Thinning Effects on the Tree Height–Diameter Allometry of Masson Pine (Pinus massoniana Lamb.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Description
2.2. Testing the Effects of Thinning on the Height–Diameter Allometry Rhythm
2.2.1. Mathematical Modeling of Height–Diameter Allometry
2.2.2. Dummy Variable Models and Standard F-tests
2.3. Analysis of the Temporal Dynamics of Thinning Effects on Height–Diameter Allometry
2.3.1. Calculation of Relative Increments and Allometric Coefficient
2.3.2. Generalized Additive Mixed Model Analysis
3. Results
3.1. Overall Trends of Thinning Effects on Height–Diameter Allometry
3.1.1. The Best Height–Diameter Allometric Model
3.1.2. Overall Effects of Thinning on Height–Diameter Allometry
3.2. The Temporal Dynamics of Thinning Effects on Height–Diameter Allometry
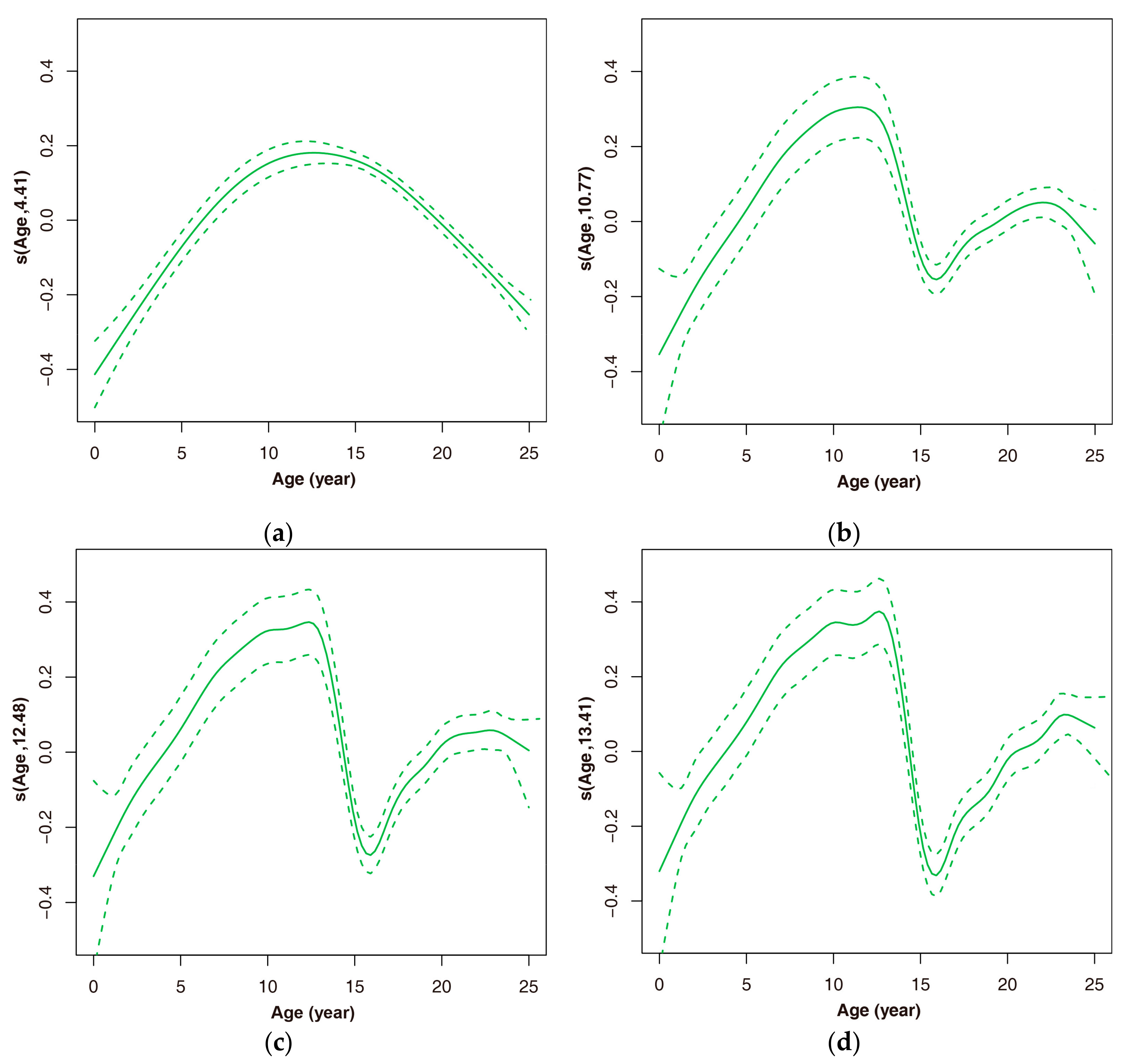
3.2.1. The Natural Allometric Trend of Height–Diameter
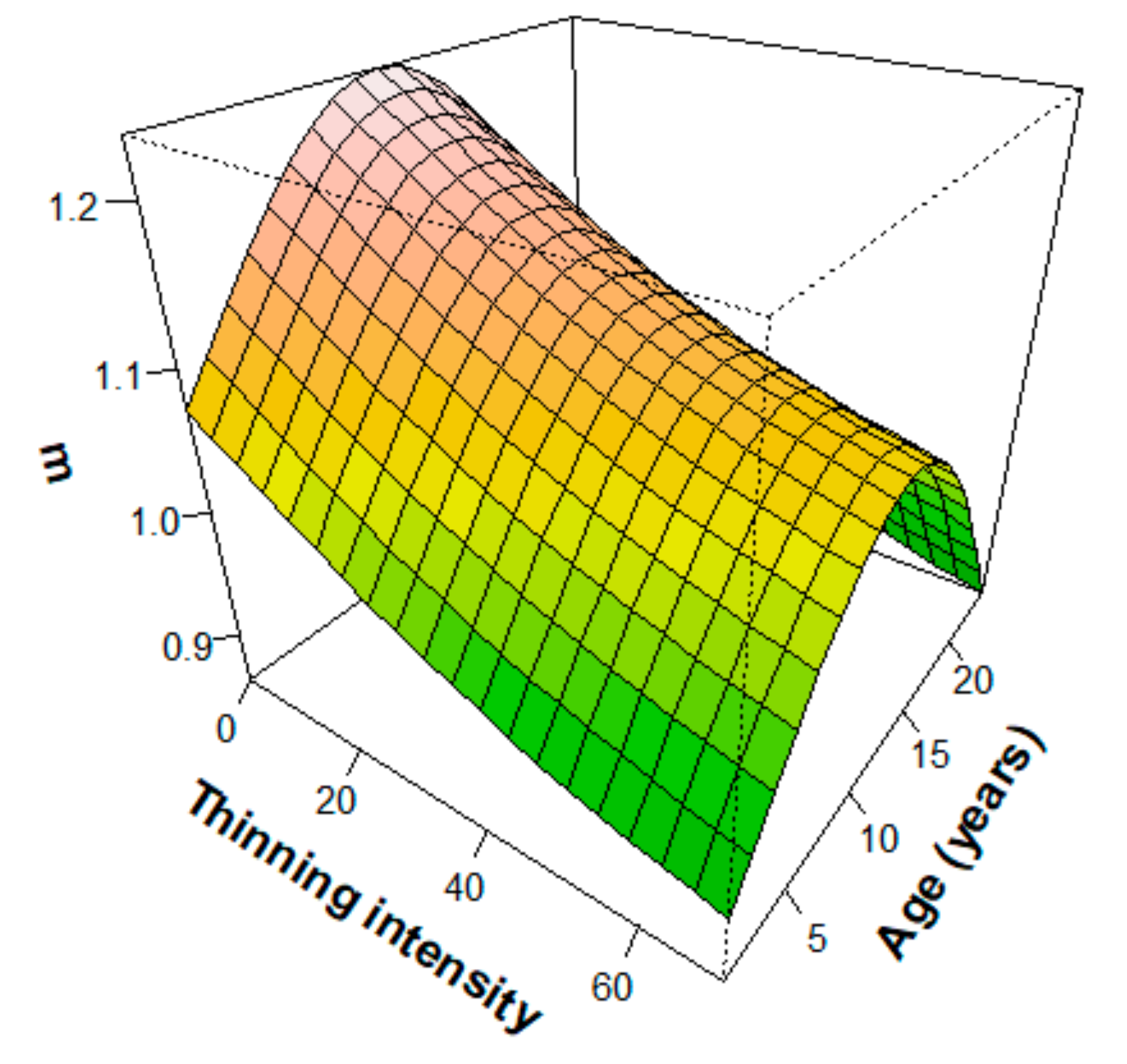
3.2.2. Effects of Thinning on Height–Diameter Allometry
4. Discussions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hulshof, C.M.; Swenson, N.G.; Weiser, M.D. Tree height-diameter allometry across the United States. Ecol. Evol. 2015, 5, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Niklas, K.J.; Spatz, H.C. Growth and hydraulic (not mechanical) constraints govern the scaling of tree height and mass. Proc. Natl. Acad. Sci. USA 2004, 101, 15661–15663. [Google Scholar] [CrossRef] [PubMed]
- Price, C.A.; Enquist, B.J.; Savage, V.M. A general model for allometric covariation in botanical form and function. Proc. Natl. Acad. Sci. USA 2007, 104, 13204–13209. [Google Scholar] [CrossRef] [PubMed]
- Feldpausch, T.R.; Banin, L.; Phillips, O.L.; Baker, T.R.; Lewis, S.L.; Quesada, C.A.; Affum-Baffoe, K.; Arets, E.J.; Berry, N.J.; Bird, M.; et al. Height-diameter allometry of tropical forest trees. Biogeosciences 2011, 8, 1081–1106. [Google Scholar] [CrossRef]
- Poorter, L.; Lianes, E. Architecture of Iberian canopy tree species in relation to wood density, shade tolerance and climate. Plant Ecol. 2012, 213, 707–722. [Google Scholar] [CrossRef]
- Vizcaíno-Palomar, N.; Ibáñez, I.; Benito-Garzón, M.; González-Martínez, S.C.; Zavala, M.A.; Alía, R. Climate and population origin shape pine tree height-diameter allometry. New For. 2017, 48, 363–379. [Google Scholar] [CrossRef]
- King, D.A. Allometry of saplings and understorey trees of a Panamanian forest. Funct. Ecol. 1990, 4, 27–32. [Google Scholar] [CrossRef]
- Harrington, C.A.; DeBell, D.S. Above-and below-ground characteristics associated with wind toppling in a young Populus plantation. Trees 1996, 11, 109–118. [Google Scholar] [CrossRef]
- Thomas, S.C.; Martin, A.R.; Mycroft, E.E.; Bellingham, P. Tropical trees in a wind-exposed island ecosystem: height-diameter allometry and size at onset of maturity. J. Ecol. 2015, 103, 594–605. [Google Scholar] [CrossRef]
- Tilman, D. Plant strategies and the dynamics and structure of plant communities. Bull. Math. Biol. 1989, 4, 28–29. [Google Scholar]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.G.; Phillips, N.; Bond, B.J. The hydraulic limitation hypothesis revisited. Plant Cell Environ. 2006, 29, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Henry, H.A.L.; Aarssen, L.W. The interpretation of stem diameter–height allometry in trees: biomechanical constraints, neighbour effects, or biased regressions? Ecol. Lett. 2010, 2, 89–97. [Google Scholar] [CrossRef]
- Moles, A.T.; Warton, D.I.; Warman, L.; Swenson, N.G.; Laffan, S.W.; Zanne, A.E.; Pitman, A.; Hemmings, F.A.; Leishman, M. Global patterns in plant height. J. Ecol. 2009, 97, 923–932. [Google Scholar] [CrossRef]
- McMahon, T. Size and shape in biology. Science 1973, 179, 1201–1204. [Google Scholar] [CrossRef]
- Niklas, K.J. The scaling of plant height: A comparison among major plant clades and anatomical grades. Annals of Botany (Lond.) 1993, 72, 165–172. [Google Scholar] [CrossRef]
- Bullock, S.H. Developmental patterns of tree dimensions in a neotropical deciduous forest. Biotropica 2000, 32, 42–52. [Google Scholar] [CrossRef]
- Vizcaíno-Palomar, N.; Ibáñez, I.; González-Martínez, S.C.; Zavala, M.A.; Alía, R. Adaptation and plasticity in aboveground allometry variation of four pine species along environmental gradients. Ecol. Evol. 2016, 6, 7561–7573. [Google Scholar] [CrossRef]
- Kobe, R.K. Light gradient partitioning among tropical tree species through differential seedling mortality and growth. Ecology 1999, 80, 187–201. [Google Scholar] [CrossRef]
- Niklas, K.J. Size-dependent allometry of tree height, diameter and trunk-taper. Ann. Bot. 1995, 75, 217–227. [Google Scholar] [CrossRef]
- Banin, L.; Feldpausch, T.R.; Phillips, O.L.; Baker, T.R.; Lloyd, J.; Affum-Baffoe, K.; Arets, E.J.; Berry, N.J.; Bradford, M.; Brienen, R.J.; et al. What controls tropical forest architecture? Testing environmental, structural and floristic drivers. Glob. Ecol. Biogeogr. 2012, 21, 1179–1190. [Google Scholar] [CrossRef]
- Fayolle, A.; Panzou, G.J.L.; Drouet, T.; Swaine, M.D.; Bauwens, S.; Vleminckx, J.; Biwole, A.; Lejeune, P.; Doucet, J.L. Taller trees, denser stands and greater biomass in semi-deciduous than in evergreen lowland central African forests. For. Ecol. Manag. 2016, 374, 42–50. [Google Scholar] [CrossRef]
- Imani, G.; Boyemba, F.; Lewis, S.; Nabahungu, N.L.; Calders, K.; Zapfack, L.; Riera, B.; Balegamire, C.; Cuni-Sanchez, A. Height-diameter allometry and above ground biomass in tropical montane forests: Insights from the Albertine Rift in Africa. PLoS ONE 2017, 12, e0179653. [Google Scholar] [CrossRef] [PubMed]
- Motallebi, A.; Kangur, A. Are allometric relationships between tree height and diameter dependent on environmental conditions and management? Trees 2016, 30, 1429–1443. [Google Scholar] [CrossRef]
- Mensah, S.; Pienaar, O.L.; Kunneke, A.; Du Toit, B.; Seydack, A.; Uhl, E.; Pretzsch, H.; Seifert, T. Height-Diameter allometry in South Africa’s indigenous high forests: Assessing generic models performance and function forms. For. Ecol. Manag. 2018, 410, 1–11. [Google Scholar] [CrossRef]
- Khamyong, N.; Wangpakapattanawong, P.; Chairuangsri, S.; Inta, A.; Tiansawat, P. Tree species composition and height-diameter allometry of three forest types in Northern Thailand. CMU J. Nat. Sci. 2018, 17, 289–306. [Google Scholar] [CrossRef]
- Allen, R.H.; Marquis, D.A. Effect of Thinning on Height and Diameter Growth of Oak and Yellow-Poplar Saplings; Northeastern Forest Experiment Station, Forest Service, USDA For. Serv.: Upper Darby, PA, USA, 1970; Res. Pap. NE-173; pp. 1–11. [Google Scholar]
- David, H.C.; Arce, J.E.; Netto, S.P.; Corte, A.P.D.; Marinheski Filho, A.; Araújo, E.J.G.D. Thinning and site quality effects on the height-diameter relationship of Pinus taeda. Floresta E Ambiente 2016, 23, 220–228. [Google Scholar] [CrossRef][Green Version]
- Medeiros, R.A.; Paiva, H.N.D.; Soares, A.A.V.; Cruz, J.D.; Leite, H.G. Thinning from below: effects on height of dominant trees and diameter distribution in Eucalyptus stands. J. Trop. For. Sci. 2017, 29, 238–247. [Google Scholar]
- Drew, T.J.; Flewelling, J.W. Stand density management: An alternative approach and its application to Douglas-fir plantations. For. Sci. 1979, 25, 518–532. [Google Scholar]
- Smith, D.M.; Larson, B.C.; Kelty, M.J.; Ashton, P.M.S. The Practice of Silviculture: Applied Forest Ecology; John Wiley and Sons, Inc.: New York, NY, USA, 1997. [Google Scholar]
- Zeide, B. Thinning and growth: A full turnaround. J. For. 2001, 99, 20–25. [Google Scholar]
- Nishizono, T. Effects of thinning level and site productivity on age-related changes in stand volume growth can be explained by a single rescaled growth curve. For. Ecol. Manag. 2010, 259, 2276–2291. [Google Scholar] [CrossRef]
- Hayes, J.P.; Chan, S.S.; Emmingham, W.H.; Tappeiner, J.C.; Kellogg, L.D.; Bailey, J.D. Wildlife response to thinning young forests in the Pacific Northwest. J. For. 1997, 95, 28–33. [Google Scholar]
- Miller, M.; Emmingham, B. Can selection thinning convert even-age Douglas-fir stands to uneven-age structures? West. J. Appl. For. 2001, 16, 35–43. [Google Scholar] [CrossRef]
- Bradford, J.B.; Palik, B.J. A comparison of thinning methods in red pine: Consequences for stand-level growth and tree diameter. Can. J. For. Res. 2009, 39, 489–496. [Google Scholar] [CrossRef]
- Gavinet, J.; Vilagrosa, A.; Chirino, E.; Granados, M.E.; Vallejo, V.R.; Prévosto, B. Hardwood seedling establishment below Aleppo pine depends on thinning intensity in two Mediterranean sites. Ann. For. Sci. 2015, 72, 999–1008. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Sánchez-Salguero, R.; Rodriguez, C.; Lazo, J.D.; Moreno-Rojas, J.M.; Palacios-Rodriguez, G.; Camarero, J.J. Is thinning an alternative when trees could die in response to drought? The case of planted Pinus nigra and P. Sylvestris stands in southern Spain. For. Ecol. Manag. 2019, 433, 313–324. [Google Scholar] [CrossRef]
- Kerr, G. The effect of heavy or ‘free growth’ thinning on oak (Quercus petraea and Q. robur). Forestry 1996, 69, 303–317. [Google Scholar] [CrossRef]
- Peltola, H.; Miina, J.; Rouvinen, I.; Kellomäki, S. Effect of early thinning on the diameter growth distribution along the stem of Scots pine. Silva Fenn. 2002, 36, 813–825. [Google Scholar] [CrossRef]
- Mäkinen, H.; Isomäki, A. Thinning intensity and growth of Norway spruce stands in Finland. Forestry 2004, 77, 349–364. [Google Scholar] [CrossRef]
- Juodvalkis, A.; Kairiukstis, L.; Vasiliauskas, R. Effects of thinning on growth of six tree species in north-temperate forests of Lithuania. Eur. J. For. Res. 2005, 124, 187–192. [Google Scholar] [CrossRef]
- Ulvcrona, K.A.; Karlsson, K.; Ulvcrona, T. Identifying the biological effects of pre-commercial thinning on diameter growth in young Scots pine stands. Scand. J. For. Res. 2014, 29, 427–435. [Google Scholar] [CrossRef]
- Ferraz, Filho, A.C.; Mola-Yudego, B.; Gonzalez-Olabarria, J.R.; Scolforo, J.R.S. Thinning regimes and initial spacing for Eucalyptus plantations in Brazil. An Acad Bras Cienc. 2018, 90, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.W. Fifteen-Year Growth Patterns after Thinning a Ponderosa-Jeffrey Pine Plantation in Northeastern California. Res. Pap. PSW-RP-141; U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: Berkeley, CA, USA, 1979; Volume 10, p. 141. [Google Scholar]
- Harrington, C.A.; Reukema, D.L. Initial shock and long-term stand development following thinning in a Douglas-fir plantation. For. Sci. 1983, 29, 33–46. [Google Scholar]
- Schubert, G.H. Growth response of even-aged ponderosa pine related to stand density levels in Arizona. J. For. 1971, 69, 857–860. [Google Scholar]
- Weber, V.P.; Finger, C.A.G.; Fleig, F.D.; Souza, C.A.M.D.; Munareto, F.F.; Silva, L.T.D.M.D. Guidelines for thinning of Pinus taeda L. because of dominant height. Ciência Florest. 2013, 23, 193–201. [Google Scholar]
- Fedorová, B.; Kadavý, J.; Adamec, Z.; Kneifl, M.; Knott, R. Response of diameter and height increment to thinning in oak-hornbeam coppice in the southeastern part of the Czech Republic. J. For. Sci. 2016, 62, 229–235. [Google Scholar] [CrossRef]
- Swift, D.E.; Knight, W.; Béland, M.; Boureima, I.; Bourque, C.P.A.; Meng, F.R. Stand dynamics and tree quality response to precommercial thinning in a northern hardwood forest of the Acadian forest region: 23 years of intermediate results. Scand. J. For. Res. 2016, 32, 45–59. [Google Scholar] [CrossRef]
- Larson, P.R. Stem form development of forest trees. For. Sci. 1963, 9, a0001–a0042. [Google Scholar] [CrossRef]
- Zhang, S.; Burkhart, H.E.; Amateis, R.L. The influence of thinning on tree height and diameter relationships in loblolly pine plantations. South. J. Appl. For. 1997, 21, 199–205. [Google Scholar] [CrossRef]
- Sharma, M.; Smith, M.; Burkhart, H.E.; Amateis, R.L. Modeling the impact of thinning on height development of dominant and codominant loblolly pine trees. Ann. For. Sci. 2006, 63, 349–354. [Google Scholar] [CrossRef]
- Russell, M.B.; Amateis, R.L.; Burkhart, H.E. Implementing regional locale and thinning response in the loblolly pine height-diameter relationship. South. J. Appl. For. 2010, 34, 21–27. [Google Scholar] [CrossRef]
- Missanjo, E.; Kamanga-Thole, G. Effect of first thinning and pruning on the individual growth of pinus patulatree species. J. For. Res. 2015, 26, 827–831. [Google Scholar] [CrossRef]
- Ginn, S.E.; Seiler, J.R.; Cazell, B.H.; Kreh, R.E. Physiological and growth responses of eight-year-old loblolly pine stands to thinning. For. Sci. 1991, 37, 1030–1040. [Google Scholar]
- Voelker, S.L.; Lachenbruch, B.; Meinzer, F.C.; Strauss, S.H. Reduced wood stiffness and strength, and altered stem form, in young antisense 4CL transgenic poplars with reduced lignin contents. New Phytol. 2011, 189, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Eckenwalder, J.E. Conifers of the World; Timber Press, Inc.: Portland, OR, USA, 2009; p. 720. [Google Scholar]
- Farjon, A. A Handbook of the World’s Conifers; Brill Academic Publishers: Leiden, The Netherlands, 2010; p. 1112. [Google Scholar]
- Payn, T.; Carnus, J.M.; Freer-Smith, P.; Kimberley, M.; Kollert, W.; Liu, S.R.; Orazio, C.; Rodriguez, L.; Silva, L.N.; Wingfield, M.J. Changes in planted forests and future global implications. For. Ecol. Manag. 2015, 352, 57–67. [Google Scholar] [CrossRef]
- Huang, S.; Titus, S.J.; Wiens, D.P. Comparison of nonlinear height-diameter functions for major alberta tree species. Can. J. For. Res. 1992, 22, 1297–1304. [Google Scholar] [CrossRef]
- Ledo, A.; Cornulier, T.; Illian, J.B.; Iida, Y.; Kassim, A.R.; Burslem, D.F. Re-evaluation of individual diameter: Height allometric models to improve biomass estimation of tropical trees. Ecol. Appl. 2016, 26, 2376–2382. [Google Scholar] [CrossRef]
- Winsor, C.P. The Gompertz Curve as a Growth Curve. Proc. Natl. Acad. Sci. USA 1932, 18, 1–8. [Google Scholar] [CrossRef]
- Pearl, R.; Reed, L.J. On the rate of growth of the population of the United States since 1790 and its mathematical representation. Proc. Natl. Acad. Sci. USA 1920, 6, 275–288. [Google Scholar] [CrossRef]
- Huxley, A. Problems of Relative Growth; The Dial Press: New York, NY, USA, 1932. [Google Scholar]
- Richards, F.J. A flexible growth function for empirical use. J. Exp. Bot. 1959, 10, 290–301. [Google Scholar] [CrossRef]
- Wykoff, W.R.; Crookston, N.L.; Stage, A.R. User’s Guide to the Stand Prognosis Model. Gen. Tech. Rep. INT-133; US Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1982; Volume 112, p. 133. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-137. 2018. Available online: https://CRAN.R-project.org/package=nlme (accessed on 20 September 2019).
- Akaike, H. A new look at the statistical model identification. Autom. Control IEEE Trans. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Mugasha, W.A.; Bollandsås, O.M.; Eid, T. Relationships between diameter and height of trees in natural tropical forest in Tanzania. South. For. A J. For. Sci. 2013, 75, 221–237. [Google Scholar] [CrossRef]
- Kearsley, E.; Moonen, P.C.; Hufkens, K.; Doetterl, S.; Lisingo, J.; Bosela, F.B.; Boeckx, P.; Beeckman, H.; Verbeeck, H. Model performance of tree height-diameter relationships in the central Congo basin. Ann. For. Sci. 2017, 74, 7. [Google Scholar] [CrossRef]
- Buford, M.A.; Burkhart, H.E. Genetic improvement effects on growth and yield of loblolly pine plantations. For. Sci. 1987, 33, 707–724. [Google Scholar]
- Gayon, J. History of the concept of allometry. Am. Zool. 2000, 40, 748–758. [Google Scholar] [CrossRef]
- Murphy, S.T.; Pommerening, A. Modelling the growth of sitka spruce (Picea sitchensis (bong.) carr.) in wales using wenk’s model approach. Allg. Forst Und Jagdztg. 2010, 181, 35–43. [Google Scholar]
- Pommerening, A.; Muszta, A. Relative plant growth revisited: Towards a mathematical standardisation of separate approaches. Ecol. Model. 2016, 320, 383–392. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, D.; Fryzlewicz, P.; Keilegom, I.V. Inference in generalized additive mixed modelsby using smoothing splines. J. R. Stat. Soc. 2010, 61, 381–400. [Google Scholar] [CrossRef]
- Groll, A.; Tutz, G. Regularization for generalized additive mixed models by likelihood-based boosting. Methods Inf. Med. 2012, 51, 168–177. [Google Scholar]
- Latimer, A.M.; Wu, S.; Gelfand, A.E.; Silander, J.A. Building statistical models to analyze species distributions. Ecol. Appl. 2006, 16, 33–50. [Google Scholar] [CrossRef]
- Simpson, S.L.; Edwards, L.J.; Muller, K.E.; Sen, P.K.; Styner, M.A. A linear exponent AR(1) family of correlation structures. Stat. Med. 2010, 29, 1825–1838. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qin, G.; Zhao, N.; Wang, C.; Song, G. Using a generalized additive model with autoregressive terms to study the effects of daily temperature on mortality. BMC Med. Res. Methodol. 2012, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.F. First Order Autoregressive Mixed Effects Zero Inflated Poisson Model for Longitudinal Data—A Bayesian Approach. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 2014; p. 12. [Google Scholar]
- Mellor, A.F.P.; Cey, E.E. Using generalized additive mixed models to assess spatial, temporal, and hydrologic controls on bacteria and nitrate in a vulnerable agricultural aquifer. J. Contam. Hydrol. 2015, 182, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Temesgen, H.; Gadow, K.V. Generalized height-diameter models—An application for major tree species in complex stands of interior British Columbia. Eur. J. For. Res. 2004, 123, 45–51. [Google Scholar] [CrossRef]
- Pretzsch, H. Re-Evaluation of Allometry: State-of-the-Art and Perspective Regarding Individuals and Stands of Woody Plants. In Progress in Botany 71; Springer: Berlin/Heidelberg, Germany, 2010; pp. 339–369. [Google Scholar]
- Pretzsch, H.; Dieler, J. Evidence of variant intra-and interspecific scaling of tree crown structure and relevance for allometric theory. Oecologia 2012, 169, 637–649. [Google Scholar] [CrossRef]
- Franceschini, T.; Schneider, R. Influence of shade tolerance and development stage on the allometry of ten temperate tree species. Oecologia 2014, 176, 739–749. [Google Scholar] [CrossRef]
- Blanchard, E.; Birnbaum, P.; Ibanez, T.; Boutreux, T.; Antin, C.; Ploton, P.; Vincent, G.; Pouteau, R.; Vandrot, H.; Hequet, V.; et al. Contrasted allometries between stem diameter, crown area, and tree height in five tropical biogeographic areas. Trees 2016, 30, 1953–1968. [Google Scholar] [CrossRef]
- Russo, S.E.; Wiser, S.K.; Coomes, D.A. Growth–size scaling relationships of woody plant species differ from predictions of the Metabolic Ecology Model. Ecol. Lett. 2007, 10, 889–901. [Google Scholar] [CrossRef]
- Watt, M.S.; Kirschbaum, M.U. Moving beyond simple linear allometric relationships between tree height and diameter. Ecol. Model. 2011, 222, 3910–3916. [Google Scholar] [CrossRef]
- Bragg, D.C. Potential relative increment (PRI): A new method to empirically derive optimal tree diameter growth. Ecol. Model. 2001, 137, 77–92. [Google Scholar] [CrossRef]
- King, D.A. Linking tree form, allocation and growth with an allometrically explicit model. Ecol. Model. 2005, 185, 77–91. [Google Scholar] [CrossRef]
- Pommerening, A.; Muszta, A. Methods of modelling relative growth rate. For. Ecosyst. 2015, 2, 5. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Multivariate Allometry. In Advances in Morphometrics; Marcus, L.F., Corti, M., Loy, A., Naylor, G.J.P., Slice, D.E., Eds.; Plenum: New York, NY, USA, 1996; pp. 23–49. [Google Scholar]
- Vospernik, S.; Monserud, R.A.; Sterba, H. Do individual-tree growth models correctly represent height: diameter ratios of Norway spruce and Scots pine? For. Ecol. Manag. 2010, 260, 1735–1753. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Dauber, E.; Biber, P. Species-specific and ontogeny-related stem allometry of European forest trees: evidence from extensive stem analyses. For. Sci. 2013, 59, 290–302. [Google Scholar] [CrossRef]
- Ryan, M.G.; Yoder, B.J. Hydraulic limits to tree height and tree growth. Bioscience 1997, 47, 235–242. [Google Scholar] [CrossRef]
- Weiner, J. Allocation, plasticity and allometry in plants. Perspectives in Plant Ecology. Evol. Syst. 2004, 6, 207–215. [Google Scholar]
- Bonser, S.P.; Aarssen, L.W. Plastic allometry in young sugar maple (Acer saccharum): adaptive responses to light availability. Am. J. Bot. 1994, 81, 400–406. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Vanderklein, D.; Mencuccini, M. Tree height and age-related decline in growth in Scots pine (Pinus sylvestris L.). Oecologia 2007, 150, 529–544. [Google Scholar] [CrossRef]
- Botero, C.A.; Weissing, F.J.; Wright, J.; Rubenstein, D.R. Evolutionary tipping points in the capacity to adapt to environmental change. Proc. Natl. Acad. Sci. USA 2015, 112, 184–189. [Google Scholar] [CrossRef]
- Cañellas, I.; Río, M.; Roig, S.; Montero, G. Growth response to thinning in Quercus pyrenaica Willd. coppice stands in Spanish central mountain. Ann. For. Sci. 2004, 61, 243–250. [Google Scholar] [CrossRef]
- Jiang, L.; Ye, M.; Zhu, S.; Zhai, Y.; Xu, M.; Huang, M.; Wu, R. Computational identification of genes modulating stem height-diameter allometry. Plant Biotechnol. J. 2016, 14, 2254–2264. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Stand Density (Stems/ha) | Number of Sample Trees | DBH | H | ||||
|---|---|---|---|---|---|---|---|---|
| 2007b | 2007a | 2016 | 2007b | 2007a | 2016 | |||
| T0 | 1400 | 224 | 17.3 | 17.3 | 25.2 | 12.2 | 12.2 | 16.0 |
| T1 | 1375 | 117 | 17.5 | 18.3 | 29.1 | 12.3 | 12.7 | 17.5 |
| T2 | 1425 | 71 | 17.2 | 18.5 | 31.4 | 12.2 | 12.9 | 18.1 |
| T3 | 1325 | 49 | 17.7 | 19.9 | 34.1 | 12.5 | 13.4 | 19.1 |
| Model | Function | References |
|---|---|---|
| Gompertz | Winsor, 1932 [63] | |
| Logistic | Pearl and Reed, 1920 [64] | |
| Power | Huxley, 1932 [65] | |
| Richards | Richards, 1959 [66] | |
| Wykoff | Wykoff et al., 1982 [67] |
| Model | α | β | γ | AIC | RSE | R2 |
|---|---|---|---|---|---|---|
| Gompertz | 21.949 | 1.990 | 0.073 | 11,764.952 | 0.863 | 0.896 |
| Logistic | 21.490 | 4.186 | 0.107 | 12,329.021 | 0.918 | 0.879 |
| Power | 2.186 | 0.612 | - | 10,960.653 | 0.791 | 0.906 |
| Richards | 32.966 | 0.019 | 0.851 | 11,055.019 | 0.801 | 0.903 |
| Wykoff | 3.258 | −15.083 | - | 12,082.396 | 0.893 | 0.892 |
| Parameters | Estimate | Standard Error | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| α0 | 1.830 | 0.036 | 1.760 | 1.899 |
| β0 | 0.665 | 0.006 | 0.653 | 0.677 |
| α1 | −0.011 | 0.057 | −0.123 | 0.101 |
| α2 | 0.162 | 0.062 | 0.040 | 0.284 |
| α3 | 0.009 | 0.064 | −0.117 | 0.135 |
| β1 | 0.006 | 0.010 | −0.014 | 0.025 |
| β2 | −0.029 | 0.010 | −0.049 | −0.009 |
| β3 | −0.007 | 0.011 | −0.028 | 0.014 |
| Equation | RSS | DF | Calculated F Value | Critical F Value | p |
|---|---|---|---|---|---|
| (1) | 3673.68 | 4625 | - | - | - |
| (2) | 3591.62 | 4622 | F2−1 = 35.29 | 2.61 | <0.05 |
| (3) | 3582.38 | 4619 | F3−2 = 4.02 | 2.61 | <0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, C.; Zhang, S.; Lu, Y.; Froese, R.E.; Ming, A.; Li, Q. Thinning Effects on the Tree Height–Diameter Allometry of Masson Pine (Pinus massoniana Lamb.). Forests 2019, 10, 1129. https://doi.org/10.3390/f10121129
Deng C, Zhang S, Lu Y, Froese RE, Ming A, Li Q. Thinning Effects on the Tree Height–Diameter Allometry of Masson Pine (Pinus massoniana Lamb.). Forests. 2019; 10(12):1129. https://doi.org/10.3390/f10121129
Chicago/Turabian StyleDeng, Cheng, Shougong Zhang, Yuanchang Lu, Robert E. Froese, Angang Ming, and Qingfen Li. 2019. "Thinning Effects on the Tree Height–Diameter Allometry of Masson Pine (Pinus massoniana Lamb.)" Forests 10, no. 12: 1129. https://doi.org/10.3390/f10121129
APA StyleDeng, C., Zhang, S., Lu, Y., Froese, R. E., Ming, A., & Li, Q. (2019). Thinning Effects on the Tree Height–Diameter Allometry of Masson Pine (Pinus massoniana Lamb.). Forests, 10(12), 1129. https://doi.org/10.3390/f10121129





