Well-Aerated Southern Appalachian Forest Soils Demonstrate Significant Potential for Gaseous Nitrogen Loss
Abstract
1. Introduction
2. Methods
2.1. Site Description
2.2. Sample Collection
2.3. Nitrogen Cycling: Potential Rates
2.4. Nitrogen Cycling: Gross Rates
2.5. Carbon Processing
2.6. Soil Chemical, N, and 15N Analyses
2.7. Statistical Procedures
3. Results
3.1. Nitrogen Cycling
3.2. Microbial Biomass and C Lability
3.3. Drivers of Gross Nitrogen Cycling
4. Discussion
4.1. Nitrogen Mineralization and Nitrification
4.2. Gaseous Nitrogen Losses
4.3. Nitrogen Retention by DNRA
4.4. Drivers of Soil Nitrogen Cycling
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cai, M.; Schwartz, J.S.; Robinson, R.B.; Moore, S.E.; Kulp, M.A. Long-term annual and seasonal patterns of acidic deposition and stream water quality in a Great Smoky Mountains high-elevation watershed. Water Air Soil Pollut. 2011, 219, 547–562. [Google Scholar] [CrossRef]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130164. [Google Scholar] [CrossRef] [PubMed]
- Gruber, N.; Galloway, J.N. An Earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293. [Google Scholar] [CrossRef] [PubMed]
- Brookshire, E.; Gerber, S.; Webster, J.R.; Vose, J.M.; Swank, W.T. Direct effects of temperature on forest nitrogen cycling revealed through analysis of long-term watershed records. Glob. Chang. Biol. 2010, 17, 297–308. [Google Scholar] [CrossRef]
- Melillo, J.M.; Steudler, P.A.; Aber, J.D.; Newkirk, K.; Lux, H.; Bowles, F.P.; Catricala, C.; Magill, A.; Ahrens, T.; Morrisseau, S. Soil warming and carbon-cycle feedbacks to the climate system. Science 2002, 298, 2173–2176. [Google Scholar] [CrossRef]
- Churkina, G.; Brovkin, V.; von Bloh, W.; Trusilova, K.; Jung, M.; Dentener, F. Synergy of rising nitrogen depositions and atmospheric CO2 on land carbon uptake moderately offsets global warming. Glob. Biogeochem. Cycles 2009, 23. [Google Scholar] [CrossRef]
- De Vries, W. Assessment of the relative importance of nitrogen deposition and climate change on the sequestration of carbon by forests in Europe: An overview. For. Ecol. Manag. 2009, 258, vii–x. [Google Scholar] [CrossRef]
- Bolstad, P.V.; Vose, J.M.; McNulty, S.G. Forest productivity, leaf area, and terrain in southern Appalachian deciduous forests. For. Sci. 2001, 47, 419–427. [Google Scholar]
- Hofmockel, K.S.; Zak, D.R.; Moran, K.K.; Jastrow, J.D. Changes in forest soil organic matter pools after a decade of elevated CO2 and O3. Soil Biol. Biochem. 2011, 43, 1518–1527. [Google Scholar] [CrossRef]
- Zaehle, S.; Ciais, P.; Friend, A.D.; Prieur, V. Carbon benefits of anthropogenic reactive nitrogen offset by nitrous oxide emissions. Nat. Geosci. 2011, 4, 601–605. [Google Scholar] [CrossRef]
- Yang, W.H.I. Quantification of and Controls on Dinitrogen and Nitrous Oxide Fluxes from Terrestrial Ecosystems. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2010. [Google Scholar]
- Keeney, D.R. Prediction of soil nitrogen availability in forest ecosystems: A literature review. For. Sci. 1980, 26, 159–171. [Google Scholar]
- Reich, P.B.; Grigal, D.F.; Aber, J.D.; Gower, S.T. Nitrogen mineralization and productivity in 50 hardwood and conifer stands on diverse soils. Ecology 1997, 78, 335–347. [Google Scholar] [CrossRef]
- Knoepp, J.; Vose, J.; Swank, W. Nitrogen deposition and cycling across an elevation and vegetation gradient in southern Appalachian forests. Int. J. Environ. Stud. 2008, 65, 391–410. [Google Scholar] [CrossRef]
- Knoepp, J.D.; Swank, W.T. Rates of nitrogen mineralization across an elevation and vegetation gradient in the southern Appalachians. Plant Soil 1998, 204, 235–241. [Google Scholar] [CrossRef]
- Knoepp, J.D.; See, C.R.; Vose, J.M.; Miniat, C.F.; Clark, J.S. Total C and N Pools and Fluxes Vary with Time, Soil Temperature, and Moisture Along an Elevation, Precipitation, and Vegetation Gradient in Southern Appalachian Forests. Ecosystems 2018, 21, 1623–1638. [Google Scholar] [CrossRef]
- Koch, O.; Tscherko, D.; Kandeler, E. Temperature sensitivity of microbial respiration, nitrogen mineralization, and potential soil enzyme activities in organic alpine soils. Glob. Biogeochem. Cycles 2007, 21. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Beusen, A.H.W.; Griffioen, J.; van Groenigen, J.W.; Hefting, M.M.; Oenema, O.; van Puijenbroek, P.J.T.M.; Seitzinger, S.; Slomp, C.P.; Stehfest, E. Global trends and uncertainties in terrestrial denitrification and N2O emissions. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130112. [Google Scholar] [CrossRef]
- Groffman, P.; Butterbach-Bahl, K.; Fulweiler, R.; Gold, A.; Morse, J.; Stander, E.; Tague, C.; Tonitto, C.; Vidon, P. Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrification models. Biogeochemistry 2009, 93, 49–77. [Google Scholar] [CrossRef]
- Seitzinger, S.; Harrison, J.; Böhlke, J.; Bouwman, A.; Lowrance, R.; Peterson, B.; Tobias, C.; Drecht, G. Denitrification across landscapes and waterscapes: A synthesis. Ecol. Appl. 2006, 16, 2064–2090. [Google Scholar] [CrossRef]
- Bonito, G.M.; Coleman, D.C.; Haines, B.L.; Cabrera, M.L. Can nitrogen budgets explain differences in soil nitrogen mineralization rates of forest stands along an elevation gradient? For. Ecol. Manag. 2003, 176, 563–574. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Bernhardt, E.S. Biogeochemistry: An Analysis of Global Change; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Tiedje, J. Ecology of denitrification and dissimilatory nitrate reduction to ammonium. Biol. Anaerob. Microorg. 1988, 179, 244. [Google Scholar]
- Cole, J. Assimilatory and dissimilatory reduction of nitrate to ammonia. In The Nitrogen and Sulphur Cycles; Cambridge University Press: Cambridge, UK, 1988; pp. 281–329. [Google Scholar]
- Davidson, E. Sources of nitric oxide and nitrous oxide following wetting of dry soil. Soil Sci. Soc. Am. J. 1992, 56, 95–102. [Google Scholar] [CrossRef]
- Verchot, L.; Holmes, Z.; Mulon, L.; Groffman, P.; Lovett, G. Gross vs net rates of N mineralization and nitrification as indicators of functional differences between forest types. Soil Biol. Biochem. 2001, 33, 1889–1901. [Google Scholar] [CrossRef]
- Pett-Ridge, J.; Silver, W.; Firestone, M. Redox Fluctuations Frame Microbial Community Impacts on N-cycling Rates in a Humid Tropical Forest Soil. Biogeochemistry 2006, 81, 95–110. [Google Scholar] [CrossRef]
- Morley, N.; Baggs, E.M. Carbon and oxygen controls on N2O and N2 production during nitrate reduction. Soil Biol. Biochem. 2010, 42, 1864–1871. [Google Scholar] [CrossRef]
- Parkin, T. Soil microsites as a source of denitrification variability. Soil Sci Soc. Am. J. 1987, 51, 1194–1199. [Google Scholar] [CrossRef]
- Sexstone, A.; Revsbech, N.; Parkin, T.; Tiedje, J. Direct measurement of oxygen profiles and denitrification rates in soil aggregates. Soil Sci Soc. Am. J. 1985, 49, 645–651. [Google Scholar] [CrossRef]
- Baas, P.; Knoepp, J.D.; Markewitz, D.; Mohan, J.E. Areas of residential development in the southern Appalachian Mountains are characterized by low riparian zone nitrogen cycling and no increase in soil greenhouse gas emissions. Biogeochemistry 2017, 133, 113–125. [Google Scholar] [CrossRef]
- Baas, P.; Markewitz, D.; Knoepp, J.D.; Mohan, J.E. Nitrogen cycling heterogeneity: An approach for plot scale assessments. Soil Sci. Soc. Am. J. 2014, 78, S237–S247. [Google Scholar] [CrossRef]
- McClain, M.E.; Boyer, E.W.; Dent, C.L.; Gergel, S.E.; Grimm, N.B.; Groffman, P.M.; Hart, S.C.; Harvey, J.W.; Johnston, C.A.; Mayorga, E.; et al. Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 2003, 6, 301–312. [Google Scholar] [CrossRef]
- Brandes, J.A.; Devol, A.H.; Deutsch, C. New Developments in the Marine Nitrogen Cycle. Chem. Rev. 2007, 107, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Laseter, S.H.; Ford, C.R.; Vose, J.M.; Swift, L.W. Long-term temperature and precipitation trends at the Coweeta Hydrologic Laboratory, Otto, North Carolina, USA. Hydrol. Res. 2012, 43, 890–901. [Google Scholar] [CrossRef]
- Swift, J.L.W.; Cunningham, G.B. Climatology and Hydrology. In Forest Hydrology and Ecology at Coweeta; Swank, W.T., Crossley, D.A., Jr., Eds.; Springer-Verlag: New York, NY, USA, 1988; Volume 66, p. 469. [Google Scholar]
- Elliott, K.J.; Swank, W.T. Long-term changes in forest composition and diversity following early logging (1919–1923) and the decline of American chestnut (Castanea dentata). Plant Ecol. 2008, 197, 155–172. [Google Scholar] [CrossRef]
- Turner, M.G.; Pearson, S.M.; Bolstad, P.; Wear, D.N. Effects of land-cover change on spatial pattern of forest communities in the Southern Appalachian Mountains (USA). Landsc. Ecol. 2003, 18, 449–464. [Google Scholar] [CrossRef]
- Soil Survey Staff; United States Department of Agriculture Natural Resources Conservation Service. Web Soil Survey. 2013. Available online: http://websoilsurvey.nrcs.usda.gov/ (accessed on 5 January 2013).
- Coweeta Long Term Ecological Research. Available online: http://coweeta.uga.edu/ (accessed on 9 December 2019).
- Groffman, P.; Altabet, M.; Böhlke, J.; Butterbach-Bahl, K.; David, M.; Firestone, M.; Giblin, A.; Kana, T.; Nielsen, L.; Voytek, M. Methods for measuring denitrification: Diverse approaches to a difficult problem. Ecol. Appl. 2006, 16, 2091–2122. [Google Scholar] [CrossRef]
- USEPA. Methods for Chemical Analysis of Water and Waste. Determination of Nitrite/Nitrate by Automated Cadmium Reduction; Method 353.2; Environmental Monitoring and Support Lab., Office of Research and Development, USEPA: Cincinnati, OH, USA, 1983.
- Groffman, P.; Holland, E.; Myrold, D.; Robertson, G.; XiaoMing, Z.; Coleman, D.; Bledsoe, C.; Sollins, P. Denitrification. In Standard Soil Methods for Long-Term Ecological Research; Robertson, G.P., Coleman, D.C., Bledsoe, C.S., Sollins, P., Eds.; Oxford University: Oxford, UK, 1999; pp. 272–288. [Google Scholar]
- Davidson, E.; Hart, S.; Shanks, C.; Firestone, M. Measuring gross nitrogen mineralization, and nitrification by 15N isotopic pool dilution in intact soil cores. Eur. J. Soil Sci. 1991, 42, 335–349. [Google Scholar] [CrossRef]
- Silver, W.; Herman, D.; Firestone, M. Dissimilatory nitrate reduction to ammonium in upland tropical forest soils. Ecology 2001, 82, 2410–2416. [Google Scholar] [CrossRef]
- Cabrera, M.L.; Beare, M.H. Alkaline Persulfate Oxidation for Determining Total Nitrogen in Microbial Biomass Extracts. Soil Sci. Soc. Am. J. 1993, 57, 1007–1012. [Google Scholar] [CrossRef]
- Bradford, M.A.; Watts, B.W.; Davies, C.A. Thermal adaptation of heterotrophic soil respiration in laboratory microcosms. Glob. Chang. Biol. 2010, 16, 1576–1588. [Google Scholar] [CrossRef]
- USEPA. Methods for Chemical Analysis of Water and Waste. Determination of Nitrogen as Ammonia; Method 350.1; Environmental Monitoring and Support Lab., Office of Research and Development, USEPA: Cincinnati, OH, USA, 1983. [Google Scholar]
- Kirkham, D.; Bartholomew, W. Equations for following nutrient transformations in soil, utilizing tracer data. Soil Sci. Soc. Am. J. 1954, 18, 33–34. [Google Scholar] [CrossRef]
- Beck, T.; Joergensen, R.G.; Kandeler, E.; Makeschin, F.; Nuss, E.; Oberholzer, H.R.; Scheu, S. An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol. Biochem. 1997, 29, 1023–1032. [Google Scholar] [CrossRef]
- Brookes, P.; Landman, A.; Pruden, G.; Jenkinson, D. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Mclean, K. Past, Present, and Future Soil Nitrogen and Phosphorus Dynamics in Southern Appalachian Forests; University of Georgia: Athens, GA, USA, 2011. [Google Scholar]
- Hart, S.C.; Nason, G.E.; Myrold, D.D.; Perry, D.A. Dynamics of gross nitrogen transformations in an old-growth forest: The carbon connection. Ecology 1994, 75, 880–891. [Google Scholar] [CrossRef]
- Poth, M.; Focht, D.D. 15N kinetic analysis of N2O production by Nitrosomonas europaea: An examination of nitrifier denitrification. Appl. Environ. Microbiol. 1985, 49, 1134–1141. [Google Scholar]
- Wrage, N.; Velthof, G.; van Beusichem, M.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Silver, W.L.; Thompson, A.W.; Reich, A.; Ewel, J.J.; Firestone, M.K. Nitrogen Cycling in Tropical Plantation Forests: Potential Controls on Nitrogen Retention. Ecol. Appl. 2005, 15, 1604–1614. [Google Scholar] [CrossRef]
- Rütting, T.; Huygens, D.; Müller, C.; van Cleemput, O.; Godoy, R.; Boeckx, P. Functional role of DNRA and nitrite reduction in a pristine south Chilean Nothofagus forest. Biogeochemistry 2008, 90, 243–258. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Matson, P.A. Nitrogen transformations in a range of tropical forest soils. Soil Biol. Biochem. 1988, 20, 361–367. [Google Scholar] [CrossRef]
- Li, Y.; Schichtel, B.A.; Walker, J.T.; Schwede, D.B.; Chen, X.; Lehmann, C.M.; Puchalski, M.A.; Gay, D.A.; Collett, J.L. Increasing importance of deposition of reduced nitrogen in the United States. Proc. Natl. Acad. Sci. USA 2016, 113, 5874–5879. [Google Scholar] [CrossRef]
- National Atmospheric Deposition Program. National Atmospheric Deposition Program 2016 Annual Summary; NADP Data Report, 2017-01; Illinois State Water Survey: Champaign, IL, USA; University of Illinois at Urbana-Champaign: Urbana, IL, USA, 2017. [Google Scholar]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef]
- Bengtson, P.; Barker, J.; Grayston, S.J. Evidence of a strong coupling between root exudation, C and N availability, and stimulated SOM decomposition caused by rhizosphere priming effects. Ecol. Evol. 2012, 2, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Brumme, R.; Borken, W.; Finke, S. Hierarchical control on nitrous oxide emission in forest ecosystems. Glob. Biogeochem. Cycles 1999, 13, 1137–1148. [Google Scholar] [CrossRef]
- Groffman, P.; Davidson, E.; Seitzinger, S. New approaches to modeling denitrification. Biogeochemistry 2009, 93, 1–5. [Google Scholar] [CrossRef]
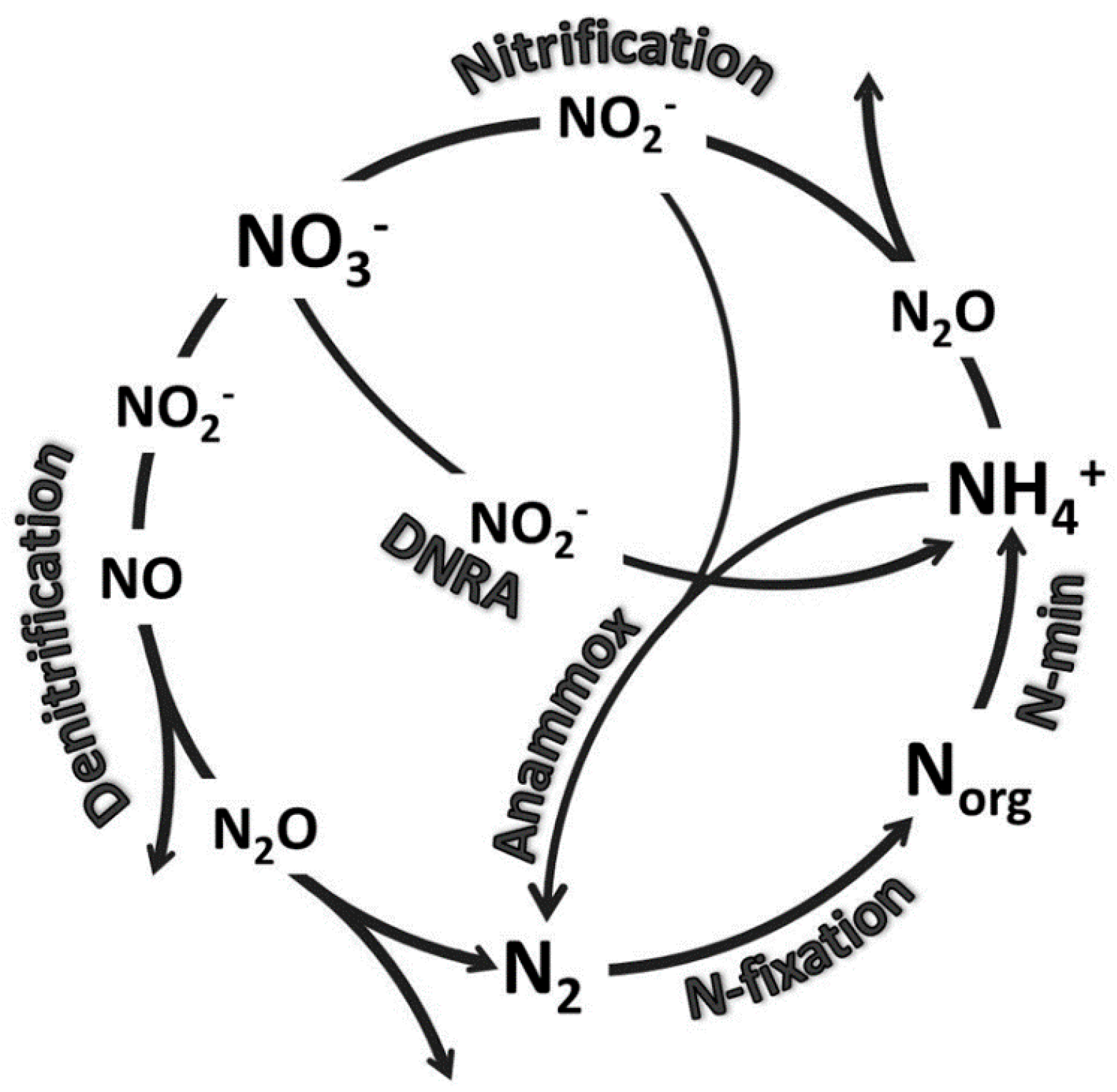
| Site | Mixed Oak–Pine | Cove Hardwood | Mixed Oak | Northern Hardwood |
|---|---|---|---|---|
| Geographic Coordinates | 83°26′ N, 35°3′ W | 83°26′ N, 35°2′ W | 83°26′ N, 35°2′ W | 83°27′ N, 35°1′ W |
| Elevation (m) | 788 | 801 | 860 | 1389 |
| Precipitation (cm) | 187 | 189 | 191 | 238 |
| Temperature (°C) | 12.9 | 12.3 | 12.7 | 9.6 |
| Aspect (degrees) | 180 | 340 | 15 | 20 |
| Slope (degrees) | 34 | 21 | 34 | 33 |
| Vegetation | Oak–pine | Cove hardwood | Mixed oak | Northern hardwood |
| Dominant Species | Pinus rigada, Quercus montana, Quercus prinus, Carya spp., Kalmia latifola | Liriodendron tulipifera, Quercus montana, Carya spp. | Quercus montana, Carya spp., Quercus rubra, Rhododendron maximum | Betula allegheniensis, Quercus rubra, Betula lenta, Tilia heterophylla |
| Moisture Regime | Xeric | Mesic | Mesic | Mesic |
| Soil Series | Evard/Cowee Chandler Edneyville/Chestnut | Saunook Tuckaseegee | Trimont | Sitet |
| Soil Texture | Fine-loamy, Coarse-loamy, Coarse-loamy | Fine-loamy, Fine-loamy | Fine-loamy | Coarse-loamy |
| Soil Subgroup | Typic Hapludults, Typic Dystrudepts | Humic Hapludults, Typic Dystrudepts | Humic Hapludults | Typic Humudepts |
| Process | NO3 | NH4 | N2O | N2 | 15NO3 | 15NH4 | 15N2 | 15N2O |
|---|---|---|---|---|---|---|---|---|
| Net Potential Nitrification | ||||||||
| Net Potential Denitrification | ||||||||
| Gross N Mineralization | ||||||||
| Gross Nitrification | ||||||||
| Gross DNRA | ||||||||
| Gross Nitrifier Denitrification | ||||||||
| Gross N2O Flux |
| Site | Mineralization | Nitrification | DNRA | Nitrifier Denitrification | N2O (× 10−3) |
|---|---|---|---|---|---|
| OP | 0.55 ± 0.33 b | 0.11 ± 0.04 b | 0.01 ± 0.00 b | 0.75 ± 0.10 b | 0.0 ± 0.0 b |
| CH | 2.15 ± 0.39 ab | 0.19 ± 0.08 b | 0.03 ± 0.02 ab | 0.97 ± 0.05 b | 0.0 ± 0.0 b |
| MO | 0.95 ± 0.41 b | 0.10 ± 0.07 b | 0.02 ± 0.02 ab | 1.71 ± 0.22 a | 0.0 ± 0.0 b |
| NH | 7.85 ± 1.34 a | 3.38 ± 1.07 a | 0.07 ± 0.03 a | 2.5 ± 0.27 a | 1.8± 0.42 a |
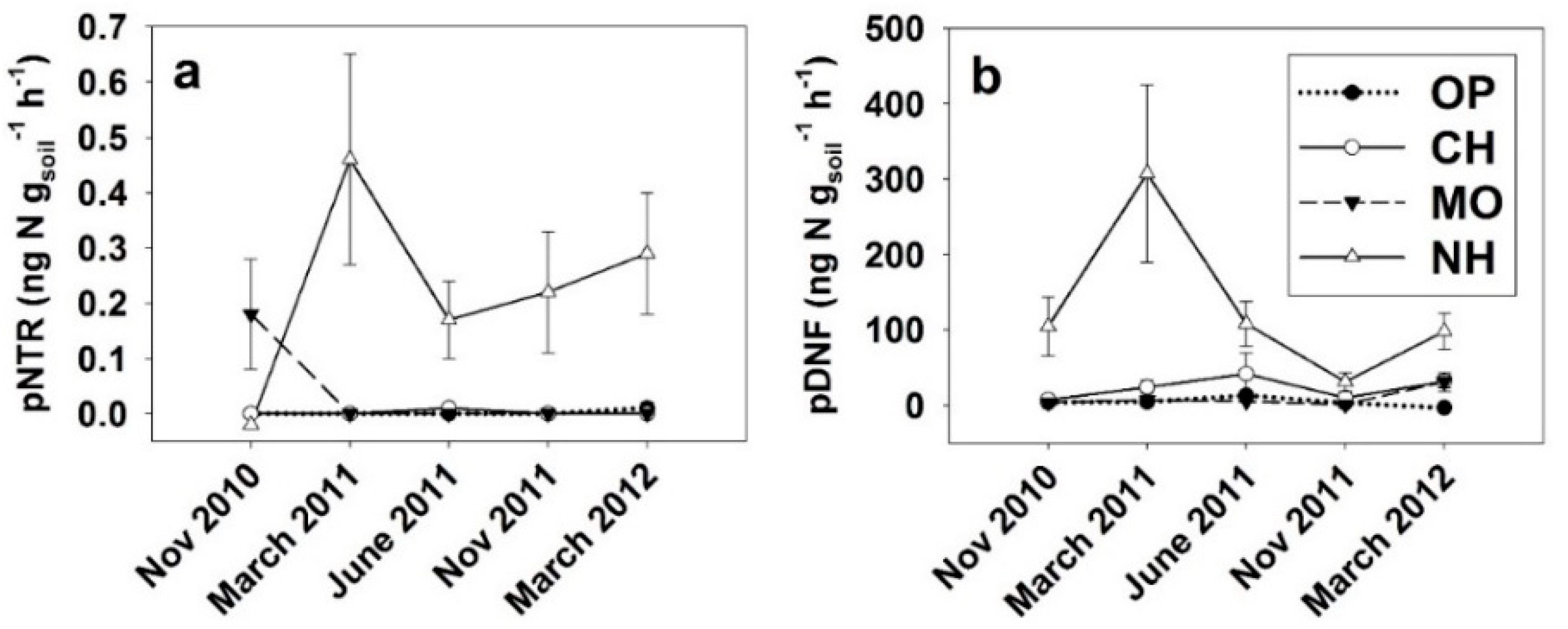
| Site | NH4+ (µg N g−1) | NO3− (µg N g−1) | MB (µg C g−1) | MBN (µg N g−1) | Cmin oxic (µg C g−1 d−1) | Cmin anoxic (µg C g−1 d−1) | pNTR (ng gsoil−1 h−1) | pDNF (ng gsoil−1 h−1) |
|---|---|---|---|---|---|---|---|---|
| OP | 0.79 ± 0.03 (3) c † | 1.26 ± 0.43 (3) b | 287 ± 69 (3) | 127 ± 16 (4) b | 239 ± 106 (3) b | 155 ± 38 (3) b | 0.0 ± 0.0 | 4.6 ± 1.4 b |
| CH | 1.31 ± 0.04 (3) b † | 1.01 ± 0.06 (3) b | 499 ± 164 (3) | 224 ± 17 (3) a | 262 ± 26 (3) b | 117 ± 12 (3) b | 0.0 ± 0.0 | 21.3 ± 7.3 b |
| MO | 0.77 ± 0.00 (3) c † | 0.67 ± 0.05 (3) b | 538 ± 45 (3) | 215 ± 20 (4) a | 455 ± 41 (3) ab | 196 ± 8 (3) ab | 0.0 ± 0.0 | 3.8 ± 0.8 b |
| NH | 5.6 ± 0.23 (3) a † | 5.4 ± 0.44 (3) a | 634 ± 57 (3) | 286 ± 22 (4) a | 632 ± 33 (2) a | 297 ± 30 (2) a | 0.26 ± 0.07 | 147.2 ± 36.8 a |
| Explanatory Variable | N Mineralization | Nitrification | DNRA | Nitrifier Denitrification | N2O | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Excl NH | All | Excl NH | All | Excl NH | All | Excl NH | All | Excl NH | |
| Cmin oxic | 0.27 | −0.32 | −0.02 | −0.40 | 0.55 † | 0.17 | 0.82 ** | 0.68 * | 0.18 | −0.71 † |
| Cmin anoxic | 0.28 | −0.32 | 0.03 | −0.33 | 0.39 | −0.10 | 0.66 * | 0.38 | 0.22 | −0.68 † |
| pDNF | 0.94 ** | 0.87 ** | 0.60 † | 0.27 | 0.69 * | 0.53 | 0.72 ** | 0.35 | 0.64 * | 0.04 |
| N Mineralization | Nitrification | DNRA | Nitrifier Denitrification | N2O ‡ | |
|---|---|---|---|---|---|
| N mineralization | 0.70 (11) * | 0.61 (12) * | 0.64 (12) * | 0.60 (10) † | |
| Nitrification | 0.47 (11) † | 0.67 (11) * | 0.72 (9) * | ||
| DNRA | 0.54 (12) † | 0.62 (10) † | |||
| Nitr. Denitr. | 0.59 (12) † | ||||
| N2O-NH4+ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baas, P.; Knoepp, J.D.; Mohan, J.E. Well-Aerated Southern Appalachian Forest Soils Demonstrate Significant Potential for Gaseous Nitrogen Loss. Forests 2019, 10, 1155. https://doi.org/10.3390/f10121155
Baas P, Knoepp JD, Mohan JE. Well-Aerated Southern Appalachian Forest Soils Demonstrate Significant Potential for Gaseous Nitrogen Loss. Forests. 2019; 10(12):1155. https://doi.org/10.3390/f10121155
Chicago/Turabian StyleBaas, Peter, Jennifer D. Knoepp, and Jacqueline E. Mohan. 2019. "Well-Aerated Southern Appalachian Forest Soils Demonstrate Significant Potential for Gaseous Nitrogen Loss" Forests 10, no. 12: 1155. https://doi.org/10.3390/f10121155
APA StyleBaas, P., Knoepp, J. D., & Mohan, J. E. (2019). Well-Aerated Southern Appalachian Forest Soils Demonstrate Significant Potential for Gaseous Nitrogen Loss. Forests, 10(12), 1155. https://doi.org/10.3390/f10121155





