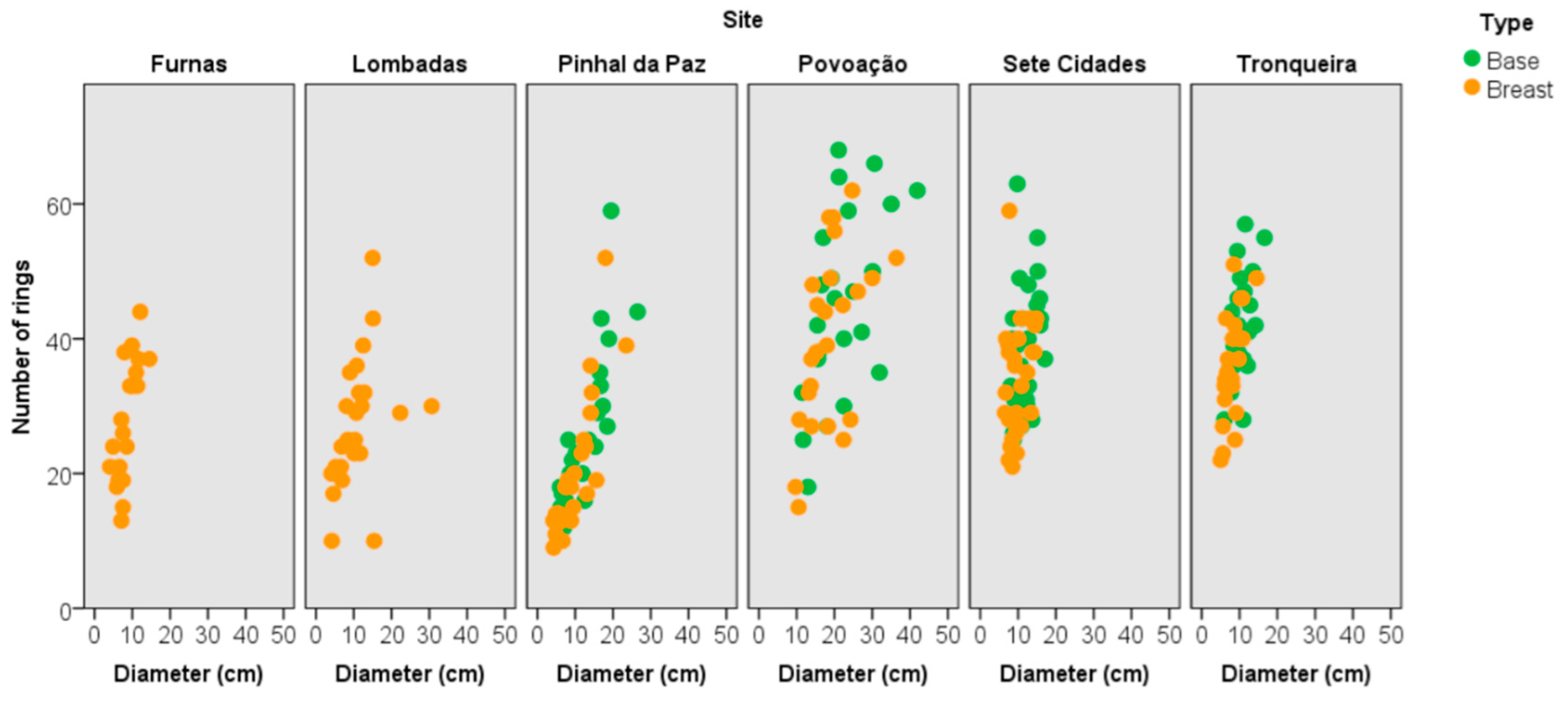
3.2. Dendrometric Traits
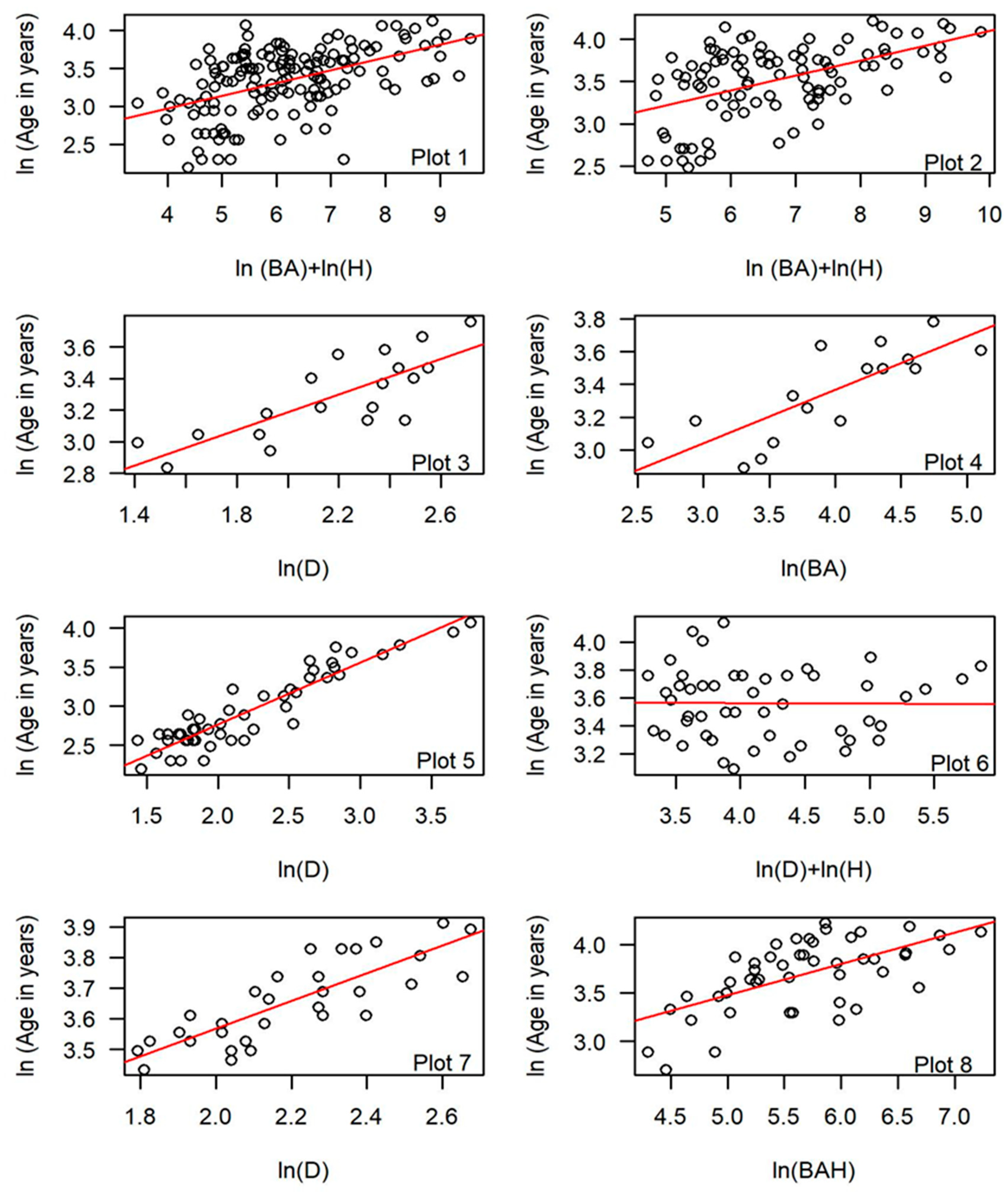
3.3. Tree Age and Dendrometric Traits
3.4. Estimated Annual Increment
4. Discussion
4.1. Laurel Forest in São Miguel Island
4.2. Growth Ring Anatomy
4.3. Dendrometry and Dendrochronology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grebner, D.L.; Bettinger, P.; Siry, J.P. Introduction to Forestry and Natural Resources; Academic Press: Cambridge, MA, USA, 2013; p. 496. [Google Scholar]
- Borges Silva, L.; Teixeira, A.; Alves, M.; Elias, R.B.; Silva, L. Tree age determination in the widespread woody plant invader Pittosporum undulatum. For. Ecol. Manage. 2017, 400, 457–467. [Google Scholar] [CrossRef]
- Borges Silva, L.; Lourenço, P.; Bicudo, N.; Alves, M.; Elias, R.B.; Medeiros, V.; Silva, L. Development of Allometric Equations for Estimating Above-Ground Biomass of Woody Plant Invaders: The Case of Pittosporum undulatum in the Azores Archipelago. In International Conference on Dynamics, Games and Science; Springer: Cham, Switzerland, 2014; pp. 463–484. [Google Scholar]
- Borges Silva, L.; Lourenço, P.; Teixeira, A.; Azevedo, E.B.; Alves, M.; Elias, R.B.; Silva, L. Biomass valorization in the management of woody plant invaders: The case of Pittosporum undulatum in the Azores. Biomass Bioenergy 2018, 109, 155–165. [Google Scholar] [CrossRef]
- Speer, J. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 2010. [Google Scholar]
- Barry, D. Refining Dendrochronology to Evaluate the Relationship between Age and Diameter for Dominant Riparian Trees in the Redwood Creek Watershed. Master’s Thesis, University of San Francisco, San Francisco, CA, USA, 2014. Available online: https://repository.usfca.edu/capstone/27 (accessed on 1 April 2017).
- Fraver, S.; Bradford, J.B.; Palik, B.J. Improving tree age estimates derived from increment cores: A case study of red pine. For. Sci. 2011, 57, 164–170. [Google Scholar]
- Maxwell, R.S.; Wixom, J.A.; Hessl, A.E. A comparison of two techniques for measuring and crossdating tree rings. Dendrochronologia 2011, 29, 237–243. [Google Scholar] [CrossRef]
- Dias, E.; Elias, R.B.; Melo, C.; Mendes, C. O elemento insular na estruturação das florestas da Macaronésia. Açores e Madeira–A floresta das ilhas 2007, 6, 15–48. [Google Scholar]
- Elias, R.B.; Dias, E. Ecologia das florestas de Juniperus dos Açores. Cadernos de Botânica n° 5; Herbário da Universidade dos Açores, Angra do Heroísmo: Azores, Portugal, 2008. [Google Scholar]
- Silva, L.; Moura, M.; Schaefer, H.; Rumsey, F.; Dias, E.F. Vascular Plants (Tracheobionta). In A List of the Terrestrial and Marine Biota from the Azores; Borges, P.A.V., Costa, A., Cunha, R., Gabriel, R., Gonçalves, V., Martins, A.F., Melo, I., Parente, M., Raposeiro, P., Rodrigues, P., et al., Eds.; Princípia: Cascais, Portugal, 2010; pp. 117–146. [Google Scholar]
- Elias, R.B.; Gil, A.; Silva, L.; Fernández-Palacios, J.M.; Azevedo, E.B.; Reis, F. Natural zonal vegetation of the Azores Islands: Characterization and potential distribution. Phytocoenologia 2016, 46, 107–123. [Google Scholar] [CrossRef]
- Borges, P.A.V.; Azevedo, E.B.; Borba, A.; Francisco, O.D.; Gabriel, R.; Silva, E. Ilhas Oceânicas. In Ecossistemas e Bem Estar Humano: Avaliação para Portugal do Millennium Ecossystem Assessment; Pereira, H.M., Domingos, T., Vicente, L., Eds.; Escolar Editora: Lisboa, Portugal, 2009; pp. 463–510. [Google Scholar]
- Ferreira, M.T.; Cardoso, P.; Borges, P.A.V.; Gabriel, R.; de Azevedo, E.B.; Reis, F.; Araújo, M.B.; Elias, R.B. Effects of climate change on the distribution of indigenous species in oceanic islands (Azores). Clim. Chang. 2016, 138, 603–615. [Google Scholar] [CrossRef]
- Silva, L.; Smith, C. A quantitative approach to the study of non-indigenous plants: An example from the Azores Archipelago. Biodivers. Conserv. 2006, 15, 1661–1679. [Google Scholar] [CrossRef]
- Silva, L.; Martins, M.; Maciel, G.; Moura, M. Flora Vascular dos Açores. Prioridades em Conservação. Azorean Vascular Flora. Priorities in Conservation; Amigos dos Açores & CCPA: Ponta Delgada, Portugal, 2009; p. 116. [Google Scholar]
- Costa, H.; Bettencourt, M.J.; Silva, C.M.N.; Teodósio, J.; Gil, A.; Silva, L. Invasive Alien Plants in the Azorean Protected Areas: Invasion Status and Mitigation Actions. In Plant Invasions in Protected Areas; Springer: Dordrecht, The Netherlands, 2013; pp. 375–394. [Google Scholar] [CrossRef]
- DRRF. Plano de Gestão Florestal do Perímetro Florestal e Matas Regionais da Ilha de São Miguel; Secretaria Regional da Agricultura e Florestas, Região Autónoma dos Açores: Ponta Delgada, Portugal, 2017. [Google Scholar]
- Silva, L.; Beech, E. Laurus Azorica. The IUCN Red List of Threatened Species 2017: e. T38397A81868030; IUCN Global Species Programme Red List Unit: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Viera, J.; Campelo, F.; Nabais, C. Dendrochronology of maritime pine in the middle of the Atlantic Ocean. Dendrochronologia 2017, 45, 73–80. [Google Scholar] [CrossRef]
- Morales, D.; Jiménez, M.S.; González-Rodríguez, A.M.; Cermák, J. Laurel forests in Tenerife, Canary islands: I Xylem structure in stems and petioles of Laurus azorica trees. Trees 2002, 16, 529–537. [Google Scholar] [CrossRef]
- Gaspar, C.; Borges, P.A.V.; Gaston, K.J. Diversity and distribution of arthropods in native forests of the Azores archipelago. Arquipélago. Life Mar. Sci. 2008, 25, 1–30. [Google Scholar]
- Rivas-Martínez, S.; Díaz, T.E.; Fernández-González, F.; Izco, J.; Loidi, J.; Lousã, M.; Penas, A. Vascular plant communities of Spain and Portugal: Addenda to the syntaxonomical checklist of 2001. Itinera Geobotanica 2002, 15, 5–922. [Google Scholar]
- Rodríguez-Sánchez, F.; Guzmán, B.; Alfredo Valido, A.; Vargas, P.; Juan Arroyo, J. Late Neogene history of the laurel tree (Laurus L., Lauraceae) based on phylogeographical analyses of Mediterranean and Macaronesian populations. J. Biogeog. 2009, 36, 1270–1281. [Google Scholar] [CrossRef]
- Moura, M.; Vieira, A.; Silva, L. Determination of the Possible Presence of Hybrids of Ilex azorica and Laurus azorica with Congener Exotic Taxa in the Target Area of the Project “Terras do Priolo”—LIFE12 NAT/PT/000527; Final Report; InBIO, CIBIO-Açores, Departamento de Biologia, Universidade dos Açores: Ponta Delgada, Portugal, 2015; p. 15. [Google Scholar]
- Vieira, A.F.; Moura, M.; Silva, C.; Silva, L. Screening for potential hybrids reveals new insights about Laurus phylogeography in the Azores. In Conference Program and Abstracts of the 2nd International Conference on Island Evolution, Ecology and Conservation: Island Biology 2016, 18–22 July 2016, Angra do Heroísmo, Azores, Portugal. Arquipelago. Life and Marine Sciences; Gabriel, R., Elias, R.B., Amorim, I.R., Borges, P.A.V., Eds.; University of the Azores: Ponta Delgada, Portugal, 2016; p. 505. [Google Scholar]
- Câmara, M. Estimativa das idades das árvores numa floresta natural invadida na Ilha de São Miguel, Açores. Master’s Thesis, Departamento de Biologia, Universidade dos Açores, Ponta Delgada, Portugal, 2016; p. 23. Available online: https://repositorio.uac.pt/handle/10400.3/4042 (accessed on 12 June 2018).
- Rego, R.; Borges Silva, L.; Medeiros, F.; Porteiro, J.; Silva, L. Ecological Characterization as the First Step Towards the Conservation of Natural Unprotected Areas: A case Study in the Azores. In Proceedings of the European Meeting of Phytosociology, Biogeography and Syntaxonomy of the Atlantic Regions, University of Cape Verde, Praia, Cape Verde, 5–7 November 2017. [Google Scholar]
- Cermák, J.; Jiménez, M.S.; González-Rodríguez, A.M.; Morales, D. Laurel forests in Tenerife, Canary Islands. II. Efficiency of the water conducting system in Laurus azorica trees. Trees 2002, 16, 538–546. [Google Scholar] [CrossRef]
- Reis-Avila, G.; Oliveira, J.M. Lauraceae: A promising family for the advance of neotropical dendrochronology. Dendrochronologia 2017, 44, 103–116. [Google Scholar] [CrossRef]
- Forjaz, V.H.; Tavares, J.M.; Azevedo, E.M.V.B.; Nunes, J.C.; Santos, R.S.; Barreiros, J.P.; Gallagher, L.; Barcelos, P.J.M.; Silva, P.H.; Cardigos, F.; et al. Atlas Básico dos Açores; Forjaz, V.H., Ed.; Observatório Vulcanológico dos Açores: Ponta Delgada, Portugal, 2014; p. 112. [Google Scholar]
- Azevedo, E. Condicionantes dinâmicas do clima do Arquipélago dos Açores. Elementos para o seu estudo. Açoreana 2001, 9, 309–317. [Google Scholar]
- Monteiro, R.; Furtado, S.; Rocha, M.; Freitas, M.; Medeiros, R.; Cruz, J.V. O Ordenamento do Território nos Açores: Política e Instrumentos; Secretaria Regional do Ambiente e do Mar, Direcção Regional do Ordenamento do Território e dos Recursos Hídricos: Ponta Delgada, Portugal, 2008. [Google Scholar]
- Lourenço, P.; Medeiros, V.; Gil, A.; Silva, L. Distribution habitat and biomass of Pittosporum undulatum, the most important woody plant invader in the Azores Archipelago. For. Ecol. Manage. 2011, 262, 178–187. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Ricardo, R.P.; Madeira, M.V.; Medina, J.M.B.; Marques, M.M.; Furtado, A.F.S. Esboço pedológico da Ilha de S. Miguel (Açores). An. Inst. Sup. Agron. 1977, 37, 275–385. [Google Scholar]
- APG. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Renner, S.S. Circumscription and phylogeny of the Laurales: Evidence from molecular and morphological data. Am. J. Bot. 1999, 86, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Rohwer, J.G. Toward a phylogenetic classification of the Lauraceae: Evidence from matK sequences. Syst. Botany. 2000, 25, 60–71. [Google Scholar] [CrossRef]
- Chanderbali, A.S.; van der Werff, H.; Renner, S.S. Phylogeny and historical biogeography of Lauraceae: Evidence from the chloroplast and nuclear genomes. Ann. Mo. Bot. Gard. 2001, 88, 104–134. [Google Scholar] [CrossRef]
- Rohwer, J.G.; Rudolph, B. Jumping genera: The phylogenetic positions of Cassytha, Hypodaphnis, and Neocinnamomum (Lauraceae) based on different analyses of trnK intron sequences. Ann. Mo. Bot. Gard. 2005, 92, 153–178. [Google Scholar]
- Quinet, A.; Andreata, H.R.P. Lauraceae Jussieu na reserva ecológica de Macaéde Cima, município de Nova Friburgo, Rio de Janeiro, Brasil. Rodriguésia 2002, 53, 59–121. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; Arévalo, J.R.; Balguerías, E.; Barone, R.; de Nascimento, L.; Elias, R.B.; Delgado, J.D.; Fernández-Lugo, S.; Méndez, J.; Naranjo Cigala, A.; et al. La Laurisilva. In Canarias, Madeira y Azores; Macaronesia Editorial: Santa Cruz de Tenerife, Spain, 2017; p. 420. [Google Scholar]
- Vieira, C.M.; Vaz, A.M.S.F.; Lima, H.C. Espécies de Interesse Conservacionista na Reserva Ecológica de Macaé de Cima. In Serra de Macaé de Cima: Diversidade Florística e Conservação; Lima, H.C., Guedes-Bruni, R.R., Eds.; Mata Atlântica, Instituto de Pesquisas Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 1997; pp. 297–305. [Google Scholar]
- Marques, C.A. Importância Econômica da Família Lauraceae. Floresta e Ambiente 2001, 8, 195–206. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2017.3. Available online: www.iucnredlist.org (accessed on 7 April 2018).
- Köhl, M.; Magnussen, S.S.; Marchetti, M. Sampling Methods, Remote Sensing and GIS Multiresource Forest Inventory. In Tropical Forestry; Springer: Berlin, Germany, 2006; p. 373. [Google Scholar]
- Rossi, S.; Anfodillo, T.; Menardi, R. Trephor: A new tool for sampling microcores from tree stems. IAWA J. 2006, 27, 89–97. [Google Scholar] [CrossRef]
- Worbes, M. Tree-Ring Analysis; Elsevier Ltd.: Amsterdam, The Netherlands, 2004. [Google Scholar] [CrossRef]
- Prislan, P.; Gričar, J.; Čufar, K. Wood Sample Preparation for Microscopic Analysis. STREeSS COST 2014, 8. [Google Scholar]
- Van der Werf, G.W.; Sass-Klaassen, U.; Mohren, G.M.J. The impact of the 2003 summer drought on the intra-annual growth pattern of beech (Fagus sylvatica L.) and oak (Quercus robur L.) on a dry site in the Netherlands. Dendrochronologia 2007, 25, 103–112. [Google Scholar] [CrossRef]
- Bettinger, P.; Boston, K.; Siry, J.P.; Grebne, L.D. Forest Management and Planning; Academic Press: Cambridge, MA, USA, 2017; Chapter 2; p. 362. [Google Scholar]
- Semenzato, P.; Cattaneo, D.; Dainese, M. Growth prediction for five tree species in an Italian urban forest. Urban For. Urban Green. 2011, 10, 169–176. [Google Scholar] [CrossRef]
- Troxel, B.; Piana, M.; Ashton, M.S.; Murphy-Dunning, C. Relationships between bole and crown size for young urban trees in the northeastern USA. Urban For. Urban Green. 2013, 12, 144–153. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Silva, L.; Ojeda-Land, E.; Rodriguez-Luengo, J.L. (Eds.) Invasive Terrestrial Flora and Fauna of Macaronesia. Top 100 in Azores; Madeira and Canaries, ARENA: Ponta Delgada, Portugal, 2008; p. 546. [Google Scholar]
- Caviedes, J.; Ibarra, J.T. Influence of Anthropogenic Disturbances on Stand Structural Complexity in Andean Temperate Forests: Implications for Managing Key Habitat for Biodiversity. PLoS ONE 2017, 12, e0169450. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.B.; Dias, E. Gap Dynamics and regeneration strategies in Juniperus-Laurus forests of the Azores Islands. Plant. Ecol. 2009, 200, 179–189. [Google Scholar] [CrossRef]
- Shishov, V.V.; Tychkov, I.I.; Popkova, M.I.; Ilyin, V.A.; Bryukhanova, M.V.; Kirdyanov, A.V. VS-oscilloscope: A new tool to parameterize tree radial growth based on climate conditions. Dendrochronologia 2016, 39, 42–50. [Google Scholar] [CrossRef]
- Schweingruber, F.H. Anatomy of European Woods; Verlag Paul Haupt: Bern, Switzerland, 1990. [Google Scholar]
- Schweingruber, F.H.; Börner, A.; Schulze, E.-D. Atlas of Stem Anatomy in Herbs, Shrubs and Trees; Springer-Verlag: Berlin, Germany, 2011; Volume 1, p. 495. [Google Scholar]
- Albuquerque, A.R.; Castro, V.R.; Lobão, M.S.; Sarto, C.; Tomazello Filho, M.; Guedes, F.T.P. Comparative analysis of anatomy and micro-densitometry of the growth rings of hardwoods and conifers, with emphasis on dendrochronology. Sci. For. 2016, 44, 595–610. [Google Scholar] [CrossRef]
- Kraus, J.E.; de Sousa, H.C.; Rezende, M.H.; Castro, N.M.; Vecchi, C.; Luque, R. Astra Blue and Basic Fuchsin Double Staining of Plant Materials. Biotech. Histochem. 1998, 73, 235–243. [Google Scholar] [CrossRef]
- Shestakova, T.A.; Gutiérrez, E.; Kirdyanov, A.V.; Camarero, J.J.; Génova, M.; Knorre, A.A.; Linares, J.C.; Resco de Dios, V.; Sánchez-Salguero, R.; Voltas, J. Forests synchronize their growth in contrasting Eurasian regions in response to climate warming. Proc. Natl. Acad. Sci. USA 2016, 113, 662–667. [Google Scholar] [CrossRef]
- Morris, H.E.R. The Structure and Function of Ray and Axial Parenchyma in Woody Seed Plants. Master’s Thesis, Ulm University, Ulm, Germany, 2016; p. 171. [Google Scholar] [CrossRef]
- Schöngart, J.; Arieira, J.; Fortes, C.F.; Cezarine de Arruda, E.; Nunes da Cunha, C. Age-related and stand-wise estimates of carbon stocks and sequestration in the aboveground coarse wood biomass of wetland forests in the northern Pantanal, Brazil. Biogeosciences 2011, 8, 3407–3421. [Google Scholar] [CrossRef]
- McPherson, E.; van Doorn, G.; Natalie, S.; Paula, J. Urban Tree Database and Allometric Equations; Gen. Tech. Rep. PSW-GTR-235; Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2016; p. 86. [Google Scholar] [CrossRef]
- Diallo, A.; Agbangba, E.C.; Ndiaye, O.; Guisse, A. Ecological Structure and Prediction Equations for Estimating Tree Age, and Dendometric Parameters of Acacia senegal in the Senegalese Semi-Arid Zone—Ferlo. Am. J. Plant Sci. 2013, 4, 1046–1053. [Google Scholar] [CrossRef]
- Gonzalez-Benecke, C.A.; Flamenco, H.N.; Wightman, M.G. Effect of Vegetation Management and Site Conditions on Volume, Biomass and Leaf Area Allometry of Four Coniferous Species in the Pacific Northwest United States. Forests 2018, 9, 581. [Google Scholar] [CrossRef]
- Yang, B.; Xue, W.; Yu, S.; Zhou, J.; Zhang, W. Effects of Stand Age on Biomass Allocation and Allometry of Quercus Acutissima in the Central Loess Plateau of China. Forests 2019, 10, 41. [Google Scholar] [CrossRef]
- Gonçalves, G.V. Dendrocronologia: Princípios teóricos, problemas práticos e aplicabilidade. CIDEHUS. Universidade de Evora, PT. Consultado julho 2012, 23. [Google Scholar]
- Tinker, D.G.; Stakes, K.A. Allometric equation development, biomass and aboveground productivity in ponderosa pine forests Black Hill, Wyoming. West J. Appl. For. 2010, 25, 112–119. [Google Scholar]
- Vahedi, A.A.; Mataji, A.; Babayi-Kafaki, S.; Eshaghi-Rad, J.; Hodjati, S.M.; Djomo, A. Allometric equations for predicting aboveground biomass of beech-hornbeam stands in the Hyrcanian forests of Iran. JFS 2014, 60, 236–247. [Google Scholar] [CrossRef]
- Sillett, S.C.; Van Pelt, R.; Koch, G.W.; Ambrose, A.R.; Carroll, A.L.; Antoine, M.E.; Mifsud, B.M. Increasing wood production through old age in tall trees. For. Ecol. Manag. 2010, 259, 976–994. [Google Scholar] [CrossRef]
- Schuster, R.; Oberhuber, W. Age-dependent climate-growth relationships and regeneration of Picea abies in a drought-prone mixed coniferous forest in the Alps. Can. J. For. Res. 2013, 43, 609–618. [Google Scholar] [CrossRef]
- Hess, A.F.; Loiola, T.; Souza, I.A.; Minatti, M.; Ricken, P.; Borsoi, G.A. Forest management for the conservation of Araucaria angustifolia in southern brazil. Floresta 2018, 48, 373–382. [Google Scholar] [CrossRef]
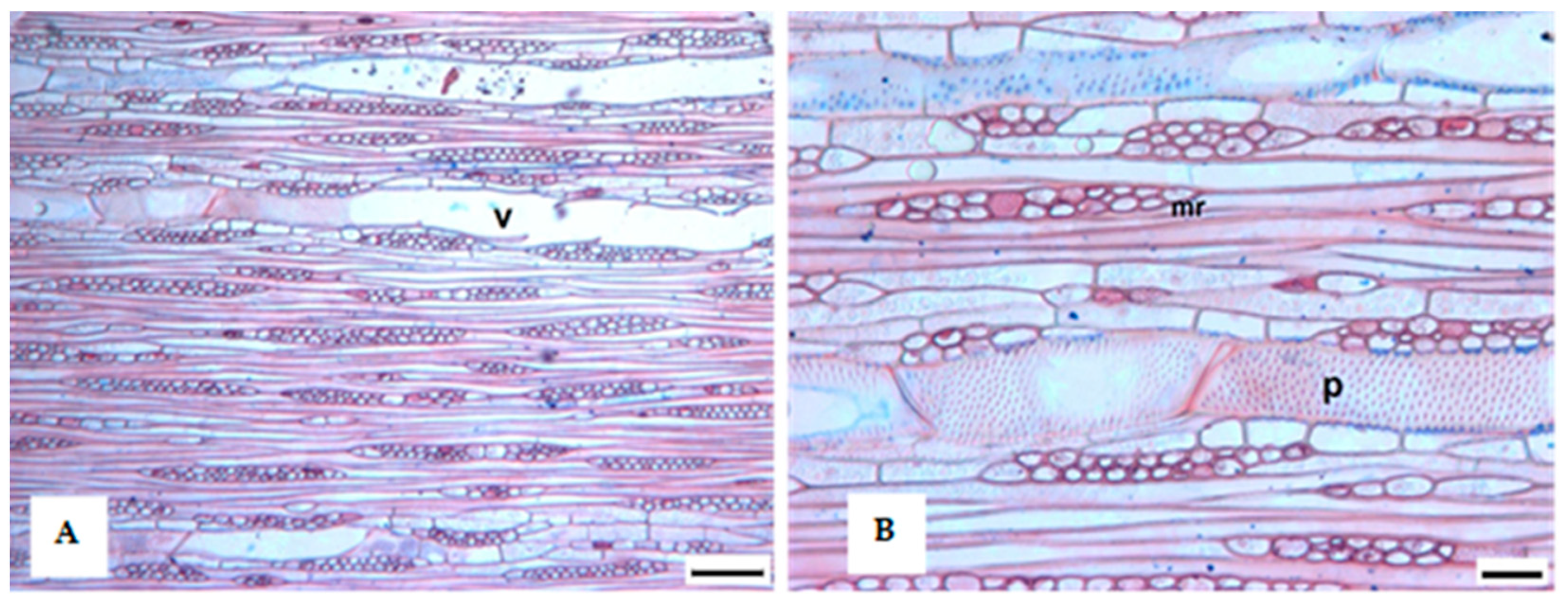
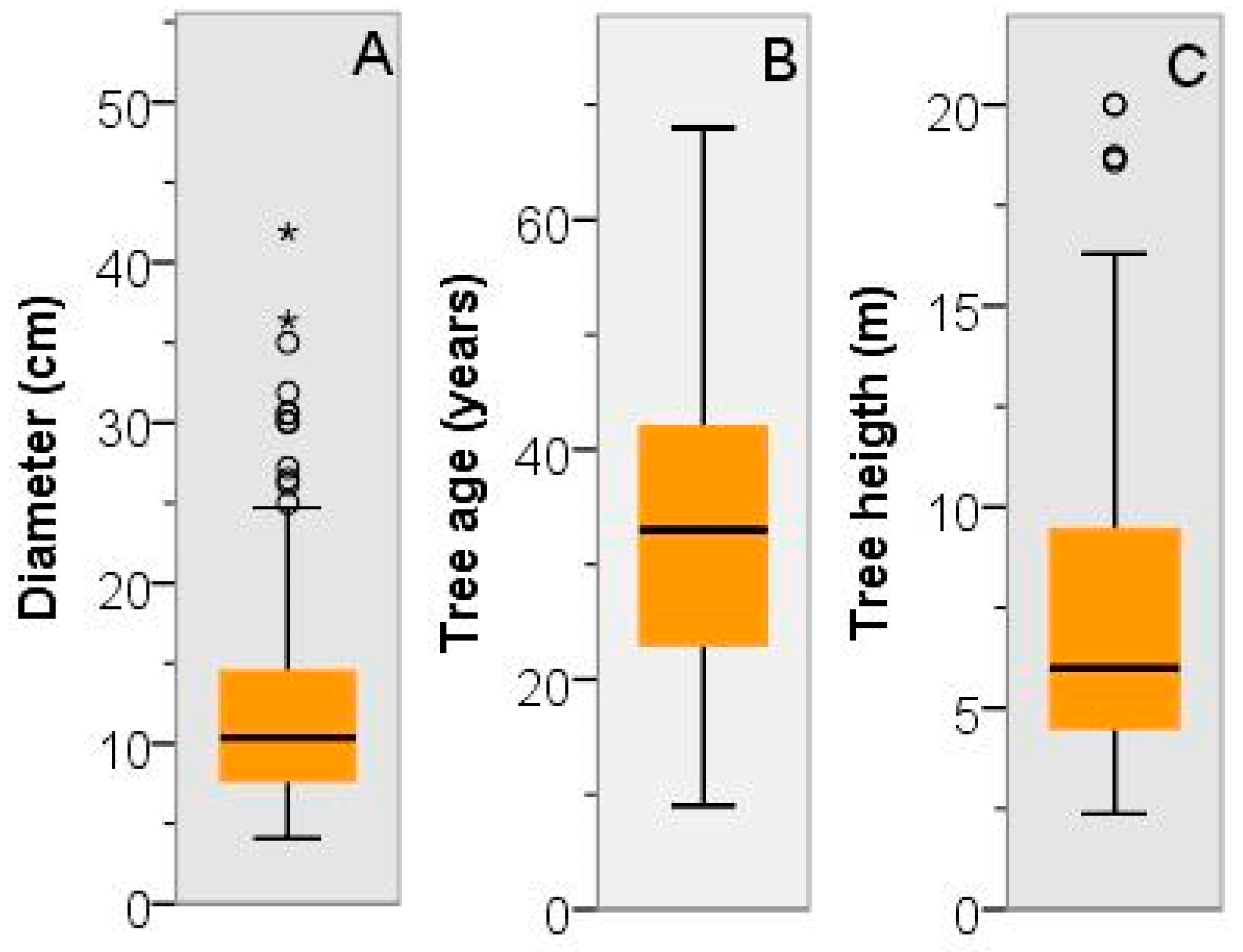
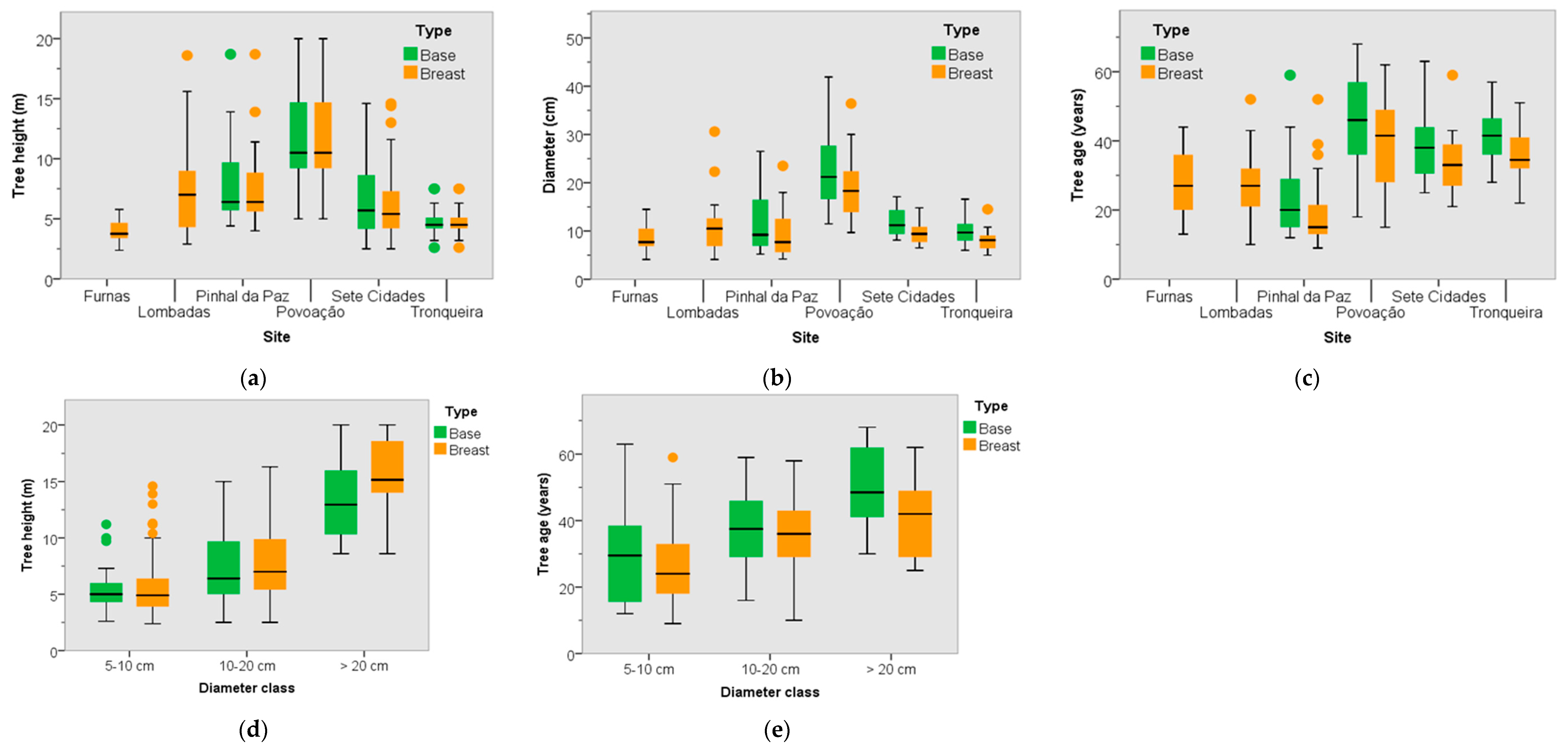
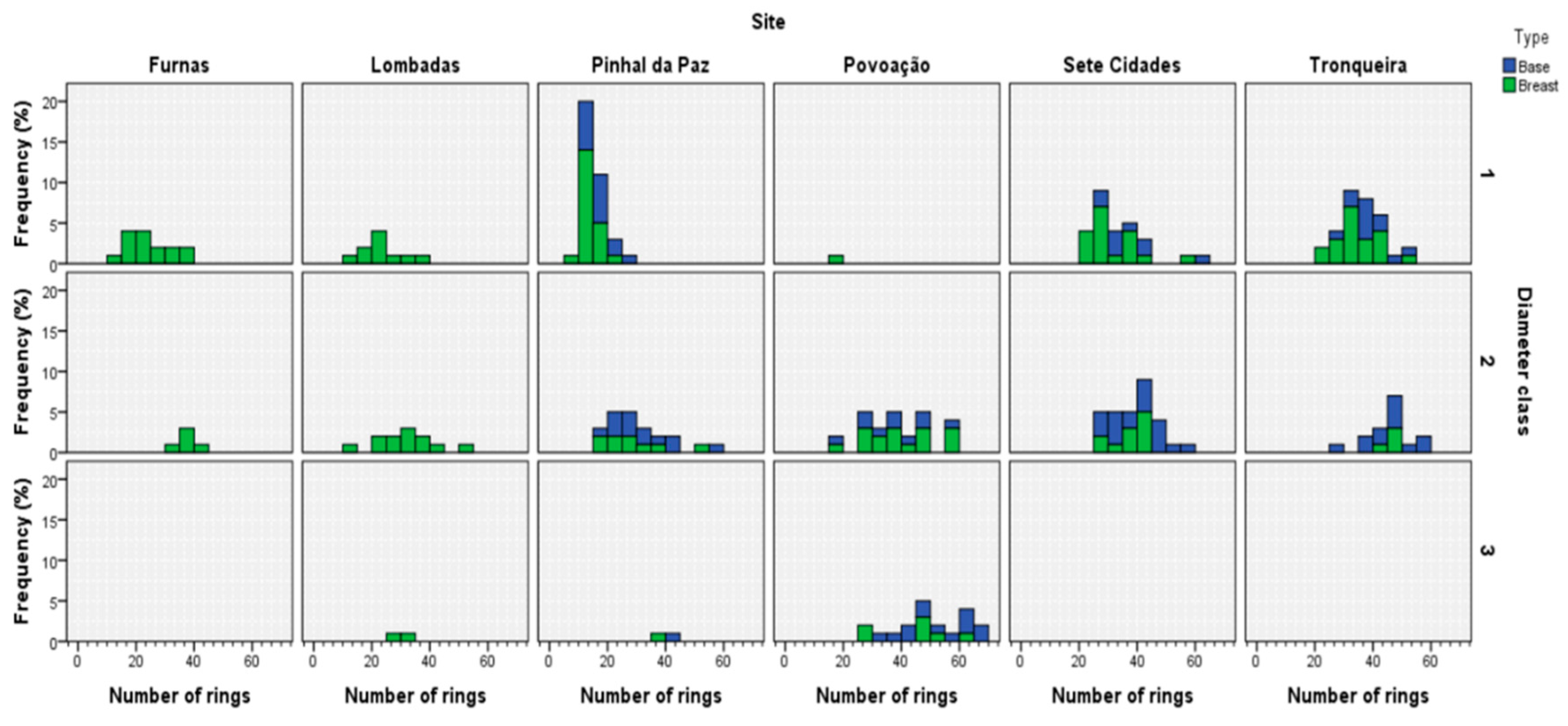
| Stands | Code | E (m) | Sampling | Physical Description | Main Species | Other Information | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | CB | CA | MT | TW | TC | TR | AP | PW | PD | Soil Type | Native | Exotic | Protection | ||||
| Lombadas | LO | 569 | 25 | 25 | 0 | 14 | 22 | 7 | 15 | 1684 | 641 | 167 | Ins And | L. azorica, I. azorica, M. faya | P. undulatum, H. gardnerianum | Habitat/Species Management Area | |
| Achada das Furnas | AF | 600 | 20 | 20 | 0 | 13 | 22 | 7 | 15 | 2108 | 789 | 221 | Shal Aloph Ins/Ferr And | L. azorica, I. azorica | C. arborea, P. undulatum, H. gardnerianum | Unprotected | |
| Pinhal da Paz | PP | 322 | 25 | 31 | 29 | 15 | 23 | 8 | 15 | 1294 | 494 | 133 | Shal Aloph Reg | L. azorica, M. faya | P. undulatum, H. gardnerianum | Recreational Forest Reserve | |
| Sete Cidades | SC | 599 | 25 | 29 | 28 | 13 | 22 | 7 | 15 | 1850 | 698 | 195 | Ins And | L. azorica, I. azorica, M. faya | C. japonica, P. undulatum, H. gardnerianum | Protected Landscape | |
| Tonqueira | TR | 629 | 25 | 25 | 25 | 13 | 22 | 6 | 15 | 2092 | 816 | 199 | Shal Aloph Ins/Ferr And | L. azorica, I. azorica, M. faya, J. brevifolia | C. arborea, H. gardnerianum | Habitat/Species Management Area | |
| Povoação | PO | 541 | 25 | 25 | 25 | 14 | 23 | 7 | 15 | 1557 | 596 | 152 | Shal Aloph Ins/Ferr And | L. azorica I. azorica | H. gardnerianum | Unprotected | |
| Total | 145 | 155 | 107 | ||||||||||||||
| Stand | BH/Base | N | Plot # | Regression Model | Adj R2 | AIC | RMSE | MRE | CF ## |
|---|---|---|---|---|---|---|---|---|---|
| All | BH | 147 | ln(Age) = a + b1ln(D) + ε * | 0.34 | 118.60 | 10.01 | −0.07 | 1.07 | |
| All | BH | 147 | ln(Age) = a + b1ln(D) + b2ln(H) + ε *** | 0.38 | 110.29 | 10.05 | −0.06 | 1.06 | |
| All | BH | 147 | 1 | ln(Age) = a + b1ln(BA) + b2ln(H) + ε ** | 0.39 | 106.87 | 9.75 | −0.06 | 1.06 |
| Null Model | 178.18 | ||||||||
| All | Base | 97 | ln(Age) = a + b1ln(D) + ε * | 0.36 | 74.47 | 11.60 | −0.06 | 1.06 | |
| All | Base | 97 | ln(Age) = a + b1ln(D) + b2ln(H) + ε *** | 0.41 | 67.46 | 11.70 | −0.06 | 1.06 | |
| All | Base | 97 | 2 | ln(Age) = a + b1ln(BA) + b2ln(H) + ε ** | 0.44 | 62.31 | 11.12 | −0.05 | 1.05 |
| Null Model | 116.37 | ||||||||
| LO | BH/Base | 19 | 3 | ln(Age) = a + b1ln(D) + ε * | 0.61 | −11.26 | 4.41 | −0.01 | 1.01 |
| AF | BH/Base | 16 | 4 | ln(Age) = a + b1ln(BA) + ε *** | 0.62 | −6.82 | 4.63 | −0.01 | 1.01 |
| PP | BH/Base | 49 | 5 | ln(Age) = a + b1ln(D) + ε * | 0.86 | −22.87 | 3.92 | −0.02 | 1.02 |
| SC | BH/Base | 49 | 6 | ln(Age) = a + b1ln(D) + b2ln(H) + ε*** | 0.13 | 1.76 | 8.60 | −0.03 | 1.03 |
| TR | BH/Base | 33 | 7 | ln(Age) = a + b1ln(D) + ε * | 0.66 | −69.53 | 3.12 | −0.03 | 1.00 |
| PO | BH/Base | 47 | 8 | ln(Age) = a + b1ln(BAH) + ε *** | 0.40 | 17.78 | 10.77 | −0.04 | 1.04 |
| Growth Parameters | Mean | Standard Deviation | Standard Error | Minimum | Maximum |
|---|---|---|---|---|---|
| PAI (cm·year−1) | 0.679 | 0.659 | 0.066 | 0.033 | 4.200 |
| Age Diference (years) | 5.010 | 3.413 | 0.340 | 0.000 | 18.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, B.; Borges Silva, L.; Camarinho, R.; Rodrigues, A.S.; Rego, R.; Câmara, M.; Silva, L. Linking Dendrometry and Dendrochronology in the Dominant Azorean Tree Laurus azorica (Seub.) Franco. Forests 2019, 10, 538. https://doi.org/10.3390/f10070538
Matos B, Borges Silva L, Camarinho R, Rodrigues AS, Rego R, Câmara M, Silva L. Linking Dendrometry and Dendrochronology in the Dominant Azorean Tree Laurus azorica (Seub.) Franco. Forests. 2019; 10(7):538. https://doi.org/10.3390/f10070538
Chicago/Turabian StyleMatos, Bárbara, Lurdes Borges Silva, Ricardo Camarinho, Armindo S. Rodrigues, Ruben Rego, Mariana Câmara, and Luís Silva. 2019. "Linking Dendrometry and Dendrochronology in the Dominant Azorean Tree Laurus azorica (Seub.) Franco" Forests 10, no. 7: 538. https://doi.org/10.3390/f10070538
APA StyleMatos, B., Borges Silva, L., Camarinho, R., Rodrigues, A. S., Rego, R., Câmara, M., & Silva, L. (2019). Linking Dendrometry and Dendrochronology in the Dominant Azorean Tree Laurus azorica (Seub.) Franco. Forests, 10(7), 538. https://doi.org/10.3390/f10070538