Effects of Disturbance on Understory Vegetation across Slovenian Forest Ecosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Vegetation Survey
- -
- Tree layer: including woody plants and all climbers; height > 5 m;
- -
- Shrub layer: including only woody plants and all climbers; height between 0.5 and 5 m;
- -
- Herb layer: including all non-woody plants, and woody plants with height ≤ 0.5 m, including tree seedlings and browsed trees;
- -
- Moss layer: separate record of mosses on the forest floor (on mineral soil) and mosses on other substrata, e.g., deadwood, living wood, rocks.
2.3. Disturbance Events and Evaluation
2.4. Data Analysis
3. Results
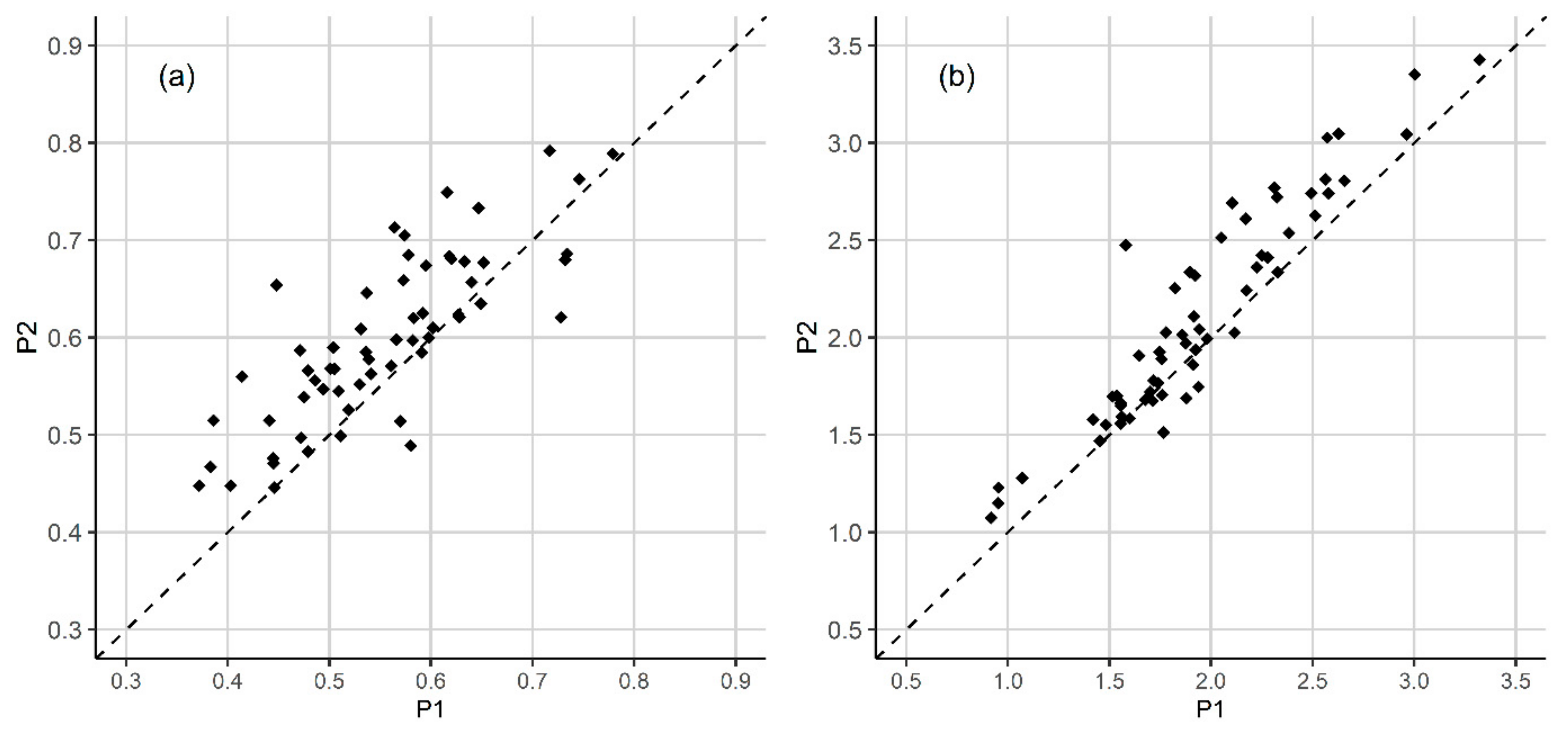
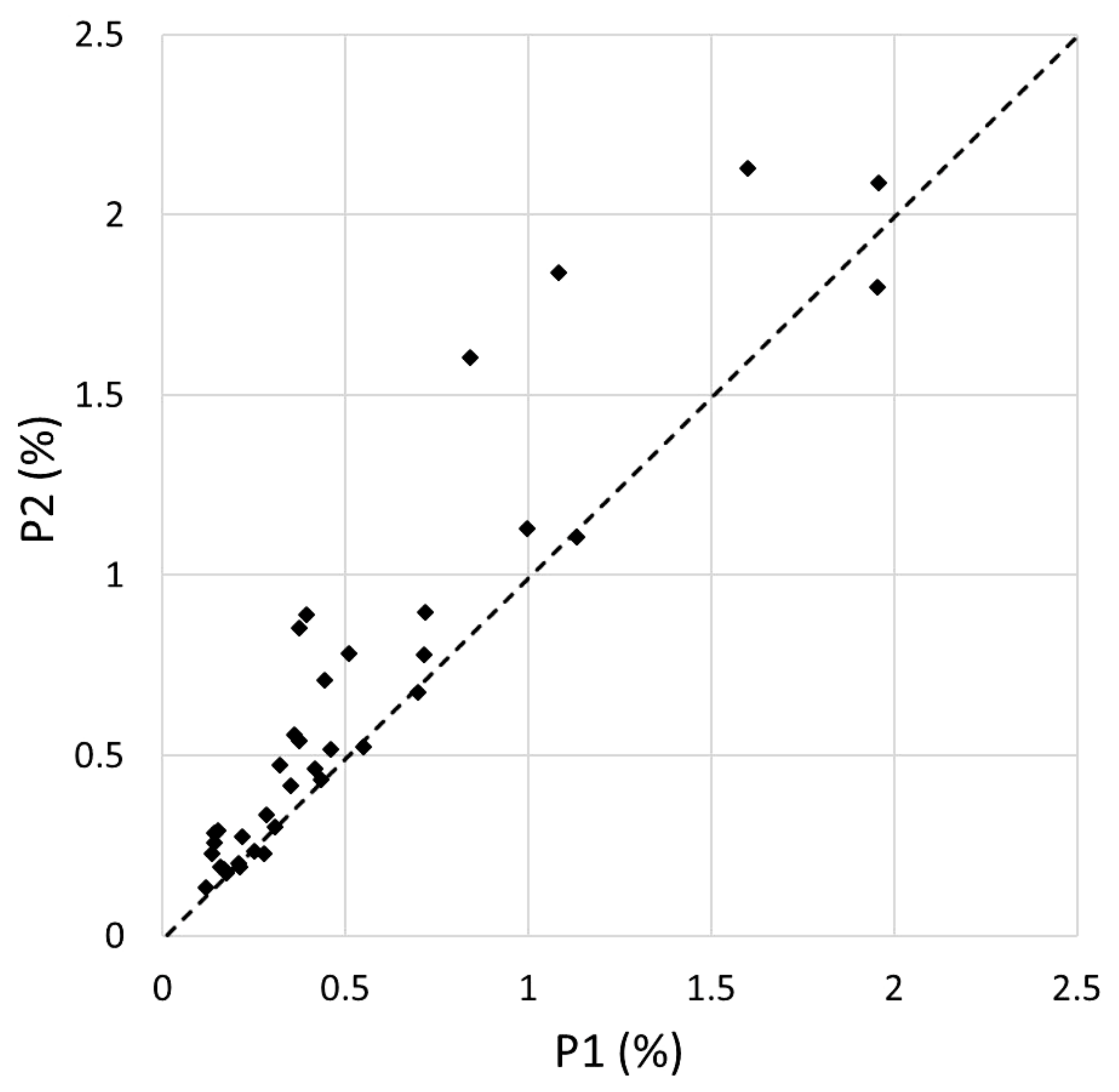
3.1. Plant Diversity
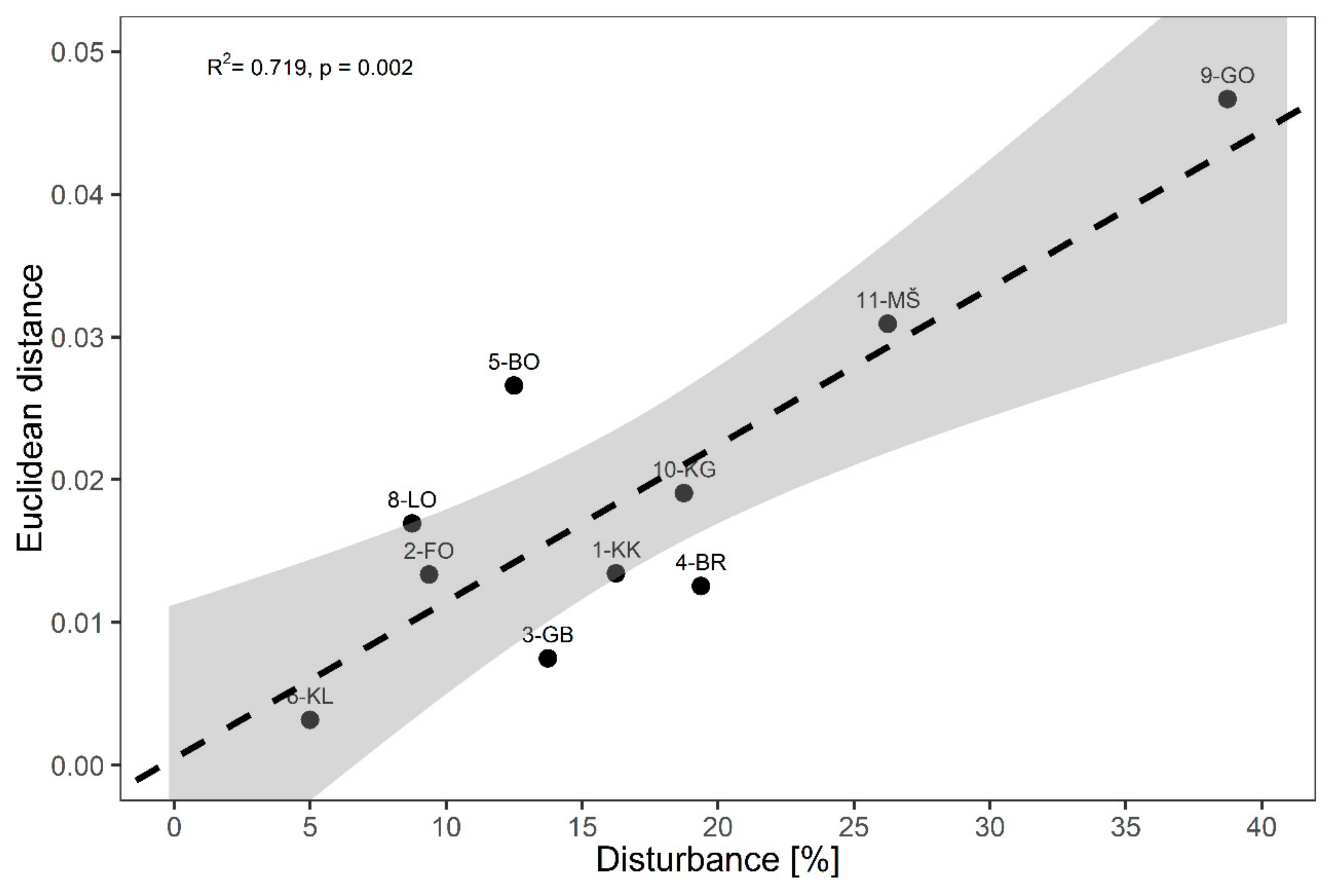
3.2. Disturbance Impacts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kovač, M.; Kutnar, L.; Hladnik, D. Assessing biodiversity and conservation status of the Natura 2000 forest habitat types: Tools for designated forestlands stewardship. For. Ecol. Manag. 2016, 359, 256–267. [Google Scholar] [CrossRef]
- Lindenmayer, D.; Margules, C.; Botkin, D. Indicators of biodiversity for ecologically sustainable forest management. Conserv. Biol. 2000, 14, 941–950. [Google Scholar] [CrossRef]
- Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Felton, A.; Knight, E.; Wood, J.; Zammit, C.; Lindenmayer, D. A meta-analysis of fauna and flora species richness and abundance in plantations and pasture lands. Biol. Conserv. 2010, 143, 545–554. [Google Scholar] [CrossRef]
- Royo, A.A.; Carson, W.P. On the formation of dense understory layers in forests worldwide: Consequences and implications for forest dynamics, biodiversity, and succession. Can. J. For. Res. 2006, 36, 1345–1362. [Google Scholar] [CrossRef]
- Durak, T. Changes in diversity of the mountain beech forest herb layer as a function of the forest management method. For. Ecol. Manag. 2012, 276, 154–164. [Google Scholar] [CrossRef]
- Verheyen, K.; Baeten, L.; De Frenne, P.; Bernhardt-Römermann, M.; Brunet, J.; Cornelis, J.; Decocq, G.; Dierschke, H.; Eriksson, O.; Hédl, R.; et al. Driving factors behind the eutrophication signal in understorey plant communities of deciduous temperate forests. J. Ecol. 2012, 100, 352–365. [Google Scholar] [CrossRef]
- van Dobben, H.F.; de Vries, W. The contribution of nitrogen deposition to the eutrophication signal in understorey communities of European forests. Ecol. Evol. 2017, 7, 214–227. [Google Scholar] [CrossRef]
- Gilliam, F.S. Excess Nitrogen in Temperate Forest Ecosystems Decreases Herbaceous Layer Diversity and Shifts Control from Soil to Canopy Structure. Forests 2019, 10, 66. [Google Scholar] [CrossRef]
- De Frenne, P.; Rodríguez-Sánchez, F.; Coomes, D.A.; Baeten, L.; Verstraeten, G.; Vellend, M.; Bernhardt-Römermann, M.; Brown, C.D.; Brunet, J.; Cornelis, J.; et al. Microclimate moderates plant responses to macroclimate warming. Proc. Natl. Acad. Sci. USA 2013, 110, 18561–18565. [Google Scholar] [CrossRef]
- Wang, H.; Liu, G.; Li, Z.; Wang, P.; Wang, Z. Comparative Assessment of Vegetation Dynamics under the Influence of Climate Change and Human Activities in Five Ecologically Vulnerable Regions of China from 2000 to 2015. Forests 2019, 10, 317. [Google Scholar] [CrossRef]
- Rooney, T.P. Deer impacts on forest ecosystems: A North American perspective. Forestry 2001, 74, 201–208. [Google Scholar] [CrossRef]
- Schulze, E.D.; Bouriaud, O.; Wäldchen, J.; Eisenhauer, N.; Walentowski, H.; Seele, C.; Heinze, E.; Pruschitzki, U.; Dănilă, G.; Marin, G.; et al. Ungulate browsing causes species loss in deciduous forests independent of community dynamics and silvicultural management in Central and Southeastern Europe. Ann. For. Res. 2014, 57, 267–288. [Google Scholar] [CrossRef]
- Bergeron, Y.; Harvey, B. Basing silviculture on natural ecosystem dynamics: An approach applied to the southern boreal mixedwood forest of Quebec. For. Ecol. Manag. 1997, 92, 235–242. [Google Scholar] [CrossRef]
- Bengtsson, J.; Nilsson, S.G.; Franc, A.; Menozzi, P. Biodiversity, disturbances, ecosystem function and management of European forests. For. Ecol. Manag. 2000, 132, 39–50. [Google Scholar] [CrossRef]
- Thom, D.; Seidl, T. Natural disturbance impacts on ecosystem services and biodiversity in temperate and boreal forests. Biol. Rev. 2016, 91, 760–781. [Google Scholar] [CrossRef]
- Woods, K.D.; Hicks, D.J.; Schultz, J. Losses in understory diversity over three decades in an old-growth cool-temperate forests in Michigan, USA. Can. J. For. Res. 2012, 42, 532–549. [Google Scholar] [CrossRef]
- Schelhaas, M.-J.; Nabuurs, G.-J.; Schuck, A. Natural disturbances in the European forests in the 19th and 20th centuries. Glob. Chang. Biol. 2003, 9, 1620–1633. [Google Scholar] [CrossRef]
- Seidl, R.; Schelhaas, M.-J.; Lexer, M.J. Unraveling the drivers of intensifying forest disturbance regimes in Europe. Glob. Chang. Biol. 2011, 17, 2842–2852. [Google Scholar] [CrossRef]
- Rees, M.; Condit, R.; Crawley, M.; Pacala, S.; Tilman, D. Long-term studies of vegetation dynamics. Science 2001, 293, 650–655. [Google Scholar] [CrossRef]
- de Vries, W.; Reinds, G.J.; Deelstra, H.D.; Klap, J.M.; Vel, E.M. Intensive Monitoring of Forest Condition in Europe: Technical Report 2000; UN/ECE EC: Brussels, Belgium; Geneva, Switzerland, 2000; p. 193. Available online: https://www.icp-forests.org/pdf/TRLII2000.pdf (accessed on 1 August 2019).
- Rautio, P.; Ferretti, M. Monitoring European forests: Results from science, policy, and society. Ann. For. Sci. 2015, 72, 875–876. [Google Scholar] [CrossRef][Green Version]
- de Vries, W.; Vel, E.M.; Reinds, G.J.; Deelstra, H.; Klap, J.M.; Leeters, E.E.J.M.; Hendriks, C.M.A.; Kerkvoorden, M.; Landmann, G.; Herkendell, J.; et al. Intensive monitoring of forest ecosystems in Europe. 1. Objectives, set-up and evaluation strategy. For. Ecol. Manag. 2003, 174, 77–95. [Google Scholar] [CrossRef]
- Canullo, R.; Starlinger, F.; Granke, O.; Fischer, R.; Aamlid, D.; Neville, P. ICP Forests Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; Part VII.1: Assessment of Ground Vegetation; UNECE ICP Forests Programme Coordinating Centre: Hamburg, Germany, 2011; p. 19. Available online: https://www.icp-forests.org/pdf/manual/2016/ICP_Manual_2016_01_part07-1.pdf (accessed on 1 August 2019).
- van Dobben, H.F.; ter Braak, C.J.F.; Dirkse, G.M. Undergrowth as a biomonitor for deposition of nitrogen and acidity in pine forest. For. Ecol. Manag. 1999, 114, 83–95. [Google Scholar] [CrossRef]
- de Vries, W.; Reinds, G.J.; Posch, M.; Sanz, M.J.; Krause, G.H.M.; Calatayud, V.; Renaud, J.P.; Dupouey, J.L.; Sterba, H.; Vel, E.M.; et al. Intensive Monitoring of Forest Condition in Europe: Technical Report 2003; UN/ECE EC: Brussels, Belgium; Geneva, Switzerland, 2003; p. 161. Available online: https://www.icp-forests.org/pdf/TRLII2003.pdf (accessed on 1 August 2019).
- Seidling, W. Ground floor vegetation assessment within the intensive (Level II) monitoring of forest ecosystems in Germany: Chances and challenges. Eur. J. For. Res. 2005, 124, 301–312. [Google Scholar] [CrossRef]
- Buriánek, V.; Novotný, R.; Hellebrandová, K.; Šrámek, V. Ground vegetation as an important factor in the biodiversity of forest ecosystems and its evaluation in regard to nitrogen deposition. J. For. Sci. 2013, 59, 238–252. [Google Scholar] [CrossRef]
- Wraber, M. Pflanzengeographische Stellung und Gliederung Sloweniens. Vegetatio 1969, 17, 176–199. [Google Scholar] [CrossRef]
- Kutnar, L. Intensive Monitoring of Vegetation of Forest Ecosystems in Slovenia. In Monitoring the Management of Forests and Forested Landscapes; Hladnik, D., Ed.; Studia Forestalia Slovenica, Biotehniška Fakulteta, Oddelek za gozdarstvo in obnovljive gozdne vire: Ljubljana, Slovenia, 2006; pp. 277–290, ISBN 9616020420, 9789616020428; (In Slovenian with English Summary). [Google Scholar]
- Kutnar, L. Diversity of woody species on forest monitoring plots in Slovenia. GozdVestn 2011, 69, 271–278, (In Slovenian with English Summary). [Google Scholar]
- Kutnar, L.; Martinčič, A. Bryophyte species diversity of forest ecosystems in Slovenia (Intensive Monitoring Programe). Zb. Gozdarstva Lesar. 2008, 85, 11–26. [Google Scholar]
- Vilhar, U.; Skudnik, M.; Ferlan, M.; Simončič, P. Influence of meteorological conditions and crown defoliation on three phenology in Intensive forest monitoring plots in Slovenia. Acta Silvae Ligni 2014, 105, 1–15. [Google Scholar] [CrossRef]
- Urbančič, M.; Kutnar, L.; Kobal, M.; Žlindra, D.; Marinšek, A.; Simončič, P. Soil and vegetation characteristics on Intensive Monitoring Plots of forest ecosystems). GozdVestn 2016, 74, 3–27, (In Slovenian with English Summary). [Google Scholar]
- Barkman, J.J.; Doing, H.; Segal, S. Kritische Bemerkungen und Vorschläge zur quantitativen Vegetationsanalyse. Acta Bot. Neerl. 1964, 13, 394–419. [Google Scholar] [CrossRef]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1964–1980; Volume 1–5. [Google Scholar]
- Tutin, T.G.; Burges, N.A.; Chater, A.O.; Edmondson, J.R.; Heywood, V.H.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Martinčič, A.; Wraber, T.; Jogan, N.; Podobnik, A.; Turk, B.; Vreš, B.; Ravnik, V.; Frajman, B.; Strgulc Krajšek, S.; Trčak, B.; et al. Mala Flora Slovenije: Ključ za določanje praprotnic in semenk; Tehniška založba Slovenije: Ljubljana, Slovenia, 2007; p. 967. ISBN 978-961-251-026-8. [Google Scholar]
- Nagel, T.A.; Mikac, S.; Dolinar, M.; Klopcic, M.; Keren, S.; Svoboda, M.; Diaci, J.; Boncina, A.; Paulic, V. The natural disturbance regime in forests of the Dinaric Mountains: A synthesis of evidence. For. Ecol. Manag. 2017, 388, 29–42. [Google Scholar] [CrossRef]
- Čater, M.; Kutnar, L.; Accetto, M. Slovenian Lowland and Floodplain Forests. In the Floodplain Forests in Europe: Current Situation and Perspectives; Klimo, E., Hager, H., Eds.; Brill: Leiden, The Netherlands, 2001; pp. 233–248. ISBN 90-04-11958-2. [Google Scholar]
- Kutnar, L.; Matijašič, D.; Pisek, R. Conservation Status and Potential Threats to Natura 2000 Forest Habitats in Slovenia. Sumar. List 2011, 135, 215–231. [Google Scholar]
- Kutnar, L.; Dakskobler, I. Evaluation of the conservation Status of forest habitat types (Natura 2000) and their forest management. GozdVestn 2014, 72, 419–439, (In Slovenian with English Summary). [Google Scholar]
- Dakskobler, I.; Kutnar, L.; Šilc, U. Floodplain Woods, Swamp Woods and Riverine Forests in Slovenia: Forests of Willows, Alders, White Elm, European and Narrow-Leaved Ash, Pedunculate Oak and Scots Pine along Rivers and Streams; Silva Slovenica-Gozdarski inštitut Slovenije, Zveza gozdarskih društev Slovenije—Gozdarska založba: Ljubljana, Slovenia, 2013; p. 127. ISBN 978-961-6425-71-1. (In Slovenian with English Summary). [Google Scholar]
- Marinšek, A.; Kutnar, L. Occurrence of invasive alien plant species in the floodplain forests along the Mura River in Slovenia. Period. Biol. 2017, 119, 251–260. [Google Scholar] [CrossRef]
- Nagel, T.A.; Firm, D.; Rozenbergar, D.; Kobal, M. Patterns and drivers of ice storm damage in temperate forests of Central Europe. Eur. J. For. Res. 2016, 135, 519–530. [Google Scholar] [CrossRef]
- de Groot, M. Trendi in napovedi gostote populacij smrekovih podlubnikov po žledolomu 2014 v Sloveniji: Stanje pomlad 2014. In Napovedi o Zdravju Gozdov; Gozdarski inštitut Slovenije: Ljubljana, Slovenia, 2014; Available online: http://www.zdravgozd.si/prognoze_zapis.aspx?idpor=20 (accessed on 1 August 2019).
- Ogris, N.; Grecs, Z. Prenamnožitev osmerozobega in šesterozobega smrekovega lubadarja v Sloveniji v 2016. In Napovedi o Zdravju Gozdov; Gozdarski inštitut Slovenije: Ljubljana, Slovenia, 2016; Available online: http://www.zdravgozd.si/prognoze_zapis.aspx?idpor=27 (accessed on 1 August 2019).
- Zavod za gozdove Slovenije (ZGS). ZGS, Poročilo Zavoda za gozdove Slovenije o gozdovih za leto 2015. 2016. Available online: http://www.zgs.si/fileadmin/zgs/main/img/PDF/LETNA_POROCILA/2015_Porocilo_o_gozdovih.pdf (accessed on 1 August 2019).
- Roženbergar, D.; Nagel, T.; Fidej, G.; Diaci, J. Influence of deer browsing on the composition, structure, and function of Slovene forests. GozdVestn 2017, 75, 373–382, (In Slovenian with English Summary). [Google Scholar]
- Veselič, Ž. Unsatisfactory regeneration due to overabundant herbivorous game is the greatest threat to the conservation of Slovenian forests. GozdVestn 2017, 75, 383–397, (In Slovenian with English Summary). [Google Scholar]
- Dell Inc. Statistica 64—Dell Statistica (Data Analysis Software System); Version 13; Dell Inc.: Round Rock, TX, USA, 2016. [Google Scholar]
- Roberts, D.W. Package »labdsv«—Ordination and Multivariate Analysis for Ecology. 2019. Available online: https://cran.r-project.org/web/packages/labdsv/labdsv.pdf (accessed on 20 September 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package ‘vegan’—Community Ecology Package. 2019. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 20 September 2019).
- Butchart, S.H.M.; Walpole, M.; Collen, B.; van Strein, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global biodiversity: Indicators of recent declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef]
- Helm, N.; Essl, F.; Mirtl, M.; Dirnböck, T. Multiple environmental changes drive forest floor vegetation in a temperate mountain forest. Ecol. Evol. 2017, 7, 2155–2168. [Google Scholar] [CrossRef]
- de Groot, M.; Kolšek, M.; Kavčič, A. Napoved ulova smrekovih lubadarjev (Ips typographus in Pityogenes chalcographus) v kontrolne feromonske pasti tipa Theysohn za leto 2016. In Napovedi o Zdravju Gozdov; Gozdarski inštitut Slovenije: Ljubljana, Slovenia, 2016; Available online: http://www.zdravgozd.si/prognoze_zapis.aspx?idpor=26 (accessed on 16 May 2019).
- Simončič, T.; Bončina, A.; Jarni, K.; Klopčič, M. Assessment of the long-term impact of deer on understory vegetation in mixed temperate forests. J. Veg. Sci. 2019, 30, 108–120. [Google Scholar] [CrossRef]
- Depauw, L.; Perring, M.P.; Brunet, J.; Maes, S.L.; Blondeel, H.; De Lombaerde, E.; De Groote, R.; Verheyen, K. Interactive effects of past land use and recent forest management on the understory community in temperate oak forests in South Sweden. J. Veg. Sci. 2019, 30, 917–928. [Google Scholar] [CrossRef]
- Vellend, M.; Baeten, L.; Myers-Smith, I.H.; Elmendorf, S.C.; Beauséjour, R.; Brown, C.D.; De Frenne, P.; Verheyen, K.; Wipf, S. Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proc. Natl. Acad. Sci. USA 2013, 110, 19456–19459. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Falkengren-Grerup, U.; Tyler, G. Herb layer vegetation of south Swedish beech and oak forests-effects of management and soil acidity during one decade. For. Ecol. Manag. 1996, 88, 259–272. [Google Scholar] [CrossRef]
- Decocq, G.; Aubert, M.; Dupont, F.; Alard, D.; Saguez, R.; Wattez-Franger, A.; De Foucault, B.; Delelis-Dusollier, A.; Bardat, J. Plant diversity in a managed temperate deciduous forest: Understorey response to two silvicultural systems. J. Appl. Ecol. 2004, 41, 1065–1079. [Google Scholar] [CrossRef]
- Kermavnar, J.; Eler, K.; Marinšek, A.; Kutnar, L. Initial understory vegetation responses following different forest management intensities in Illyrian beech forests. Appl. Veg. Sci. 2019, 22, 48–60. [Google Scholar] [CrossRef]
- Marinšek, A.; Celarc, B.; Grah, A.; Kokalj, Ž.; Nagel, T.A.; Ogris, N.; Oštir, K.; Planinšek, Š.; Roženbergar, D.; Veljanovski, T.; et al. Impacts of Ice Storms on Forest Development—A Review. GozdVestn 2015, 73, 392–405, (In Slovenian with English Summary). [Google Scholar]
- Kelemen, K.; Mihók, B.; Gálhidy, L.; Standovár, T. Dynamic response of herbaceous vegetation to gap opening in a Central European beech stand. Silva Fenn. 2012, 46, 53–65. [Google Scholar] [CrossRef]
- Kutnar, L.; Eler, K.; Marinšek, A. Effects of different silvicultural measures on plant diversity—The case of the Illyrian Fagus sylvatica habitat type (Natura 2000). iForest 2015, 9, 318–324. [Google Scholar] [CrossRef]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Environ. 2011, 9, 222–228. [Google Scholar] [CrossRef]
- Sonnier, G.; Johnson, S.E.; Amatangelo, K.L.; Rogers, D.A.; Waller, D.M. Is taxonomic homogenization linked to functional homogenization in temperate forests? Glob. Ecol. Biogeogr. 2014, 23, 894–902. [Google Scholar] [CrossRef]
- Olden, J.D.; Comte, L.; Giam, X. The Homogocene: A research prospectus for the study of biotic homogenisation. NeoBiota 2018, 37, 23–36. [Google Scholar] [CrossRef]
- Closset-Kopp, D.; Hattab, T.; Decocq, G. Do drivers of forestry vehicles also drive herb layer changes (1970–2015) in a temperate forest with contrasting habitat and management conditions? J. Ecol. 2019, 107, 1439–1456. [Google Scholar] [CrossRef]
- Velázquez, J.; Tejera, R.; Hernando, A.; Núñez, M.A. Environmental diagnosis: Integrating biodiversity conservation in management of Natura 2000 forest spaces. J. Nat. Conserv. 2010, 18, 309–317. [Google Scholar] [CrossRef]
- Schulze, E.D. Effects of forest management on biodiversity in temperate deciduous forests: An overview based on Central European beech forests. J. Nat. Conserv. 2018, 43, 213–226. [Google Scholar] [CrossRef]
- Vertačnik, G.; Berlanič, R. Climate Variability in Slovenia in Period 1961–2011; Chapter 3: Climate Characteristics in Slovenia; Ministrtvo za okolje in prostor, Agencija RS za okolje: Ljubljana, Slovenia, 2017; p. 198. Available online: https://meteo.arso.gov.si/uploads/probase/www/climate/text/sl/publications/PSSbrosura_spread_SLO.pdp (accessed on 1 August 2019).
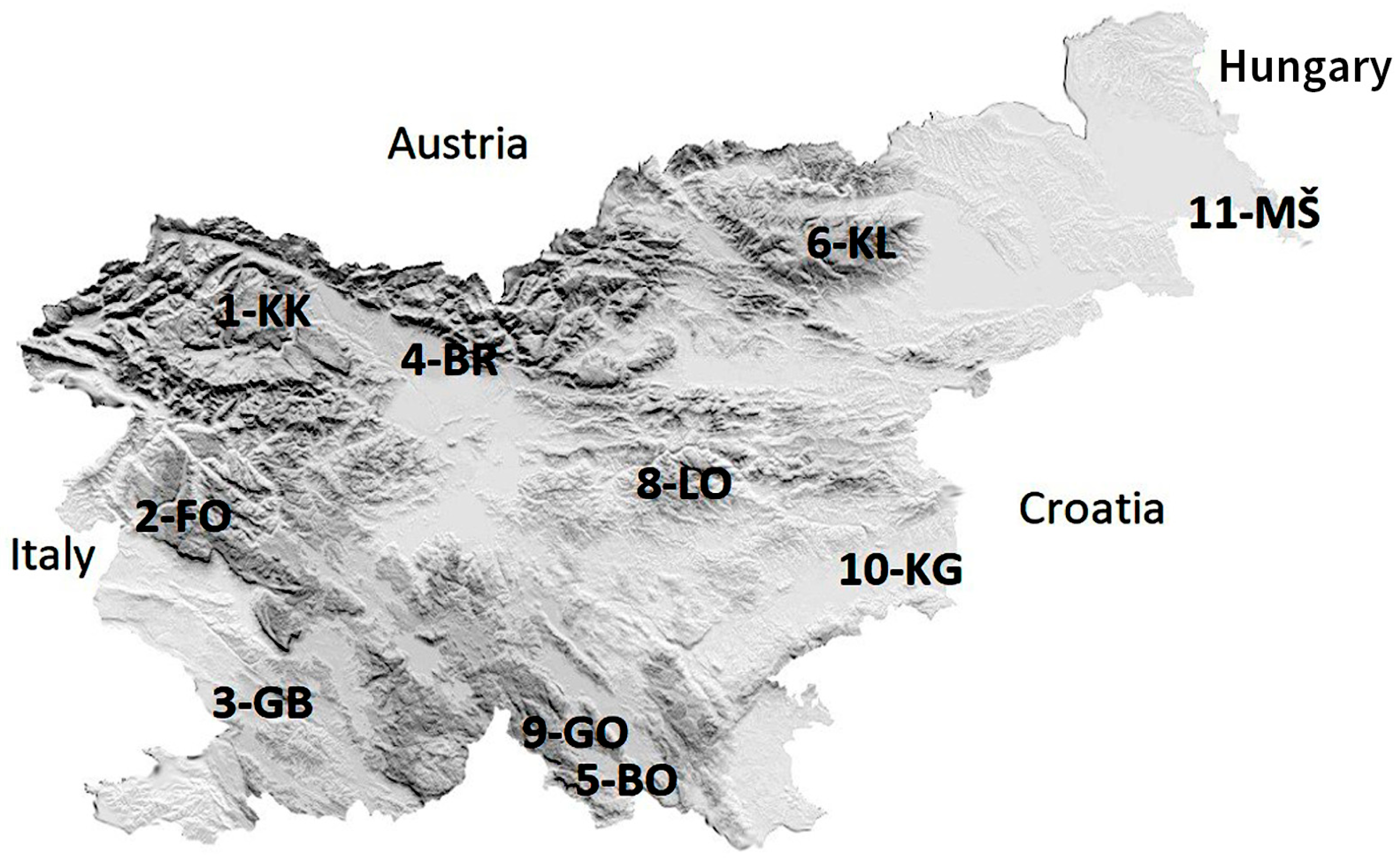
| Name (Abbreviation) of IM Site | Area & Phyto-geographic Region [28] | Latitude (X) | Longitude (Y) | Elevation (m) | Temperature (10-Years Mean) (deg. C) | Bedrock | Soil Unit | Dominant Tree Species | Number of Plots |
|---|---|---|---|---|---|---|---|---|---|
| 1. KRUCMANOVE KONTE (1-KK) | Pokljuka, Alpine region | 418,719 | 136,466 | 1397 | 4.4 | Moraine | Eutric Cambisols, Rendzic Leptosols | Picea abies | 4 |
| 2. FONDEK (2-FO) | Trnovski gozd, Dinaric region | 402,239 | 95,690 | 827 | 11.2 | Limestone | Rendzic Leptosols, Eutric Cambisols | Fagus sylvatica | 8 |
| 3. GROPAJSKI BORI (3-GB) | Sežana, Sub-Mediterranean region | 411,589 | 59,052 | 420 | 11.9 | Limestone | Chromic Cambisols | Pinus nigra | 4 |
| 4. BRDO (4-BR) | Kranj, Pre-Alpine region | 454,133 | 127,146 | 471 | 10.3 | Fluvioglacial gravels and sands | Dystric Cambisols | Pinus sylvestris | 8 |
| 5. BOROVEC (5-BO) | Kočevska Reka, Dinaric region | 484,737 | 43,605 | 705 | 8.7 | Limestone, Dolomite | Rendzic Leptosols, Eutric Cambisols | Fagus sylvatica | 8 |
| 6. KLADJE (6-KL) | Pohorje, Alpine region | 530,522 | 147,809 | 1304 | 4.9 | Dioritoid (Tonalite) | Dystric Cambisols | Picea abies | 4 |
| 8. LONTOVŽ (8-LO) | Kum, Pre-Dinaric region | 505,362 | 105,871 | 958 | 7.4 | Dolomite | Rendzic Leptosols, Eutric Cambisols | Fagus sylvatica | 8 |
| 9. GORICA (9-GO) | Loški potok, Dinaric region | 471,818 | 54,755 | 955 | 8.2 | Dolomite | Rendzic Leptosols, Eutric Cambisols | Fagus sylvatica | 4 |
| 10. KRAKOVSKI GOZD (10-KG) | Kostanjevica na Krki, Sub-Pannonian region | 532,688 | 82,059 | 160 | 9.8 | Pleistocene sediments | Gleysols | Carpinus betulus (Quercus robur) | 4 |
| 11. MURSKA ŠUMA (11-MŠ) | Lendava, Sub-Pannonian region | 616,509 | 151,426 | 170 | 10.5 | Alluvium | Gleysols, Fluvisol | Quercus robur (Carpinus betulus) | 8 |
| IM Site | Disturbances in 1st Half of Period | Disturbances in 2nd Half of Period |
|---|---|---|
| 1. KRUCMANOVE KONTE | Windthrows (2005, 2007, 2009, 2015)—mostly outside the plots; Intensive logging activities after all windthrows—mostly outside the plots | Windthrows (y. 2015)—mostly outside the plots; Intensive logging activities after all windthrows—mostly outside the plots |
| 2. FONDEK | Windthrow and logging of a few trees (2011) | |
| 3. GROPAJSKI BORI | Mortality of single trees of Pinus nigra (all years) due to different fungal diseases and various insects | Mortality of single trees of Pinus nigra (all years); Windthrow and logging of a few trees (2010) |
| 4. BRDO | Snow-break of trees (2010), Windthrow (2012); Ice storm (2014) and logging after disturbances; Waterpipe-system reconstruction across plots area (2013/2014) | |
| 5. BOROVEC | Windthrow (2008)—mostly outside the plots; | Ice storm damaged parts of tree crowns (2014), logging activities after disturbances—mostly outside the plots |
| 6. KLADJE | Windthrows damaged a few trees (2005, 2006, 2009)—all outside the plots | Windthrows damaged a few trees (2014)—all outside the plots |
| 8. LONTOVŽ | Regular selective management (2012)—party in plots, but mostly outside | |
| 9. GORICA | Logging activities and forest-road construction near the plots (2008/2009) | Ice storm (2014) and intensive logging activities in all plots and in a wider area (2014/2015) |
| 10. KRAKOVSKI GOZD | Mortality of Fraxinus angustifolia trees (all years), mainly caused by altered flooding regime (soil drying because of lower groundwater level) and fungal infections (e.g., ash dieback) | Mortality of Fraxinus angustifolia trees (all years); Main logging activities in 2014 and 2015 |
| 11. MURSKA ŠUMA | Extreme flooding (2005, 2009), Intensive logging activities (2008) | Extreme flooding (2013) |
| Period 1 (2004/2005) | Period 2 (2014/2015) | |||
|---|---|---|---|---|
| Mean (Cover in %) | Mean (Cover in %) | Z | Significance Level | |
| (n = 10) | (n = 10) | |||
| Deadwood cover | 4.4 | 5.9 | 0.291 | ns. |
| Total vegetation cover | 99.3 | 96.3 | 0.775 | * |
| Tree-layer cover | 84.1 | 78.4 | 0.787 | * |
| Shrub-layer cover | 7.7 | 9.3 | 0.032 | ns. |
| Herb-layer cover | 72.9 | 71.5 | 0.355 | ns. |
| Ground moss cover | 4.0 | 3.6 | 0.085 | ns. |
| Other moss cover | 1.7 | 1.8 | 0.465 | ns. |
| N of species | 37.7 | 37.5 | 0.048 | ns. |
| Evenness E | 0.55 | 0.60 | 0.838 | ** |
| Shannon H’ | 1.94 | 2.12 | 0.838 | ** |
| PLANT SPECIES | VERTICAL LAYER | N plots (P1) | N plots (P2) | COVER (P1) | COVER (P2) | COV. DIFF. (P2-P1) | Z | Significance Level |
|---|---|---|---|---|---|---|---|---|
| Fagus sylvatica L. | UT | 28 | 28 | 34.4 | 31.9 | −2.5 | 2.310 | * |
| Fagus sylvatica L. | LT | 25 | 27 | 5.5 | 4.9 | −0.6 | 1.014 | ns. |
| Daphne mezereum L. | S | 30 | 25 | 0.3 | 0.2 | −0.1 | 1.193 | ns. |
| Acer pseudoplatanus L. | S | 24 | 24 | 0.4 | 0.9 | 0.5 | 0.698 | ns. |
| Fagus sylvatica L. | S | 24 | 24 | 2.0 | 1.8 | −0.2 | 0.876 | ns. |
| Acer campestre L. | S | 17 | 16 | 0.4 | 0.9 | 0.5 | 2.845 | ** |
| Acer pseudoplatanus L. | H | 34 | 35 | 1.6 | 2.1 | 0.5 | 1.870 | ns. |
| Anemone nemorosa L. | H | 29 | 29 | 0.4 | 0.7 | 0.3 | 2.636 | ** |
| Fagus sylvatica L. | H | 28 | 29 | 1.0 | 1.8 | 0.8 | 2.490 | * |
| Viola reichenbachiana Jord. ex Boreau | H | 28 | 29 | 0.1 | 0.3 | 0.2 | 2.542 | * |
| Cyclamen purpurascens Mill. | H | 27 | 28 | 0.8 | 1.6 | 0.8 | 3.432 | *** |
| Mercurialis perennis L. | H | 27 | 27 | 2.0 | 2.1 | 0.1 | 0.734 | ns. |
| Asarum europaeum L. | H | 25 | 25 | 0.7 | 0.8 | 0.1 | 0.621 | ns. |
| Hedera helix L. | H | 24 | 25 | 0.4 | 0.5 | 0.1 | 2.310 | * |
| Polygonatum multiflorum (L.) All. | H | 26 | 23 | 0.2 | 0.2 | 0.0 | 0.201 | ns. |
| Cardamine trifolia L. | H | 24 | 24 | 0.7 | 0.7 | 0.0 | 0.135 | ns. |
| Daphne mezereum L. | H | 22 | 26 | 0.2 | 0.3 | 0.1 | 2.329 | * |
| Galium odoratum (L.) Scop. | H | 25 | 23 | 0.7 | 0.9 | 0.2 | 0.971 | ns. |
| Carex sylvatica Huds. | H | 23 | 23 | 0.1 | 0.3 | 0.1 | 2.511 | * |
| Oxalis acetosella L. | H | 21 | 22 | 0.5 | 0.5 | 0.0 | 1.826 | ns. |
| Galeobdolon flavidum (L.) Crantz | H | 21 | 21 | 0.5 | 0.8 | 0.3 | 2.701 | ** |
| Rosa arvensis Huds. | H | 21 | 20 | 0.4 | 0.4 | 0.0 | 0.059 | ns. |
| Aremonia agrimonoides (L.) DC. | H | 20 | 20 | 0.2 | 0.2 | 0.0 | 0.114 | ns. |
| Picea abies (L.) H. Karst. | H | 21 | 19 | 0.4 | 0.4 | 0.0 | 0.210 | ns. |
| Mycelis muralis Dumort | H | 21 | 18 | 0.2 | 0.2 | 0.0 | 0.489 | ns. |
| Solidago virgaurea L. | H | 19 | 20 | 0.1 | 0.1 | 0.0 | 0.853 | ns. |
| Carex digitata L. | H | 19 | 19 | 0.3 | 0.3 | 0.0 | 1.098 | ns. |
| Euphorbia amygdaloides L. | H | 19 | 19 | 0.3 | 0.4 | 0.1 | 1.604 | ns. |
| Fragaria vesca L. | H | 19 | 18 | 0.2 | 0.2 | 0.0 | 0.357 | ns. |
| Salvia glutinosa L. | H | 19 | 18 | 0.3 | 0.5 | 0.2 | 2.192 | * |
| Acer campestre L. | H | 17 | 19 | 0.4 | 0.6 | 0.2 | 1.922 | ns. |
| Brachypodium sylvaticum (Huds.) Beauv. | H | 17 | 19 | 0.1 | 0.3 | 0.2 | 2.585 | ** |
| Dryopteris filix-mas (L.) Schott | H | 18 | 17 | 0.3 | 0.3 | 0.0 | 0.535 | ns. |
| Sorbus aucuparia L. | H | 16 | 19 | 0.1 | 0.2 | 0.1 | 1.922 | ns. |
| Sanicula europaea L. | H | 19 | 15 | 0.5 | 0.5 | 0.0 | 0.153 | ns. |
| Lathyrus vernus L.) Bernh. subsp. vernus | H | 16 | 17 | 0.2 | 0.2 | 0.0 | 0.296 | ns. |
| Senecio fuchsii Gmelin | H | 16 | 17 | 0.2 | 0.3 | 0.1 | 0.612 | ns. |
| Hacquetia epipactis (Scop.) DC. | H | 17 | 15 | 1.1 | 1.1 | 0.0 | 0.000 | ns. |
| Gentiana asclepiadea L. | H | 16 | 15 | 0.2 | 0.2 | 0.0 | 0.730 | ns. |
| Omphalodes verna Moench | H | 16 | 15 | 1.0 | 1.1 | 0.131 | 0.676 | ns. |
| Period 1 (2004/2005) | Period 2 (2014/2015) | |
|---|---|---|
| IM Ste | Mean (%) ± SE | Mean (%) ± SE |
| 1. KRUCMANOVE KONTE (1-KK) | 2.5 ± 2.5 | 16.3 ± 3.2 |
| 2. FONDEK (2-FO) | 0.6 ± 0.6 | 9.4 ± 2.1 |
| 3. GROPAJSKI BORI (3-GB) | 0.0 ± 0.0 | 13.8 ± 4.3 |
| 4. BRDO (4-BR) | 1.3 ± 0.8 | 19.4 ± 2.6 |
| 5. BOROVEC (5-BO) | 0.6 ± 0.6 | 12.5 ± 0.9 |
| 6. KLADJE (6-KL) | 0.0 ± 0.0 | 5.0 ± 3.5 |
| 8. LONTOVŽ (8-LO) | 0.0 ± 0.0 | 8.8 ± 3.2 |
| 9. GORICA (9-GO) | 2.5 ± 2.5 | 38.8 ± 1.3 |
| 10. KRAKOVSKI GOZD (10-KG) | 1.3 ± 1.3 | 18.8 ± 3.8 |
| 11. MURSKA ŠUMA (11-MŠ) | 0.6 ± 0.6 | 26.3 ± 3.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutnar, L.; Nagel, T.A.; Kermavnar, J. Effects of Disturbance on Understory Vegetation across Slovenian Forest Ecosystems. Forests 2019, 10, 1048. https://doi.org/10.3390/f10111048
Kutnar L, Nagel TA, Kermavnar J. Effects of Disturbance on Understory Vegetation across Slovenian Forest Ecosystems. Forests. 2019; 10(11):1048. https://doi.org/10.3390/f10111048
Chicago/Turabian StyleKutnar, Lado, Thomas A. Nagel, and Janez Kermavnar. 2019. "Effects of Disturbance on Understory Vegetation across Slovenian Forest Ecosystems" Forests 10, no. 11: 1048. https://doi.org/10.3390/f10111048
APA StyleKutnar, L., Nagel, T. A., & Kermavnar, J. (2019). Effects of Disturbance on Understory Vegetation across Slovenian Forest Ecosystems. Forests, 10(11), 1048. https://doi.org/10.3390/f10111048





