Optimization of Pollen Germination in Tectona grandis (Teak) for Breeding Programs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Effect of Dehydration on the Viability and Germination of Pollen Inside and Outside the Anther
2.2. Effect of pH on Viability and in vitro Germination of Pollen
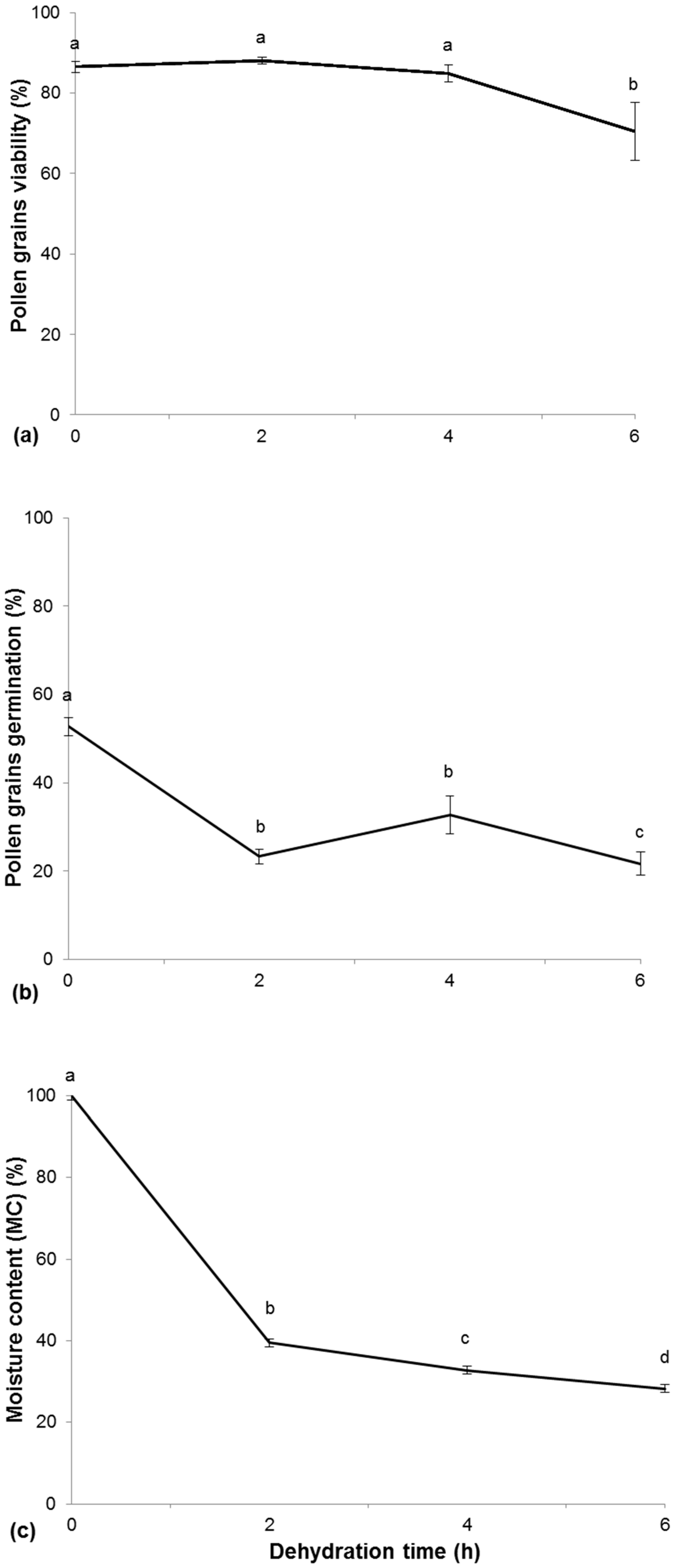
2.3. Determination of the Dehydration Curve and Survival of Pollen
2.4. Effect of the Culture Medium on the Viability and Germination of Pollen
2.5. Statistic Analysis
3. Results
3.1. Effect of Dehydration on the Viability and Germination of Pollen Analyzed Inside and Outside the Anther
3.2. Effect of pH on the Viability and In Vitro Germination of Pollen
3.3. Determination of the Dehydration Curve and Survival of Pollen
3.4. Effect of the Culture Media on the Viability and Germination of Pollen
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fonseca, W. Manual para productores de teca (Tectona grandis L.f) en Costa Rica [Manual for teak (Tectona grandis L.f) producers en Costa Rica]. SIREFOR (Forest Resources Information System//Sistema de Información de Recursos Forestales). Costa Rica; 2004. Available online: www.sirefor.go.cr/Documentos/Reforestacion/2004_Fonseca_ManualProductoresTeca.pdf (accessed on 20 October 2017).
- Keogh, R. Capítulo 2: La teca y su importancia económica a nivel mundial [Chapter 2: Teak and its economic relevance worldwide]. In Las Plantaciones de Teca en América Latina: Mitos y Realidades; de Camino, R., Morales, J.P., Eds.; CATIE: Turrialba, Costa Rica, 2013; pp. 8–28. [Google Scholar]
- Murillo, O.; Wright, J.; Monteuuis, O.; Montenegro, F. Capítulo 6: Mejoramiento genético de la teca en América Latina [Chapter 6: Genetic improvement of teak in Latin America]. In Las Plantaciones de Teca en América Latina: Mitos y Realidades; De Camino, R., Morales, J.P., Eds.; CATIE: Turrialba, Costa Rica, 2013; pp. 86–111. [Google Scholar]
- Keogh, R. Teak (Tectona grandis) provenances of the Caribbean, Central America, Venezuela and Colombia. In Proceedings of the Rio Piedras IUFRO Meeting, Working Group S1.07.09 Rio Piedras, Puerto Rico, USA, 8–12 September 1980; pp. 343–358. [Google Scholar]
- Murillo, O.; Badilla, Y.; Rojas, F. Desarrollo del mejoramiento genético forestal en Costa Rica y liderazgo regional con especies tropicales. In Proceedings of the XIV Congreso Nacional Agropecuario, Forestal y Ambiental, (CONAFA), Belén, Costa Rica, 25–27 October 2016. [Google Scholar]
- Guzmán, N.; Moya, R.; Murillo, O. Evaluation of bent trees in juvenile teak (Tectona grandis L.f.) plantations in Costa Rica: Effects on tree morphology and wood properties. Forests 2017, 8, 79. [Google Scholar] [CrossRef]
- Resende, M.; Murillo, O.; Badilla, Y. Genética Cuantitativa y Selección en el Mejoramiento Forestal; Editorial Tecnológica de Costa Rica: Cartago, Costa Rica, 2018; p. 302. [Google Scholar]
- Moya, R.; Marín, J.D.; Murillo, O.; Leandro, L. Wood physical properties, color, decay resistance and stiffness in Tectona grandis clones with evidence of genetic control. Silvae Genet. 2013, 62, 142–152. [Google Scholar] [CrossRef]
- Tangmitcharoen, S.; Owens, J. Floral Biology, Pollinization, pistil Receptivity, and Pollen-tube growth of teak (Tectona grandis Linn f). Annu. Bot. 1997, 79, 401–410. [Google Scholar] [CrossRef]
- Tangmitcharoen, S.; Owens, J. Pollen viability and pollen-tube growth following controlled pollination and their relation to low fruit production in teak (Tectona grandis Linn f). Annu. Bot. 1997, 80, 401–420. [Google Scholar] [CrossRef]
- Palupi, E.; Owens, J. Pollination, fertilization and embryogenesis of teak (Tectona grandis Linn f). Int. J. Plant Sci. 1997, 158, 259–273. [Google Scholar] [CrossRef]
- Indira, E.P.; Mohanadas, K. Intrinsic and extrinsic factors affecting pollination and fruit productivity in teak (Tectona grandis Linn.f.). Indian J. Genet. Plant Breed. 2002, 62, 208–2014. [Google Scholar]
- Vasudeva, R.; Hanumantha, M.; Gunaga, R.P. Genetic variation for floral traits among teak (Tectona grandis Linn.f.) clones: implications to seed orchard fertility. Curr. Sci. 2004, 87, 358–362. [Google Scholar]
- Palupi, E.; Owens, J.; Sadjad, S.; Sudarsono, S.; Solihin, D. The importance of fruit set, fruit abortion, and pollination of teak (Tectona grandis). Can. J. For. Res. 2010, 40, 2204–2214. [Google Scholar] [CrossRef]
- Rejón, J.; Suárez, C.; Alché, J.; Castro, A.; Rodríguez, M. Evaluación de diferentes métodos para estimar la calidad del polen en distintos cultivares de olivo [Evaluation of different methods for estimating the quality of pollen in various olive cultivars] (Olea Europaea L). Polen 2010, 20, 61–72. [Google Scholar]
- Shivanna, K.R.; Tandon, R. Reproductive Ecology of Flowering Plants: A Manual; Springer: New Delhi, India, 2014; p. 46. [Google Scholar] [CrossRef]
- Ganeshan, S.; Rajasekharan, P.E.; Shashikumar, S.; Decruz, W. Cryopreservation of Pollen. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 443–464. [Google Scholar] [CrossRef]
- Volk, G.M. Collecting pollen for genetic resources conservation. In Collecting Plant Genetic Diversity: Technical Guidelines 2011 Update; Guarino, L., Ramanatha, V.R., Goldberg, E., Eds.; Bioversity International: Rome, Italy, 2011; pp. 1–10. [Google Scholar]
- Gónzalez, M.E.; Estévez, A.; Castillo, J.; Salomón, J.; Moré, O.; Hernández, M. La Calidad de la papa: Requerimiento Indispensable del Mejoramiento Genético Tradicional de la papa en Cuba [Potato quality: essential requirement for traditional genetic improvement of potatoes in Cuba]. Rev. Latinoam. Papa 2002, 13, 75–94. [Google Scholar]
- Brewbacker, J.L.; Kwack, B.H. The essential role of calcium ion in pollen germination and pollen tube growth. Am. J. Bot. 1963, 50, 859–865. [Google Scholar] [CrossRef]
- Johnson, M.A.; Kost, B. Pollen Tube Development. In Plant Developmental Biology, Methods in Molecular Biology; Hennig, L., Köhler, C., Eds.; Springer: New York, NY, USA, 2010; p. 155. [Google Scholar] [CrossRef]
- Abdelnour, A.; Rojas, G.; Alfaro, U. Estudios preliminares para la crioconservación de especies forestales arbóreas. Tecnol. Marcha 2007, 20, 98–103. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat versión. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. 2009. Available online: http://www.infostat.com.ar (accessed on 22 July 2019).
- Statsoft Inc. Statistica (Data Analysis Software System), Version 13.1. 2013. Available online: www.statsoft.com/textbook/ (accessed on 25 July 2019).
- Taiz, L.; Zeiger, E.; Møller, I.; Murphy, A. Plant Physiology, 6th ed.; Sinauer Associates. Inc.: Sunderland, MA, USA, 2015; p. 705. [Google Scholar]
- Yang, W.C.; Shi, D.Q. Plant Developmental Biology—Biotechnological Perspectives: Pollen Germination and Tube Growth; Pua, E.C., Michael, R.D., Eds.; Springer: Heidelberg, Germany, 2010; p. 249. [Google Scholar] [CrossRef]
| Medium | Formulation |
|---|---|
| Medium 1 | 10% sucrose (g/100 mL) in aqueous solution at a pH of 7 |
| Medium 2 | 14% sucrose (g/100 mL) in aqueous solution at a pH of 7 |
| Medium 3 | BK medium at 10% of its salts + 10% sucrose (g/100 mL) in aqueous solution at a pH of 7 |
| Dehydration (h) | Viability (%) | Germination (%) | ||
|---|---|---|---|---|
| Inside | Outside | Inside | Outside | |
| 0 | 75.47 ± 10.29 c | 86.45 ± 1.38 a | 43.22 ± 2.18 a | 52.72 ± 2.01 a |
| 2 | 90.00 ± 0.00 a | 87.96 ± 0.85 a | 20.77 ± 3.71 b | 23.28 ± 1.74 bc |
| 4 | 89.00 ± 3.47 a | 84.83 ± 2.07 b | 4.68 ± 1.3 c | 32.73 ± 4.33 ab |
| 6 | 83.20 ± 13.87 b | 70.46 ± 7.24 c | 10.07 ± 1.95 c | 21.69 ± 2.57 c |
| Time (h) | pH | Variability (%) | Germination (%) |
|---|---|---|---|
| 0 | 6 | 74.72 ± 1.91 abcd | 19.61 ± 2.59 abc |
| 6.3 | 72.25 ± 6.01 bcd | 16.35 ± 2.36 bc | |
| 6.5 | 77.95 ± 4.92 abcd | 29.44 ± 3.87 abc | |
| 6.8 | 68.33 ± 1.62 cd | 19.48 ± 5.05 abc | |
| 7 | 65.54 ± 6.30 d | 29.96 ± 2.66 abc | |
| 2 | 6 | 73.60 ± 1.73 bcd | 32.23 ± 2.97 abc |
| 6.3 | 83.58 ± 2.76 abc | 36.28 ± 10.55 abc | |
| 6.5 | 86.11 ± 3.89 ab | 41.15 ± 7.69 ab | |
| 6.8 | 90.00 ± 0.00 a | 29.00 ± 8.50 abc | |
| 7 | 83.88 ± 3.78 abc | 45.78 ± 9.99 a | |
| 4 | 6 | 74.44 ± 2.12 abcd | 13.62 ± 1.56 c |
| 6.3 | 77.67 ± 0.83 abcd | 42.36 ± 5.29 ab | |
| 6.5 | 81.42 ± 0.5 abcd | 12.81 ± 1.9 c | |
| 6.8 | 79.74 ± 2.58 abcd | 15.57 ± 2.77 bc | |
| 7 | 78.14 ± 0.77 abcd | 12.81 ± 1.12 c |
| Medium | Viability (%) | Germination (%) |
|---|---|---|
| Medium 1 | 90 | 5.14 ± 1.33 b |
| Medium 2 | 90 | 5.35 ± 1.26 b |
| Medium 3 | 90 | 28.99 ± 1.53 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hine, A.; Rojas, A.; Suarez, L.; Murillo, O.; Espinoza, M. Optimization of Pollen Germination in Tectona grandis (Teak) for Breeding Programs. Forests 2019, 10, 908. https://doi.org/10.3390/f10100908
Hine A, Rojas A, Suarez L, Murillo O, Espinoza M. Optimization of Pollen Germination in Tectona grandis (Teak) for Breeding Programs. Forests. 2019; 10(10):908. https://doi.org/10.3390/f10100908
Chicago/Turabian StyleHine, Ana, Alejandra Rojas, Lorenzo Suarez, Olman Murillo, and Mario Espinoza. 2019. "Optimization of Pollen Germination in Tectona grandis (Teak) for Breeding Programs" Forests 10, no. 10: 908. https://doi.org/10.3390/f10100908
APA StyleHine, A., Rojas, A., Suarez, L., Murillo, O., & Espinoza, M. (2019). Optimization of Pollen Germination in Tectona grandis (Teak) for Breeding Programs. Forests, 10(10), 908. https://doi.org/10.3390/f10100908





