1. Introduction
Chinese fir (
Cunninghamia lanceolata (Lamb.) Hook.) is an evergreen conifer, which has been widely planted for timber in southeastern Asia for more than 1000 years [
1]. It is the most commercially important afforestation tree species in southern China with fast growth [
2], high yield, and good material quality and pest resistance [
3,
4]. Because of its fast-growing characteristics, Chinese fir has a high nutrient requirement for its optimal growth, especially nitrogen and phosphorus, which are scarce in forest soils in southern China [
5,
6]. Furthermore, successive rotation of Chinese fir plantations amplifies the lack of available N and P in forest soil due to clear-cutting and subsequent burning practice [
7]. It had been found that the yield of Chinese fir plantation decreased by 5–15% with successive planting in recent years and the N and P deficiency was the most important factor limiting growth of the species. Thus, application of N and P fertilizers has been commonly used in Chinese fir plantations, which is costly for tree growers and causing serious issues of soil and water pollution. In addition, the extremely harsh weather conditions typical of southern China coupled with seasonal droughts due to global climate change seriously affect the survival of seedlings at the beginning of reforestation [
8]. Most previous studies on Chinese fir focused on morphological and physiological adaptation mechanisms to nutrient stress including root morphological plasticity [
9], root secretion of organic acids [
10], formation of cortical aerenchyma [
11], and physiological plasticity and biochemical changes [
12]. However, studies on gene expression and molecular biology in Chinese fir are scarce. Thus, it is essential to understand the mechanisms underlying the response of Chinese fir to abiotic stresses using gene expression analysis in order to develop stress tolerant genotypes.
Transcriptome analysis is mainly used to detect the gene expression profiles and quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) is a stable method with the advantages of high precision, accuracy, and specificity for detecting target gene expression [
13]. It has become one of the most powerful tools used in molecular biology research [
14]. However, the results of qRT-PCR are easily prone to interference with experimental errors such as RNA quality, sample loading, and reverse transcription efficiency. Therefore, it requires internal reference genes to correct these experimental errors between samples [
15]. The ideal reference genes are thought to be equally expressed in different samples, developmental stages, and hence they can be used to measure the expressions of the target genes [
16]. The accuracy and reliability of qRT-PCR results is greatly dependent on a suitable reference gene to normalize gene expression and avoid errors caused by different experimental procedure [
17,
18]. The more stable the expression of the internal reference gene is, the better the accuracy of expression level of the target gene will be [
18]. Usually, housekeeping genes like
Actin,
EF1a (elongation factor 1-a),
GAPDH (glyceraldehyde-3-phosphate dehydrogenase),
18S rRNA (18S ribosomal RNA) etc. are used as reference genes [
19,
20] but previous studies have reported that the stability of expression of these reference genes is relative, and the stability of expression of housekeeping genes varies considerably in different plant species, different tissues, and different experimental conditions [
15,
21]. Particularly in some non-model plant species, like Chinese fir, for which there is a lack of genome sequence and poor genetic background information, the reference genes are usually used by the orthologous sequence of common housekeeping genes reported in model plant species [
22], which is the probable case of deviation of the accuracy in qRT-PCR experiment [
23,
24]. Therefore, it is important to screen and evaluate the stability of reference gene expression according to experimental conditions to improve the accuracy of qRT-PCR analyses [
25,
26,
27].
Due to the importance of reference gene selection, there are an increasing number of attempts to identify reference genes in different experimental conditions. Nicot et al. [
28] analyzed the expression stability of seven housekeeping genes of
Actin,
APRT (Adeninephosphorribosyltransferase),
18S rRNA,
EF1a,
TUB (Tubulin),
CYP (cytochrome), and
Ribosomal protein L2 under biological stress (late blight) and abiotic stress (salt, drought) in potato, and found variation in the expression level of target gene
hsp20.2 according to the standard of reference genes with different stability. Hong et al. [
29] analyzed the expression stability of nine reference genes in 21 tissues and organs at different developmental stages under abiotic stresses (drought, salt, cold, heat) and hormone conditions in
Brachpodium distachyon, and reported that
UBC18 (ubiquitin-conjugating enzyme) was the most stable expression in all samples, and the expression stability of
UBQ4 (ubiquitin) and
UBQ10 were the best in different tissues, organs and developmental stages, and
SamDC was the most stable gene in environmental stress samples. Additionally, reference gene validation has been done in many plant species, such as
Gossypium barbadense [
30],
Saccharum officinarum [
31],
Lycopersicon esculentum [
32],
Vitis viniferag [
33],
Glycine max [
34],
Musa acuminata [
35],
Oryza sativa [
36],
Linum usitatissimum [
37],
Kosteletzkya virginica [
20],
Sesamum indicum [
38], and
Populus euphratica [
39]. However, few studies have been conducted on the selection and expression stability of reference genes in Chinese fir, such as selection of reference genes under cold, high temperature, abscisic acid (ABA), polyethylene glycol (PEG), and sodium chloride (NaCl), and the selected reference was different for different experimental conditions [
40,
41].
Gene expression studies on forest trees have become important to unravel the adaptation mechanisms to environmental conditions at molecular level. Drought stress and N and P starvation are key problems of forest tree growth in general and Chinese fir cultivation in particular, and the molecular research to address these problems depends on a prerequisite of screening and evaluation of the stability of reference gene for normalization of qRT-PCR gene expression analysis. Based on the transcriptome data of Chinese fir roots under drought stress and P and N starvation, we have chosen nine candidate reference genes including
GAPDH,
UBQ,
18S rRNA,
28S rRNA,
EF1a,
UBC,
Actin1,
elF-3, and
CYP and examined the expression stability. Data were analyzed using geNorm, NormFinder, and BestKeeper for comprehensive and accurate determination of the most stability genes out of the nine reference genes under drought stress and N and P starvation in the root of Chinese fir seedlings [
40]. To validate our results, the most stable and unstable genes were used to standardize the expression levels of
14-3-3-4,
ClPht1;3 and
NRT 1.1 genes that are related to drought stress and N and P starvation on different experimental conditions. Our results provide a basis for normalization of gene expression in other coniferous species, such as Douglas fir.
2. Materials and Methods
2.1. Plant Material and Experimental Setup
One year old seedlings of Chinese fir clone Yang 061 were bought from Yangkou State-owned forest farm in Fujian Province, China and cultivated in the greenhouse in Fujian Agriculture and Forestry University. The seedlings were cultivated in polyethylene pots with a volume of 1 L filled with cleaned sand and supplied with a modified (one-third content) Hoagland solution for two weeks. The solution contained 1 mmol/L KH2PO4, 5 mmol/L KNO3, 2 mmol/L MgSO4-7H2O, 5 mmol/L Ca(NO3)2-H2O, 1 mmol/L Na2-EDTA, 0.001 mmol/L FeSO4-7H2O, and micro nutrients including 0.463 mmol/L H3BO3, 0.003 mmol/L CuSO4-5H2O, 0.007 mmol/L ZnSO4-7H2O, 0.091 mmol/L MnCl2-4H2O, 0.005 mmol/L H8MoN2O4, and the pH of the nutrient solution was adjusted to 5.6. After two weeks of growth, the seedlings were transferred to another pot filled with thoroughly cleaned sand to make sure that traces of N and P were negligible. The concentration gradient of nutrient solution in P starvation treatment was as follows: 0, 0.5, and 1.0 mmol/L KH2PO4, representing P-starved, low P supply and normal P supply, respectively, and the roots were harvested after 0, 1, 3, 7, and 15 days post-treatment. The N treatment included different nitrogen sources and concentrations of nitrogen. As N sources, 1 mmol/L (NH4)2SO4, 2 mmol/L KNO3, and 1 mmol/L NH4NO3 were used to represent NH4+, NO3−, and mixed nitrogen sources, respectively. The concentration gradient of nutrient solution in N starvation test was as follows: 0, 0.5, and 1.0 mmol/L NH4NO3, representing N-starved, low N supply and normal N supply, and the roots were harvested after 0, 1, 3, 7, 15, and 25 days post-treatment. The gradient of drought stress was established by adding 0%, 10%, and 15% PEG6000, representing non-stress, moderately stress and severely stress condition, to one-third content of modified Hoagland solution, and the roots were harvest after 12, 24, and 48 h post-treatment. The fine roots of all samples were harvested, washed and surface dried and then immediately frozen in liquid nitrogen and stored at −80 °C until analysis. All experimental treatments had three biological replicates.
2.2. RNA Isolation and cDNA Synthesis
Total RNAs were extracted from Chinese fir roots using the RNA purification reagent (TIANGEN, Beijing, China). The frozen specimens were ground in liquid nitrogen to a fine powder with a pestle and mortar, and the powder was completely dissolved in the plant lysate, following the manufacturer’s protocol. The integrity of obtained RNA samples was examined by 1% agarose gel electrophoresis. The concentration and purity of total RNA was examined using an ultramicro spectrophotometer (Eppendorf, Hamburg, Germany). The A260/A280 ratio of RNA between 1.8 and 2.0 was considered to be the required quality for further experiments. Complementary DNA (cDNA) was synthesized using the GoScriptTM Reverse Transcription System Kit (Promega, Madison, WI, USA), following the manufacturer’s protocol. The reverse transcription system was based on 2 µg of total RNA in a 20 µL reaction volume. The resulting cDNAs were diluted to 100 ng/μL with nuclease-free water and stored at −20 °C.
2.3. Selection of Candidate Reference Genes
Based on the transcriptome data of Chinese fir, the commonly used internal reference genes were selected as the candidate internal reference genes for this experiment. They were
GAPDH,
UBQ,
18S rRNA,
28S rRNA,
EF1a,
UBC,
Actin1,
elF-3, and
CYP (
Table 1). According to the sequence of each gene, fluorescent quantitative PCR primers were designed using MEGA6.0. The primer sequence was synthesized by Qingke Bioengineering (Fuzhou, China) Co., Ltd. A five-fold cDNA dilution series with three replicates per concentration was used to make standard curves for estimation of amplification efficiency (E) and the correlation coefficient (R
2).
2.4. Quantitative Real-Time PCR (qRT-PCR)
Real-time fluorescence quantitative PCR was performed on the Thermo Fisher’s StepOnePlus real-time fluorescence quantitative PCR instrument. Promega’sGoTagqPCR Master Mix kit was used for PCR. The reaction system was 20 µL, including: 10 µL 2 × qPCR Master Mix, 0.4 µL upstream primer (10 µM), 0.4 µL downstream primer (10 µM), 1 µL DNA, 0.2 µL ROX reference dye (containing SYBR), and 8 µL ddH2O. The procedure of PCR reaction was as follows: 95 °C for 5 min, 40 cycles of 95 °C for 15 s, and 60 °C for 1 min, 95 °C for 15 s, 60 °C for 15 s, temperature gradually increased to 95 °C. Each reaction had three biological replicates and three technical replicates (i.e., each biological replicate was analyzed three times).
2.5. Validation of the Candidate Reference Genes
In order to verify the results of our experiments, the most stable reference genes were selected to validate the expression of the
14-3-3-4,
ClPht1;3, and
NRT1.1 genes in drought stress, P, and N starvation, respectively. The time and degree of experimental treatment were consistent with the three stress treatments mentioned above. The gene
14-3-3-4 belongs to
14-3-3 family, which binds to signal molecules to regulate plant response to drought stress [
42]. The gene
ClPht1;3 belongs to
PHT1 family, which is the key transporter for uptake and transport of inorganic phosphorus from soil solution to the cell membranes of root. Moreover, the gene
NRT1.1 belongs to
NPF family and functions as NO
3− transporter, and also was the substrates of polypeptides, amino acids, sugar ligands, and plant hormones [
43].
2.6. Data Analysis
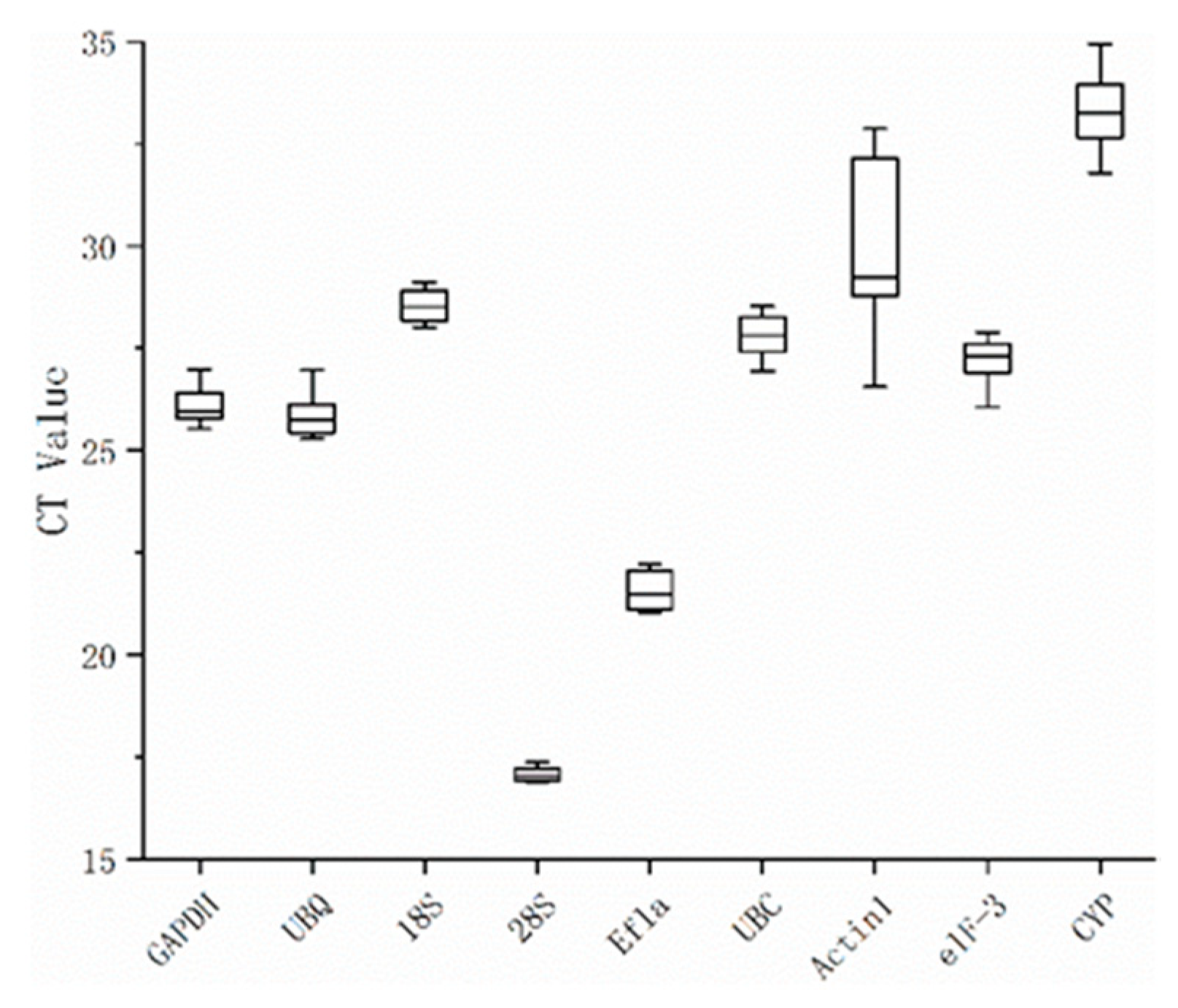
Quantitative real-time PCR was performed and analyzed using GoTagqPCR Master Mix (Promega) and a StepOnePlus™ real-time PCR instrument (Corbett Research, Australia). The expression stability of the nine candidate reference genes under different conditions were analyzed by geNorm, NormFinder, and BestKeeper, the most commonly used statistical procedures for screening reference genes. Expression levels of each reference gene were shown by Ct values. Before applying the geNorm and NormFinder analyses, the raw Ct values, weighted by amplification efficiency (
Table 1), were used to calculate relative quantities by the equation:
where
E is the amplification efficiency and Δ
Ct is the difference between the sample with the lowest Ct (highest expression) from the data set and the Ct value of the sample in question.
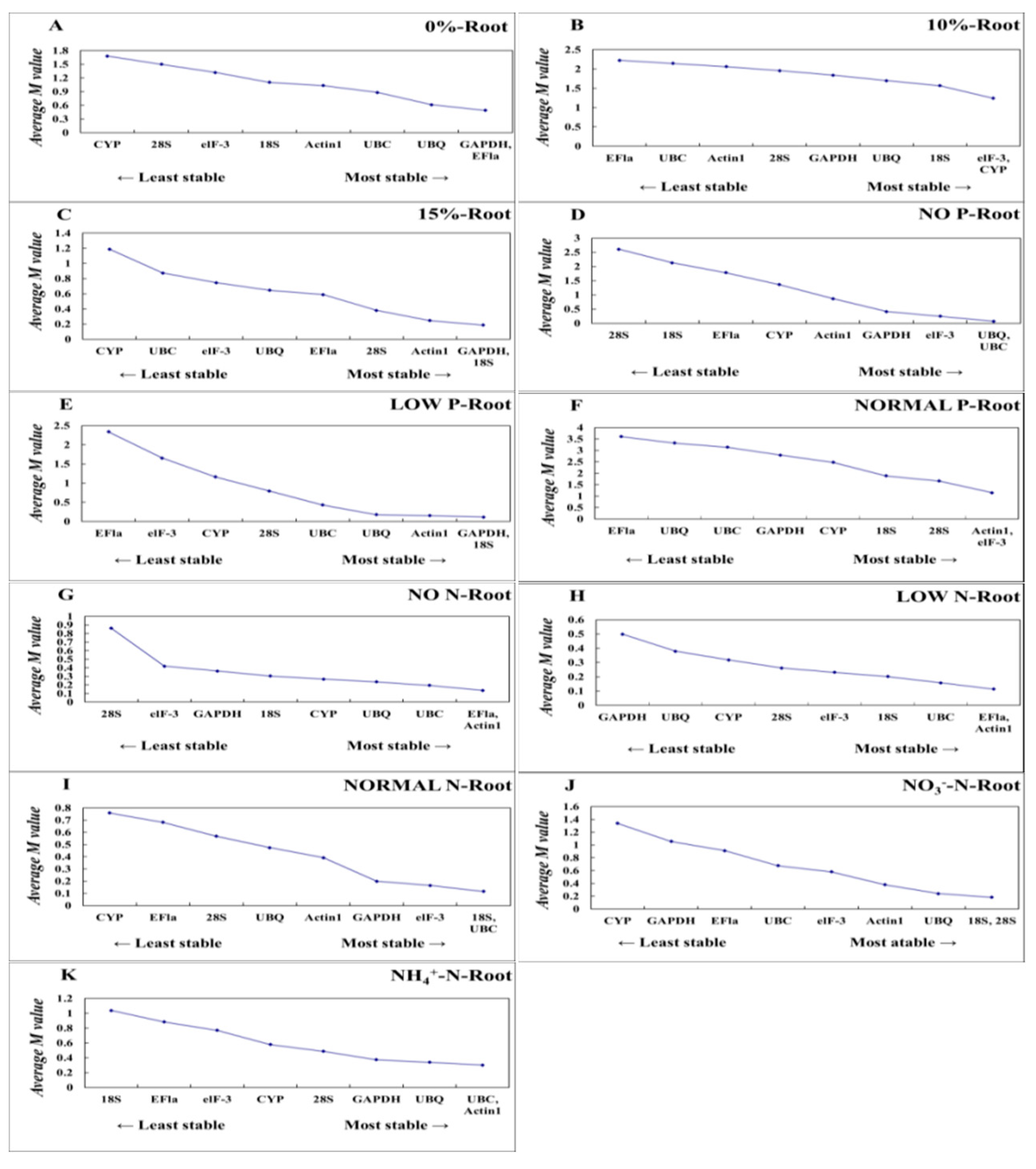
The geNorm analysis compares the stability of the candidate gene by calculating the average stability index,
M, of the candidate gene. The stability boundary is usually set at M value equal to 1.5 and if the
M value is lower than 1.5, the stability of the candidate gene will be higher. According to this principle, the geNorm analysis will step-wisely exclude the least stable gene and be repeated until only two genes remain. The lower the M value is the more stable gene expression will be [
44,
45,
46]. NormFinder was used to evaluate the expression stability of the candidate reference genes on the experimental samples. Raw Ct values were first index-transformed and used as input for the NormFinder. This algorithm takes into account the intra- and inter-group variations for normalization factors (NFs), which requires input data from a minimum of three candidate reference genes and a minimum of two tested samples per group. The calculated results of this analysis were not influenced by random co-regulated genes and the best candidate reference gene displayed lower stability values that were close to zero. The more stable reference gene will have lower stability value and inter- and intra-group variation. In our case, we calculated the intra group variations as there are no distinct groups. BestKeeper analysis was applied to rank the stability of candidate genes by calculating coefficient of variance (CV). The candidate reference genes are considered to be the most stably expressed genes when they present the lowest CV. The relative expression level of the target genes,
ClPht1;3,
NRT1.1,
14-3-3-4, in Chinese fir roots were calculated by 2
−ΔΔCt method, which is applicable when the amplification efficiencies of the target and reference genes are approximately equal [
47].
4. Discussion
The qRT-PCR is widely used in gene expression analysis because of its high specificity and sensitivity. qRT-PCR analysis results are closely related to the selection of reference genes and improper selection of reference genes may lead to deviation of analysis results and even wrong conclusions [
48,
49,
50,
51]. Therefore, to ensure the validity of the gene expression analysis results, it is necessary to screen the reference genes before using qRT-PCR to analyze the expression of the target genes. A large number of studies have shown that the screening of reference genes should be accurately evaluated and validated according to plant type [
39], tissue location [
32], and experimental conditions [
21], so as to establish an evaluation system of multiple reference genes, which is an important step towards improving the accuracy of qRT-PCR test [
52,
53].
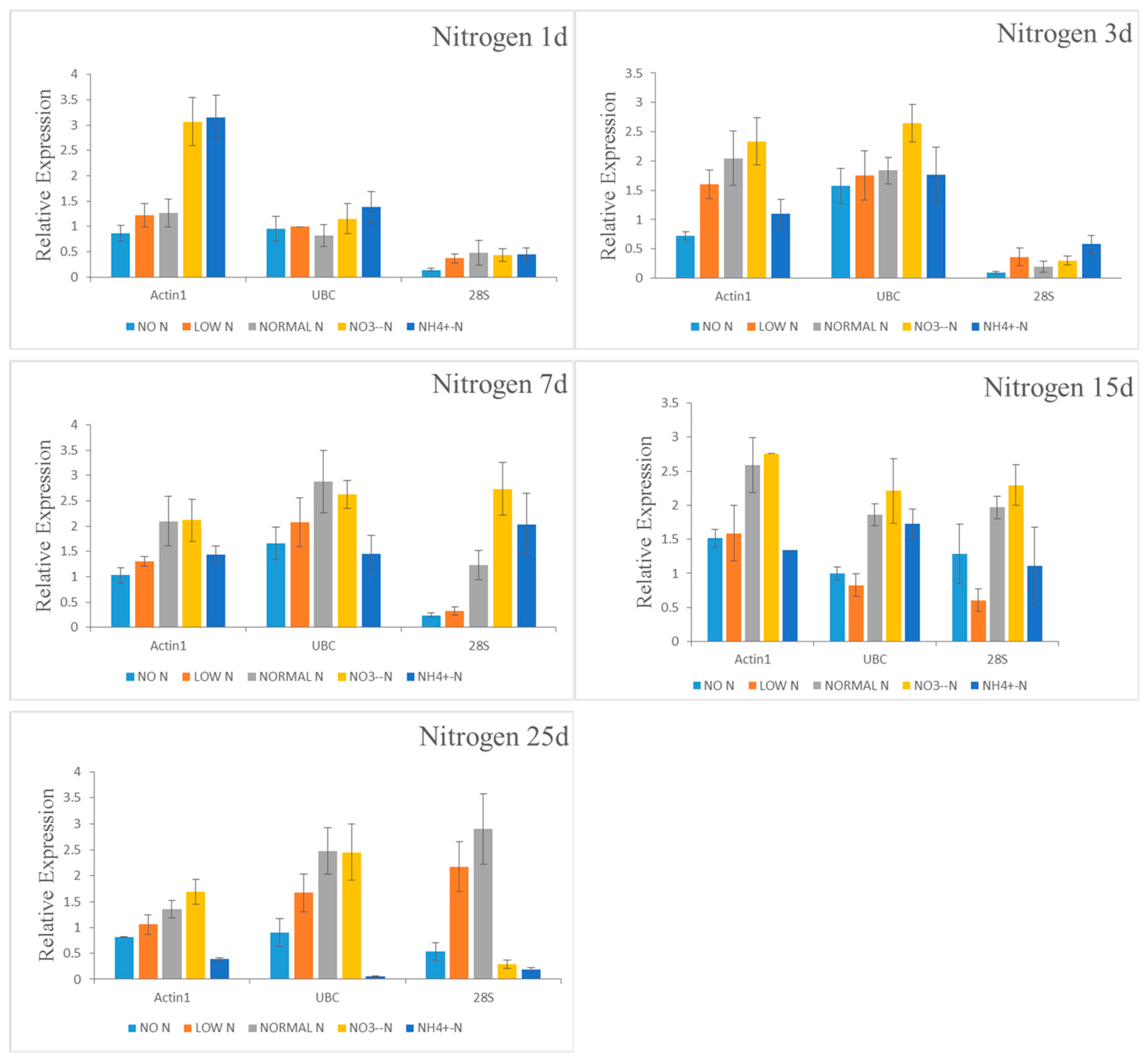
In this study, nine commonly used candidate reference genes (
GAPDH,
UBQ,
18S,
28S,
EF1a,
UBC,
Actin1,
elF-3, and
CYP) were screened from fine roots of Chinese fir under three abiotic stresses (drought, P, and N stress treatments) at different time and concentrations. In a large number of papers, the stability of these commonly used reference genes is different in different plants, different tissues, different developmental stages and different experimental treatments [
42,
54]. Our study also showed that the reference genes of Chinese fir roots under different stress treatments were not the same, and the stability of gene expression is highly dependent on external conditions. We identified different stable reference genes for drought stress and nutrient starvation, which effectively normalize the expression of target genes. A previous study screened and validated the stability of five housekeeping genes (
Actin,
18S,
UBQ,
EF1a, and
GAPDH) in root and leaf tissues of Chinese fir under different abiotic stresses and found
Actin and
GAPDH as stable reference genes for root and leaf tissues of Chinese fir, respectively [
41]. We also found
GAPDH and
UBQ as the two most stable genes under drought stress,
Actin1 and
GAPDH as the two most stable genes under P starvation, and
Actin1 and
UBC as the two most stable genes under N starvation among the nine candidate reference genes. Thus, not all housekeeping genes are equally valuable to serve as reference genes to normalize the expression level of target genes, as reported previously [
55].
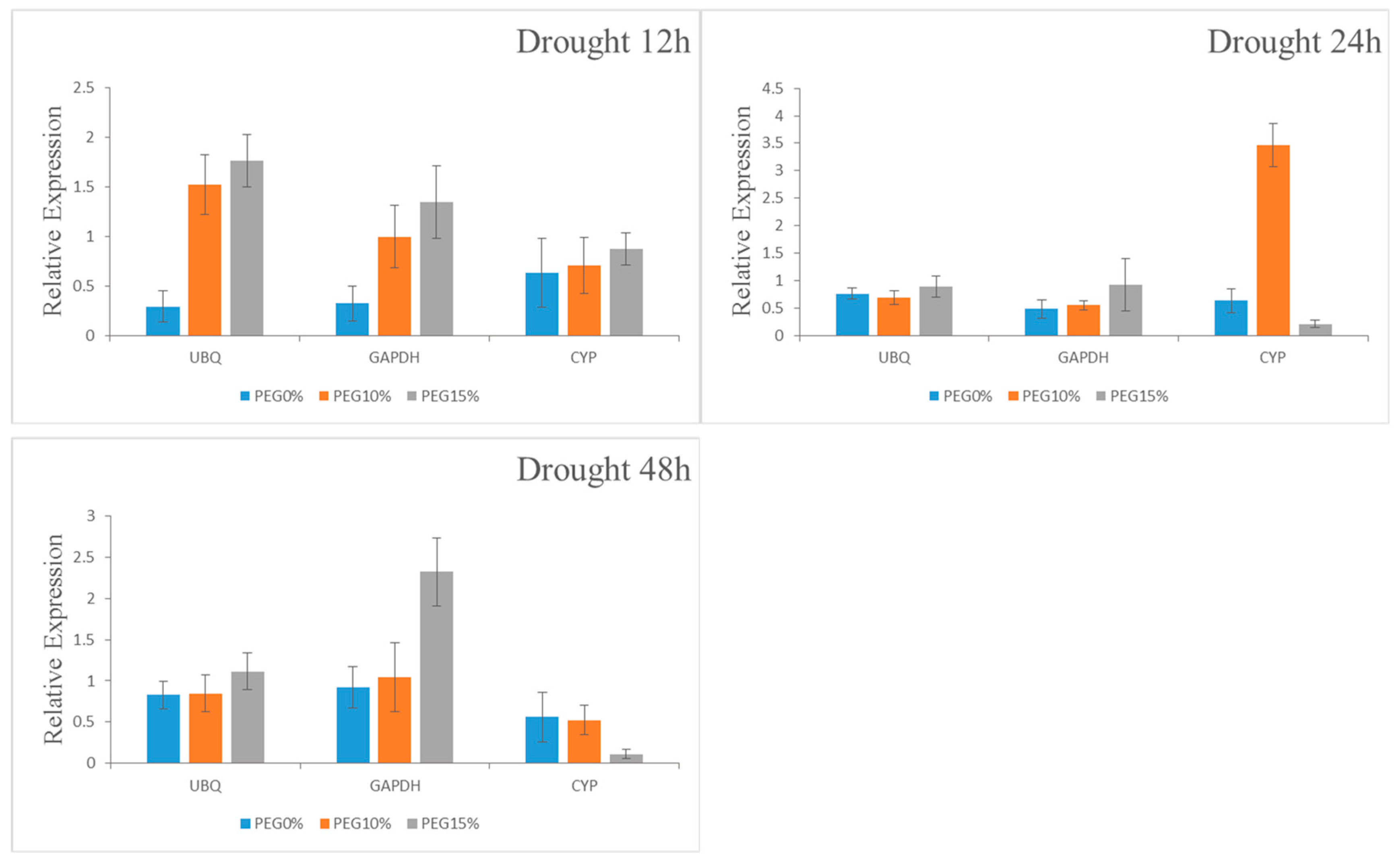
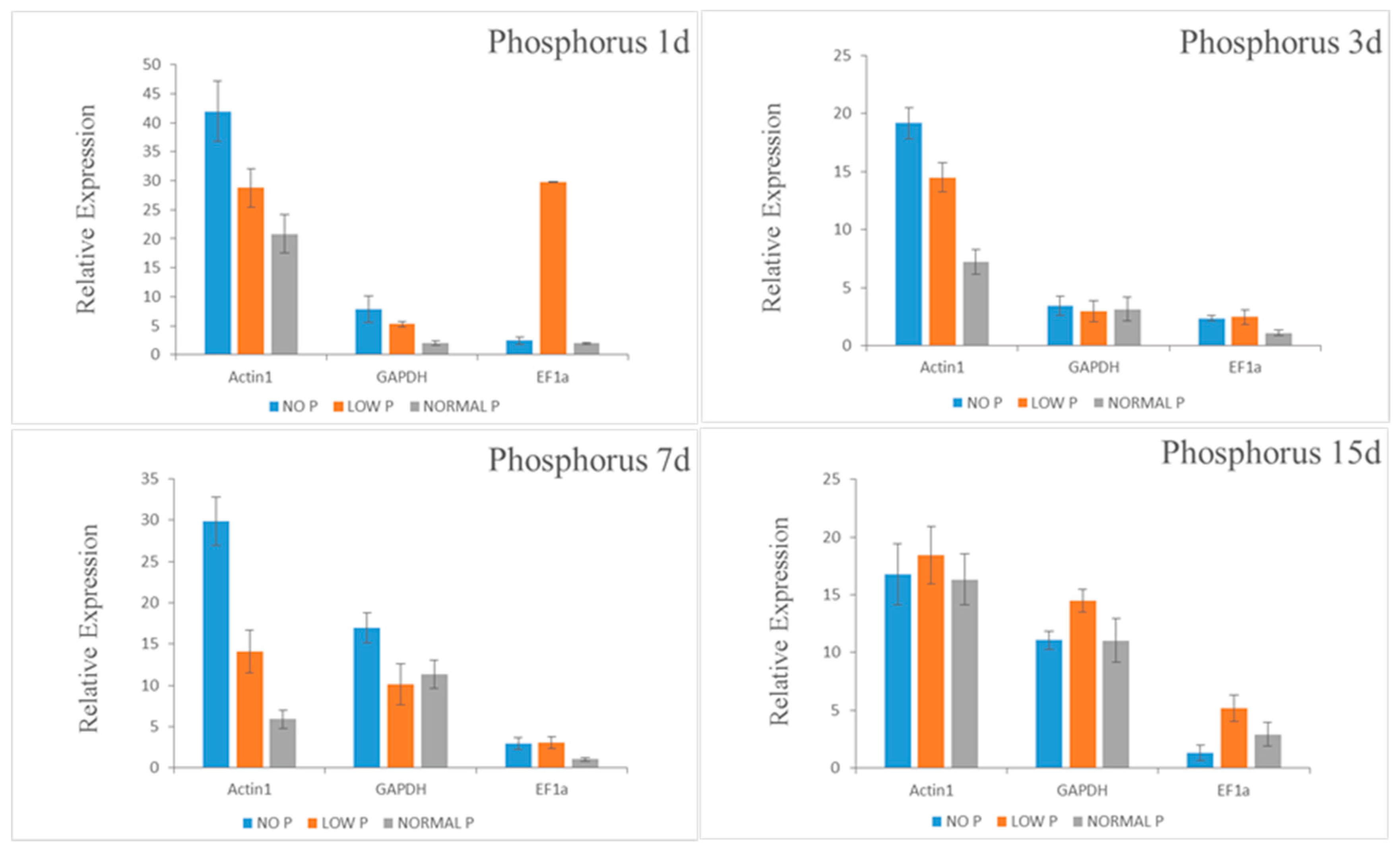
To ensure accurate normalization, some authors have recommended that multiple reference genes be used in gene expression analysis [
18,
56,
57,
58]. In this experiment, we selected the best two reference genes and the worst one to verify the expression levels of genes related to drought and nutrient stresses. The expression levels of genes related to three abiotic stresses were verified by examining the expression level of 14-3-3-4,
ClPht1;3 and
NRT1.1 in drought stressed, P, and N stressed roots, respectively. According to the verification results of target gene expression,
UBQ and
GAPDH were selected as single standardized reference genes under drought stress;
Actin1 and
GAPDH were selected as single standardized reference genes under P starvation; and
Actin1 and
UBC were selected as single standardized reference genes under N starvation. The expression patterns of 14-3-3-4,
ClPht1;3 and
NRT1.1 were similar under the same abiotic stress. The result also showed that the expression pattern of 14-3-3-4,
ClPht1;3, and
NRT1.1 were almost the same as that of one or two reference genes after normalization. Thus, our results suggest that
UBQ gene can be used to standardize Chinese fir root under drought stress, while the
Actin gene is the best internal control gene of Chinese fir root under N and P starvation. The stable reference gene selected in this study will be very useful for revealing the gene expression profiles in Chinese fir and related conifer species under abiotic stress; promoting the realization of it at cellular and gene levels. As abiotic stresses, such as drought and low nutrient availability, are major factors limiting tree growth, understanding the genetic basis for coping with such stresses has paramount importance in identifying stress tolerant genotypes. Genes that are up or down regulated during stress have not been fully understood in trees, which necessities further gene expression studies. For gene expression analysis to be accurate, it needs to be normalized using stable reference genes. In this regard, our study will lay the foundation for further investigation of stress-tolerance mechanisms at the molecular level in conifers by providing stable reference genes for normalization of gene expression levels in qRT-PCR analysis.