Arbuscular Mycorrhizal Fungi Associated with Tree Species in a Planted Forest of Eastern China
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Sites and Sampling
2.2. Soil Physicochemical Analysis
2.3. Assessment of AMF Colonization
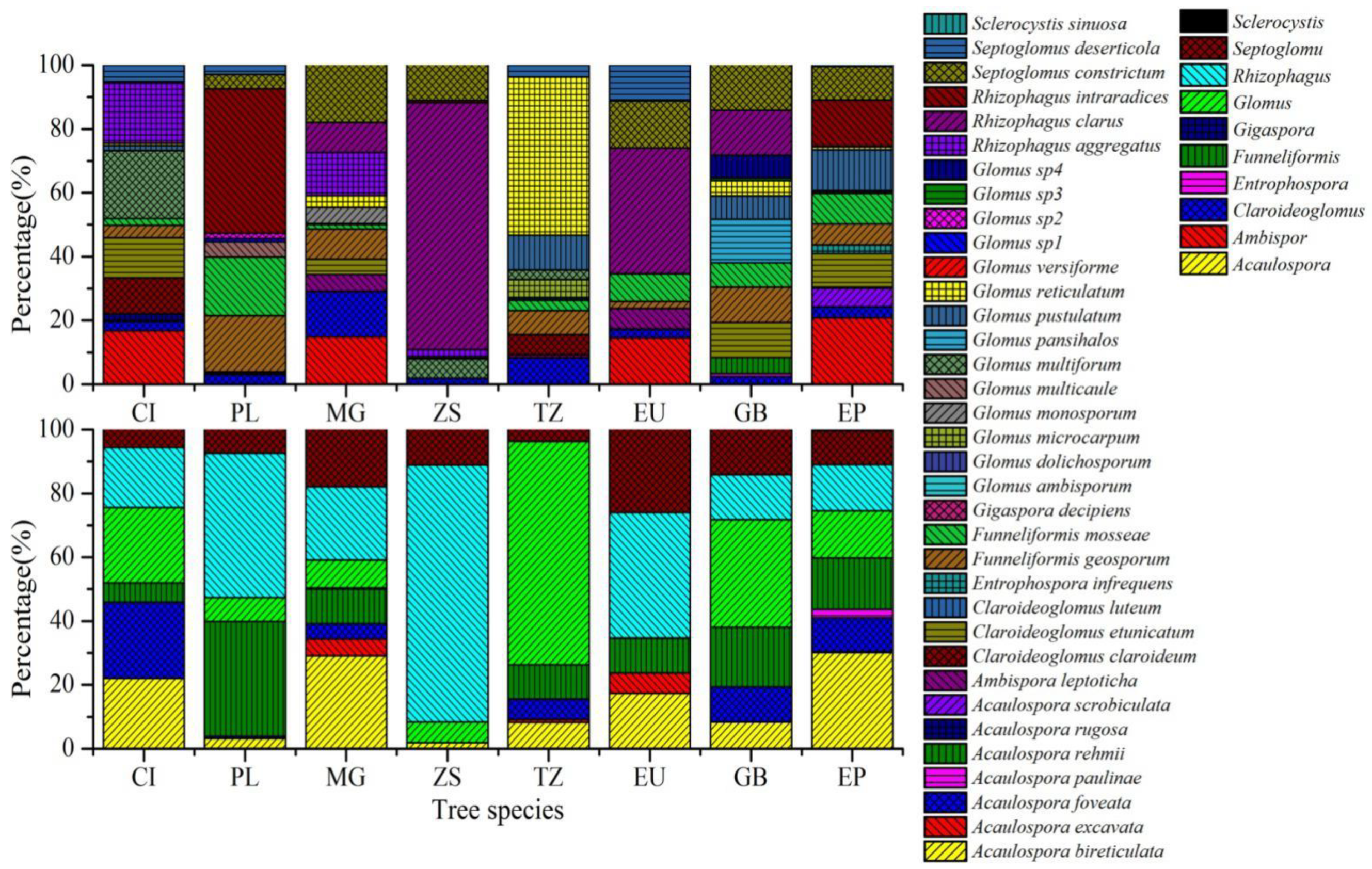
2.4. AMF Spore Quantification and Identification
2.5. Diversity Studies and Statistical Analyses
- RA = (spore number of species or genus/total spore number) × 100%
- IV = (FO + RA)/2
- SD = spore number/100 g air-dried soil
- SR = species number/soil sample
- H = ; /N, where is the spore number of a species and N is the total number of identified spore samples [48].
- E = H/ = ln S, where S is the total number of identified species.
3. Results
3.1. AMF Colonization and SD
3.2. Identification of Spores and AMF Community Composition and Diversity
3.3. Chemical and Physical Soil Parameters
3.4. Relationship between Soil Factors and AMF
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Purin, S.; Rillig, M.C. The arbuscular mycorrhizal fungal protein glomalin: Limitations, progress, and a new hypothesis for its function. Pedobiologia 2007, 51, 123–130. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Y.; Fang, S.; Tian, Y. Tress species composition influenced microbial diversity and nitrogen availability in rhizosphere soil. Plant Soil Environ. 2015, 10, 438–443. [Google Scholar]
- Fernández, N.; Fontenla, S.; Messuti, M.I. Co-occurrence of arbuscular mycorrhizas and dark septate endophytes in pteridophytes from a Valdivian Temperate Rainforest in Patagonia, Argentina. In Mycorrhiza: Occurrence in Natural and Restored Environments; Pagano, M., Ed.; Nova Science Publishers: New York, NY, USA, 2011; pp. 99–126. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008; pp. 13–41. [Google Scholar]
- Jakobsen, I. Transport of Phosphorus and Carbon in VA mycorrhizas. In Mycorrhiza; Varma, A., Hock, B., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 297–324. [Google Scholar]
- Cardoso, E.J.B.N.; Vasconcellos, R.L.F.; Bini, D.; Miyauchi, M.Y.H.; Santos, C.A.; Alves, P.R.L.; Paula, A.M.; Nakatani, A.S.; Pereira, J.M.; Nogueira, M.A. Soil health: Looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Sci. Agric. 2013, 70, 219–303. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Sansom, C.E.; Jones, E.E.; Perry, N.B.; Monk, J.; Ridgway, H.J. Arbuscular mycorrhizal fungi associated with Leptospermum scoparium (mānuka): Effects on plant growth and essential oil content. Symbiosis 2018, 75, 39–50. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Y.J.; Zhang, K.; Tian, C.Y.; Guo, J.X. Arbuscular mycorrhizal fungi improve plant growth of Ricinus communis by altering photosynthetic properties and increasing pigments under drought and salt stress. Ind. Crop. Prod. 2018, 117, 13–19. [Google Scholar] [CrossRef]
- Sarkar, A.; Asaeda, T.; Wang, Q.; Kaneko, Y.; Rashid, M.H. Response of Miscanthus sacchariflorus to zinc stress mediated by arbuscular mycorrhizal fungi. Flora 2017, 234, 60–68. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Jiang, X.; Chen, B.; Zhang, X. Arbuscular mycorrhizal fungi alleviate arsenic toxicity to Medicago sativa by influencing arsenic speciation and partitioning. Ecotoxicol. Environ. Saf. 2018, 157, 235–243. [Google Scholar] [CrossRef]
- Leifheit, E.F.; Veresoglou, S.D.; Lehmann, A.; Morris, E.K.; Rillig, M.C. Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation-a meta-analysis. Plant Soil 2014, 374, 523–537. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Strietwolf Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal fungal diversity determines the plant diversity, ecosystem variability and productivity. Nature 1998, 398, 39–72. [Google Scholar] [CrossRef]
- Bever, J.D.; Schultz, P.A.; Pringle, A.; Morton, H.B. Arbuscular mycorrhizal fungi: More diverse than meets the eye, and the ecological tale of why. Bioscience 2001, 51, 923–932. [Google Scholar] [CrossRef]
- Jiang, J.; Moore, J.A.M.; Priyadarshi, A.; Classen, A.T. Plant-mycorrhizal interactions mediate plant community coexistence by altering resource demand. Ecology 2017, 98, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.O.; Buscardo, E.; Nagy, L.; Maciel, A.B.S.; Carrenho, R.; Luizão, R.C.C. Erratum to: Arbuscular mycorrhizal fungal communities along a pedo-hydrological gradient in a Central Amazonian terra firme forest. Mycorrhiza 2014, 24, 21–32. [Google Scholar] [CrossRef]
- Pereira, C.M.R.; Silva, D.K.A.D.; Goto, B.T.; Maia, L.C. Diversity of arbuscular mycorrhizal fungi in Atlantic forest areas under different land uses. Agric. Ecosyst. Environ. 2014, 185, 245–252. [Google Scholar] [CrossRef]
- Álvarez-Sánchez, J.; Sánchez-Gallen, I.; Hernández-Cuevas, L.; Hernández, L.; Cruz, C. What can the arbuscular mycorrhizal fungi community tell us about plant biodiversity loss? In Recent Advances on Mycorrhizal Fungi, Fungi Bioloyg; Pagano, M., Ed.; Springer: Cham, Switzerland, 2016; pp. 23–33. [Google Scholar]
- Álvarez-Sánchez, J.; Johnson, N.C.; Antoninka, A.; Chaudhary, V.B.; Lau, M.K.; Owen, S.M.; Sánchez-Gallen, I.; Guadarrama, P.; Castillo, S. Large-scale diversity patterns in spore communities of arbuscular mycorrhizal fungi. In Mycorrhiza: Ocurrence in Natural and Restored Environments; Pagano, M., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2010; pp. 29–47. [Google Scholar]
- Senapati, M.; Das, A.B.; Das, P. Association of vesicular arbuscular mycorrhizal fungi with 21 forest tree species. Indian J. For. 2000, 23, 326–331. [Google Scholar]
- Singh, S.S.; Tiwari, S.C.; Dkhar, M.S. Species diversity of vesicular-arbuscular mycorrhizal (VAM) fungi in jhum fallow and natural forest soils of Arunachal Pradesh, north eastern India. Trop. Ecol. 2003, 44, 207–215. [Google Scholar]
- Dhar, P.P.; Mridha, M.A.U. Arbuscular mycorrhizal associations in different forest tree species of Hazarikhil forest of Chittagong, Bangladesh. J. For. Res. 2012, 23, 115–122. [Google Scholar] [CrossRef]
- Wubet, T.; Weiss, M.; Kottke, I.; Teketay, D.; Oberwinkler, F. Molecular diversity of arbuscular mycorrhizal fungi in Prunus africana, an endangered medicinal tree species in dry Afromontane forests of Ethiopia. New Phytol. 2004, 161, 517–528. [Google Scholar] [CrossRef] [Green Version]
- Cong, L.C.; Ye, Z.S.; Lei, L.; Huang, J.S. Arbuscular mycorrhizal fungi associated with common tree species in a tropical rain forest in Bawangling of Hainan Island, China. Chin. J. Ecol. 2010, 29, 269–273. [Google Scholar]
- Tian, H.; Gai, J.P.; Zhang, J.L.; Christie, P.; Li, X.L. Arbuscular mycorrhizal fungi associated with wild forage plants in typical steppe of eastern Inner Mongolia. Eur. J. Soil Biol. 2009, 45, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Xue, Z.K.; He, X.L.; Liu, C.M.; Steinberger, Y. Shifts in composition and diversity of arbuscular mycorrhizal fungi and glomalin contents during revegetation of desertified semiarid grassland. Appl. Soil Ecol. 2017, 115, 60–67. [Google Scholar] [CrossRef]
- Cui, X.; Hu, J.; Wang, J.; Yang, J.S.; Lin, X.G. Reclamation negatively influences arbuscular mycorrhizal fungal community structure and diversity in coastal saline-alkaline land in Eastern China as revealed by Illumina sequencing. Appl. Soil Ecol. 2016, 98, 140–149. [Google Scholar] [CrossRef]
- Wang, F.Y.; Liu, R.J.; Lin, X.G.; Zhou, J.M. Arbuscular mycorrhizal status of wild plants in saline-alkaline soils of the Yellow River Delta. Mycorrhiza 2004, 14, 133–137. [Google Scholar]
- Yang, A.N.; Lu, L.; Zhang, N. The diversity of arbuscular mycorrhizal fungi in the subtropical forest of Huangshan (Yellow Mountain), East-Central China. World J. Microb. Biot. 2011, 27, 2351–2358. [Google Scholar] [CrossRef]
- Liu, H.G.; Wang, Y.J.; Tang, M. Arbuscular mycorrhizal fungi diversity associated with two halophytes Lycium barbarum L. and Elaeagnus angustifolia L. in Ningxia, China. Arch. Agron. Soil Sci. 2016, 63, 796–806. [Google Scholar] [CrossRef]
- Wang, M.Y.; Jiang, P. Colonization and diversity of AM fungi by morphological analysis on medicinal plants in southeast China. Sci. World J. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Gai, J.P.; Cai, X.B.; Li, X.L.; Christie, P.; Zhang, F.Z.; Zhang, J.L. Molecular diversity of arbuscular mycorrhizal fungi associated with two co-occurring perennial plant species on a Tibetan altitudinal gradient. Mycorrhiza 2014, 24, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Jamiołkowska, A.; Księżniak, A.; Gałązka, A.; Hetman, B.; Kopacki, M.; Skwary-Bednarz, B. Impact of abiotic factors on development of the community of arbuscular mycorrhizal fungi in the soil: A Review. Int. Agrophys. 2018, 32, 133–140. [Google Scholar] [CrossRef]
- Benucci, G.M.; Bonito, G.; Baciarelli Falini, L.; Bencivenga, M. Mycorrhization of pecan trees (Carya illinoinensis) with commercial truffle species: Tuber aestivum Vittad. and Tuber borchii Vittad. Mycorrhiza 2012, 22, 383–392. [Google Scholar] [CrossRef]
- Ding, N.N.; Wang, B.S.; Liang, Z.H.; Liu, D.H. Effects of different Amelioration measures on coastal saline soil in the David’s Deer Reserve of Dafeng County of Jiangsu province. Soils 2011, 43, 487–492. [Google Scholar]
- Xiao, L.; Huang, Y.M.; Zeng, Q.C.; Zhao, J.F.; Zhou, J.Y. Soil enzyme activities and microbial biomass response to crop types on the terraces of the Loess Plateau, China. J. Soil Sediment. 2018, 18, 1971–1980. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, H.; Pumpanen, J.; Sietiö, O.M.; Heinonsalo, J.; Köster, K.; Berninger, F. The impact of wildfire on the microbial C:N:P stoichiometry and the fungal-to-bacterial ratio in permafrost soil. Biogeochemistry 2019, 142, 1–17. [Google Scholar] [CrossRef]
- Walkley, A.J.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Gong, M.Q.; Chen, Y.L.; Zhong, C.L. Mycorrhizal Research and Application; China Forestry Publishing House: Beijing, China, 1997; p. 223. [Google Scholar]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Mäder, P.; Boller, T.; Wiemken, A. Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Appl. Environ. Microb. 2003, 69, 2816–2824. [Google Scholar] [CrossRef]
- Morton, J.B. Taxonomy of VA mycorrhizal fungi: Classification, nomenclature, and identification. Mycotaxon 1988, 32, 267–324. [Google Scholar]
- Schenck, N.C.; Perez-Collins, Y. Manual for the Identification of va Mycorrhizal Fungi, 3rd ed.; Synergistic Publications: Gainesville, FL, USA, 1990. [Google Scholar]
- Schüßler, A.; Walker, C. The Glomeromycota: A Species List with New Families and New Genera; Libraries at the Royal Botanic Garden Edinburgh, Gloucester, Botanische Staatssammlung Munich and Oregon State University: Kew, OR, USA, 2010. [Google Scholar]
- Oehl, F.; Sieverding, E.; Palenzuela, J.; Ineichen, K.; Alves da silva, G. Advances in Glomeromycota taxonomy and classification. IMA Fungus 2011, 2, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Krüger, M.; Krüger, C.; Walker, C.; Stockinger, H.; Schüßler, A. Phylogenetic reference data for systematic and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol. 2012, 193, 970–984. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.D.; Liu, R.J. Survey of arbuscular mycorrhizal fungi in deforested and natural forest land in the subtropical region of Dujiangyan, southwest China. Plant Soil 2004, 261, 257–263. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949; pp. 623–656. [Google Scholar]
- Muthukumar, T.; Udaiyan, K. Arbuscular mycorrhizas of plants growing in the Western Ghats region, Southern India. Mycorrhiza 2000, 9, 297–313. [Google Scholar] [CrossRef]
- Tawaraya, K.; Hashimoto, K.; Wagatsuma, T. Effect of root exudate fractions from P-deficient and P-sufficient onion plants on root colonisation by the arbuscular mycorrhizal fungus Gigaspora margarita. Mycorrhiza 1998, 8, 67–70. [Google Scholar] [CrossRef]
- Kowalska, I.K.; Konieczny, A.K.; Gastol, M.K.; Sady, W.K.; Hanusfajerska, E.K. Effect of mycorrhiza and phosphorus content in nutrient solution on the yield and nutritional status of tomato plants grown on rockwool or coconut coir. Agric. Food. Sci. 2015, 24, 39–51. [Google Scholar] [CrossRef]
- Gong, M.G.; Ming, T.; Zhang, Q.M.; Feng, X.X. Effects of climatic and edaphic factors on arbuscular mycorrhizal fungi in the rhizosphere of Hippophae rhamnoides in the Loess Plateau, China. Acta Ecol. Sin. 2012, 32, 62–67. [Google Scholar] [CrossRef]
- Shi, G.; Liu, Y.; Johnson, N.C.; Olsson, P.A.; Mao, L.; Cheng, G.; Jiang, S.J.; An, L.Z.; Du, G.Z.; Feng, G.Y. Interactive influence of light intensity and soil fertility on root-associated arbuscular mycorrhizal fungi. Plant Soil 2014, 378, 173–188. [Google Scholar] [CrossRef]
- Wang, S.F.; He, X.L.; Chen, T.S. Ecological research of arbuscular mycorrhizal fungi from the rhizosphere of Raspberry and Blackberry. Acta Agric. Boreal.-Occident. Sin. 2007, 16, 219–221. [Google Scholar]
- Bhatia, N.P.; Sundari, K.; Adholeya, A. Diversity and selective dominance of vesicular-arbuscular mycorrhizal fungi. In Concepts in Mycorrhizal Research; Mukerji, K.G., Ed.; Springer: Dordrecht, Netherlands, 1996; pp. 133–178. [Google Scholar]
- Abbott, L.K.; Robson, A.D. Factors influencing the occurrence of vesicular-arbuscular mycorrhizas. Agric. Ecosyst. Environ. 1991, 35, 121–150. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Wang, G.H.; Yang, L. Biodiversity of arbuscular mycorrhizal fungi in a tropical rainforest of Xishuangbanna, southwest China. Fungal Divers. 2003, 13, 233–242. [Google Scholar]
- Martin, J.; Bereau, M.E.; Ocampo, J.A. Arbuscular mycorrhizas in Dicorynia guianensis and Eperua falcata treesfrom primary tropical rain forest of French Guiana. Symbiosis 2001, 31, 283–291. [Google Scholar]
- Louis, I.; Lim, G. Spore density and root colonization of vesicular-arbuscular mycorrhizas in tropical soil. Trans. Br. Mycol. Soc. 1987, 88, 207–212. [Google Scholar] [CrossRef]
- Chaiyasen, A.; Douds, D.D.; Gavinlertvatana, P.; Lumyong, S. Diversity of arbuscular mycorrhizal fungi in Tectona grandis Linn.f. plantations and their effects on growth of micropropagated plantlets. New For. 2017, 48, 1–16. [Google Scholar] [CrossRef]
- Silva, I.R.; Souza, F.A.; Silva, D.K.A.; Oehl, F.; Maia, L.C. Patterns of arbuscular mycorrhizal fungal distribution on mainland and island sandy coastal plain ecosystems in Brazil. Microb. Ecol. 2017, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pontes, J.S.; Oehl, F.; Pereira, C.D.; Machado, C.T.; Coyne, D.; da Silva, D.K.; Maia, L.C. Diversity of arbuscular mycorrhizal fungi in the Brazilian’s Cerrado and in soybean under conservation and conventional tillage. Appl. Soil Ecol. 2017, 117–118, 178–189. [Google Scholar] [CrossRef]
- Bonfim, J.A.; Gumiere, T.; Oehl, F. Diversity of arbuscular mycorrhizal fungi in a Brazilian atlantic forest toposequence. Microb. Ecol. 2016, 71, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jasper, D.A.; Abbott, L.K.; Robson, A.D. The survival of infective hyphae of vesicular–arbuscular mycorrhizal fungi in dry soil: An interaction with sporulation. New Phytol. 1993, 124, 473–479. [Google Scholar] [CrossRef]
- Öpik, M.; Moora, M.; Zobel, M.; Saks, Ü.; Wheatley, R.; Wright, F.; Daniell, T. High diversity of arbuscular mycorrhizal fungi in a boreal herb-rich coniferous forest. New Phytol. 2008, 179, 867–876. [Google Scholar] [PubMed] [Green Version]
- Moreira, M.; Baretta, D.; Siumui, T.; Cardoso, E.J.B.N. Arbuscular mycorrhizal fungal communities in native and in replanted Araucaria forest. Sci. Agric. 2009, 66, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Hazard, C.; Gosling, P.; Gast, C.J.; Mitchell, D.T.; Doohan, F.M.; Bending, G.D. The role of local environment and geographical distance in determining community composition of arbuscular mycorrhizal fungi at the landscape scale. ISME J. 2013, 7, 498–508. [Google Scholar] [CrossRef]
- Murray, T.R.; Frank, D.A.; Gehring, C.A. Ungulate and topographic control of arbuscular mycorrhizal fungal spore community composition in a temperate grassland. Ecology 2010, 91, 815–827. [Google Scholar] [CrossRef]
- Bonfim, J.A.; Rlf, V.; Stürmer, S.L.; Cardoso, E.J.B.N. Arbuscular mycorrhizal fungi in the Brazilian Atlantic forest: A gradient of environmental restoration. Appl. Soil Ecol. 2013, 71, 7–14. [Google Scholar] [CrossRef]
- Ramos, A.C.; Façanha, A.R.; Feijó, J.A. Proton (H+) flux signature for the presymbiotic development of the arbuscular mycorrhizal fungi. New Phytol. 2008, 178, 177–188. [Google Scholar] [CrossRef]
- Tawaraya, K.; Takaya, Y.; Turjaman, M.; Tuah, S.J.; Limin, S.H.; Tamain, Y.; Cha, J.Y.; Wagatsuma, T.; Osaki, M. Arbuscular mycorrhizal colonization of tree species grown in peat swamp forests of Central Kalimantan, Indonesia. For. Ecol. Manag. 2003, 182, 381–386. [Google Scholar] [CrossRef]
- Silva, D.K.A.; Souza, R.G.D.; Velez, B.A.D.A.; Silva, G.A.; Oehl, F.; Maia, L.C. Communities of arbuscular mycorrhizal fungi on a vegetation gradient in tropical coastal dunes. Appl. Soil Ecol. 2015, 96, 7–17. [Google Scholar] [CrossRef]
- Estrada, B.; Beltrán-Hermoso, M.; Palenzuela, J.; Iwase, K.; Ruiz-Lozano, J.M.; Barea, J.M.; Oehl, F. Diversity of arbuscular mycorrhizal fungi in the rhizosphere of Asteriscus maritimus (L.) Less., a representative plant species in arid and saline Mediterranean ecosystems. J. Arid Environ. 2013, 97, 170–175. [Google Scholar] [CrossRef]
- Fritz, O.; Endre, L.; Arno, B.; Karl, S.; Robert, B.; Marcel vander, H.; Ewald, S. Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol. Biochem. 2010, 42, 724–738. [Google Scholar]

| Tree Species | AMF Colonization/% | Colonization Intensity | SD (No. Per 100 g Air-Dried Soil) | |||
|---|---|---|---|---|---|---|
| RLC | RLV | RLA | RLH | |||
| CI | 90.3 ± 11.6 a | 45.6 ± 40.9 ab | 71.8 ± 14.3 ab | 81.8 ± 8.6 ab | Medium-strong | 934.7 ± 608.2 b |
| PL | 67.2 ± 35.7 a | 38.5 ± 38.8 ab | 54.7 ± 33.9 ab | 67.2 ± 35.9 ab | Inferior-strong | 2075.7 ± 403.6 ab |
| MG | 52.1 ± 34.3 a | 32.2 ± 28.0 ab | 37.7 ± 29.9 bc | 47.6 ± 30.1 b | Inferior-medium | 1876.0 ± 734.0 ab |
| ZS | 73.1 ± 15.3 a | 39.1 ± 14.5 ab | 68.4 ± 25.0 ab | 73.1 ± 15.3 ab | Inferior | 3971.0 ± 2684.9 a |
| TZ | 88.7 ± 9.8 a | 59.0 ± 8.7 a | 74.5 ± 14.6 ab | 86.5 ± 12.1 a | Medium-strong | 1478.7 ± 1125.3 ab |
| EU | 87.4 ± 19.0 a | 31.5 ± 25.6 ab | 83.9 ± 24.9 a | 87.4 ± 19.0 a | Strong | 4207.0 ± 3069.4 a |
| GB | 12.3 ± 15.4 b | 8.9 ± 9.6 b | 6.7 ± 5.7 c | 11.2 ± 13.4 c | Inferior-medium | 918.7 ± 247.4 b |
| EP | 86.7 ± 6.7 a | 56.7 ± 13.5 ab | 64.6 ± 16.6 ab | 86.7 ± 6.7 a | Medium-strong | 1880.0 ± 284.0 ab |
| Species No. | AM Fungi | Tree Species | FO (%) | RA (%) | IV (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CI | PL | MG | ZS | TZ | EU | GB | EP | |||||
| 1 | Acaulospora bireticulata | − | − | − | − | − | − | + | − | 4.17 | 0.008 | 2.09 |
| 2 | Acaulospora excavata | + | − | + | − | − | + | − | + | 25 | 8.317 | 16.66 |
| 3 | Acaulospora foveata | + | + | + | + | + | + | + | + | 50 | 4.352 | 27.18 |
| 4 | Acaulospora paulinae | − | − | − | − | − | − | + | − | 4.17 | 0.047 | 2.11 |
| 5 | Acaulospora rehmii | − | + | − | − | − | + | + | − | 12.5 | 0.318 | 6.41 |
| 6 | Acaulospora rugosa | + | − | − | − | − | − | − | − | 4.17 | 0.124 | 2.15 |
| 7 | Acaulospora scrobiculata | − | − | − | − | − | − | − | + | 4.17 | 0.628 | 2.40 |
| 8 | Ambispora leptoticha | − | − | + | − | + | + | − | + | 26.67 | 2.219 | 14.44 |
| 9 | Claroideoglomus claroideum | + | − | − | − | + | − | − | − | 12.5 | 1.156 | 6.83 |
| 10 | Claroideoglomus etunicatum | + | + | + | − | − | − | + | + | 33.33 | 2.964 | 18.15 |
| 11 | Claroideoglomus luteum | − | + | − | − | − | − | − | − | 4.17 | 0.031 | 2.10 |
| 12 | Entrophospora infrequens | − | − | − | − | − | − | − | + | 4.17 | 0.287 | 2.23 |
| 13 | Funneliformis geosporum | + | + | + | − | + | + | + | + | 37.5 | 5.842 | 21.67 |
| 14 | Funneliformis mosseae | + | + | + | − | + | + | + | + | 37.5 | 6.307 | 21.90 |
| 15 | Gigaspora decipiens | − | − | + | − | − | − | − | − | 4.17 | 0.039 | 2.10 |
| 16 | Glomus ambisporum | − | − | − | − | − | + | − | − | 4.17 | 0.008 | 2.09 |
| 17 | Glomus dolichosporum | − | − | − | − | + | − | − | − | 4.17 | 0.062 | 2.12 |
| 18 | Glomus microcarpum | − | − | − | − | + | − | − | − | 4.17 | 0.489 | 2.33 |
| 19 | Glomus monosporum | − | − | + | − | − | − | − | − | 8.33 | 0.551 | 4.44 |
| 20 | Glomus multicaule | − | + | − | − | − | − | − | + | 8.33 | 0.628 | 4.48 |
| 21 | Glomus multiforum | + | − | − | + | + | − | − | + | 16.67 | 2.832 | 9.75 |
| 22 | Glomus pansihalos | − | − | − | − | − | − | + | − | 4.17 | 0.721 | 2.45 |
| 23 | Glomus pustulatum | + | − | − | − | + | − | + | + | 29.17 | 2.785 | 15.98 |
| 24 | Glomus reticulatum | + | − | + | + | + | − | + | + | 29.17 | 5.112 | 17.14 |
| 25 | Glomus versiforme | − | − | − | − | − | − | − | + | 4.17 | 0.031 | 2.10 |
| 26 | Glomus sp1 | − | + | − | − | − | − | − | − | 4.17 | 0.116 | 2.14 |
| 27 | Glomus sp2 | − | + | − | − | − | − | − | − | 4.17 | 0.209 | 2.19 |
| 28 | Glomus sp3 | − | − | − | − | − | − | + | − | 4.17 | 0.039 | 2.10 |
| 29 | Glomus sp4 | − | − | − | − | − | − | + | − | 4.17 | 0.380 | 2.28 |
| 30 | Rhizophagus aggregatus | + | − | + | + | − | − | − | − | 16.67 | 3.049 | 9.86 |
| 31 | Rhizophagus clarus | − | − | + | + | − | + | + | − | 29.17 | 28.991 | 29.08 |
| 32 | Rhizophagus intraradices | − | + | − | + | − | − | − | + | 20.83 | 7.145 | 13.99 |
| 33 | Septoglomus constrictum | + | + | + | + | − | + | + | + | 54.17 | 10.512 | 32.34 |
| 34 | Septoglomus deserticola | + | + | − | − | + | + | − | − | 16.17 | 3.662 | 9.92 |
| 35 | Sclerocystis sinuosa | − | − | − | − | − | − | − | + | 4.17 | 0.039 | 2.10 |
| Species richness | 13 | 12 | 12 | 7 | 11 | 10 | 14 | 16 | ||||
| CI | PL | MG | ZS | TZ | EU | GB | EP | |
|---|---|---|---|---|---|---|---|---|
| SR | 6.33 ± 2.08 a | 5.67 ± 2.31 ab | 5.33 ± 0.58 ab | 4.00 ± 1.00 ab | 500 ± 1.73 ab | 3.33 ± 0.58 b | 5.67 ± 1.15 ab | 6.67 ± 1.53 a |
| H | 1.37 ± 0.36 a | 1.29 ± 0.24 ab | 1.52 ± 0.11 a | 0.81 ± 0.40 b | 1.11 ± 0.34 ab | 0.82 ± 0.10 b | 1.54 ± 0.17 a | 1.30 ± 0.31 ab |
| E | 0.76 ± 0.06 ab | 0.80 ± 0.11 ab | 0.91 ± 0.05 a | 0.58 ± 0.22 b | 0.74 ± 0.27 ab | 0.71 ± 0.17 ab | 0.89 ± 0.03 ab | 0.70 ± 0.19 ab |
| Species | pH | SM (%) | EC (µS/cm) | NN (mg/kg) | TN (%) | AP (mg/kg) | TP (mg/kg) | AK (mg/kg) | TK (g/kg) | OM (g/kg) | C (%) | S (‰) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CI | 8.05 a | 28.13 a | 298.3 a | 37.99 a | 0.400 ab | 12.50 ab | 854.7 b | 156.4 a | 14.35 a | 4.23 c | 1.60 c | 0.285 abc |
| PL | 7.95 a | 21.22 ab | 259.0 a | 35.47 a | 0.300 ab | 1.75 b | 737.0 b | 134.2 a | 15.54 a | 4.91 c | 1.67 bc | 0.252 abcd |
| MG | 7.78 a | 22.71 ab | 200.6 a | 52.43 a | 0.253 ab | 17.11 a | 906.5 b | 175.9 a | 14.40 a | 11.32 ab | 2.00 ab | 0.299 ab |
| ZS | 7.77 a | 29.26 a | 248.3 a | 33.72 a | 0.297 ab | 2.31 b | 829.5 b | 184.6 a | 13.46 a | 12.87 a | 2.20 a | 0.307 a |
| TZ | 7.97 a | 20.04 ab | 191.1 a | 26.63 a | 0.327 ab | 3.00 b | 788.9 b | 110.9 a | 15.60 a | 5.26 bc | 1.72 bc | 0.225 cd |
| EU | 7.88 a | 14.78 b | 178.4 a | 69.14 a | 0.187 b | 4.93 b | 811.8 b | 167.1 a | 13.96 a | 7.09 abc | 1.84 abc | 0.232 bcd |
| GB | 8.13 a | 19.89 b | 145.6 a | 38.24 a | 0.417 ab | 7.77 ab | 1272.8 a | 134.4 a | 14.06 a | 2.98 c | 1.58 c | 0.192 d |
| EP | 8.11 a | 25.75 ab | 180.4 a | 13.48 a | 0.727 a | 6.07 b | 931.9 b | 120.2 a | 14.02 a | 4.35 c | 1.73 bc | 0.215 cd |
| pH | SM | EC | NN | TN | AP | TP | AK | TK | OM | C | S | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RLC | −0.092 | −0.044 | 0.067 | −0.246 | 0.086 | −0.112 | −0.635 ** | −0.094 | 0.284 | −0.051 | −0.006 | 0.170 |
| RLV | −0.220 | −0.061 | −0.045 | −0.266 | 0.235 | −0.161 | −0.378 | −0.190 | 0.483 * | −0.030 | −0.026 | 0.067 |
| RLA | −0.151 | −0.151 | 0.030 | −0.223 | 0.033 | −0.184 | −0.568 ** | −0.076 | 0.192 | 0.029 | 0.028 | 0.163 |
| RLH | −0.105 | −0.060 | 0.078 | −0.233 | 0.081 | −0.173 | −0.627 ** | −0.114 | 0.298 | −0.020 | 0.019 | 0.160 |
| COI | 0.331 | −0.417 * | −0.257 | 0.020 | −0.018 | −0.064 | −0.269 | −0.101 | 0.400 | −0.467 * | −0.496 * | −0.453 * |
| SD | −0.112 | −0.340 | −0.074 | −0.020 | −0.117 | 0.012 | −0.056 | −0.074 | −0.115 | 0.100 | 0.034 | −0.038 |
| SR | 0.478 * | 0.006 | −0.337 | −0.291 | 0.279 | −0.005 | 0.095 | −0.129 | −0.074 | −0.300 | −0.415 * | −0.373 |
| H | 0.169 | 0.002 | −0.332 | −0.102 | 0.274 | 0.318 | 0.374 | −0.215 | −0.130 | −0.316 | −0.408 * | −0.305 |
| E | −0.146 | −0.004 | −0.131 | 0.115 | 0.121 | 0.365 | 0.346 | −0.212 | −0.028 | −0.228 | −0.218 | −0.099 |
| Acaulospora | 0.169 | −0.108 | −0.232 | −0.031 | −0.072 | −0.009 | −0.248 | 0.179 | −0.110 | 0.216 | 0.198 | −0.041 |
| Ambispora | −0.108 | −0.445 * | −0.126 | 0.051 | −0.190 | 0.272 | −0.037 | −0.026 | −0.069 | −0.002 | −0.033 | −0.076 |
| Claroideoglomus | 0.600 ** | 0.232 | 0.274 | −0.116 | 0.104 | 0.066 | 0.000 | −0.055 | 0.070 | −0.280 | −0.329 | 0.011 |
| Funneliformis | −0.211 | −0.080 | 0.092 | 0.368 | 0.012 | −0.056 | −0.062 | 0.009 | −0.033 | −0.326 | −0.086 | −0.148 |
| Glomus | 0.047 | −0.099 | −0.087 | −0.169 | 0.050 | −0.231 | 0.048 | −0.168 | 0.483 * | −0.019 | 0.050 | 0.003 |
| Rhizophagus | −0.360 | −0.026 | 0.148 | −0.108 | −0.039 | −0.099 | −0.073 | 0.110 | −0.215 | 0.347 | 0.334 | 0.295 |
| Septoglomus | −0.243 | −0.415 * | −0.151 | −0.063 | −0.080 | 0.073 | −0.103 | 0.055 | −0.263 | 0.274 | 0.215 | 0.027 |
| Glomeraceae | −0.394 | −0.175 | 0.078 | −0.074 | −0.036 | −0.142 | −0.087 | 0.059 | −0.116 | 0.282 | 0.328 | 0.220 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, G.G.; Zhang, B.; Yuan, Z.; Fu, Z.; Yuan, Y.; Zhu, L.; Ma, S.; Zhang, J. Arbuscular Mycorrhizal Fungi Associated with Tree Species in a Planted Forest of Eastern China. Forests 2019, 10, 424. https://doi.org/10.3390/f10050424
Wang J, Wang GG, Zhang B, Yuan Z, Fu Z, Yuan Y, Zhu L, Ma S, Zhang J. Arbuscular Mycorrhizal Fungi Associated with Tree Species in a Planted Forest of Eastern China. Forests. 2019; 10(5):424. https://doi.org/10.3390/f10050424
Chicago/Turabian StyleWang, Jinping, G. Geoff Wang, Bo Zhang, Zhongming Yuan, Zhiyuan Fu, Yingdan Yuan, Lingjun Zhu, Shilin Ma, and Jinchi Zhang. 2019. "Arbuscular Mycorrhizal Fungi Associated with Tree Species in a Planted Forest of Eastern China" Forests 10, no. 5: 424. https://doi.org/10.3390/f10050424
APA StyleWang, J., Wang, G. G., Zhang, B., Yuan, Z., Fu, Z., Yuan, Y., Zhu, L., Ma, S., & Zhang, J. (2019). Arbuscular Mycorrhizal Fungi Associated with Tree Species in a Planted Forest of Eastern China. Forests, 10(5), 424. https://doi.org/10.3390/f10050424




