Topography Affects Tree Species Distribution and Biomass Variation in a Warm Temperate, Secondary Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Data Collection
2.2. Habitat Classification
2.3. Forest Characteristics
2.4. Relative Contributions of Species to Total Biomass in Different Habitats
3. Results
3.1. Habitat Types
3.2. Forest Characteristics across Habitat Types
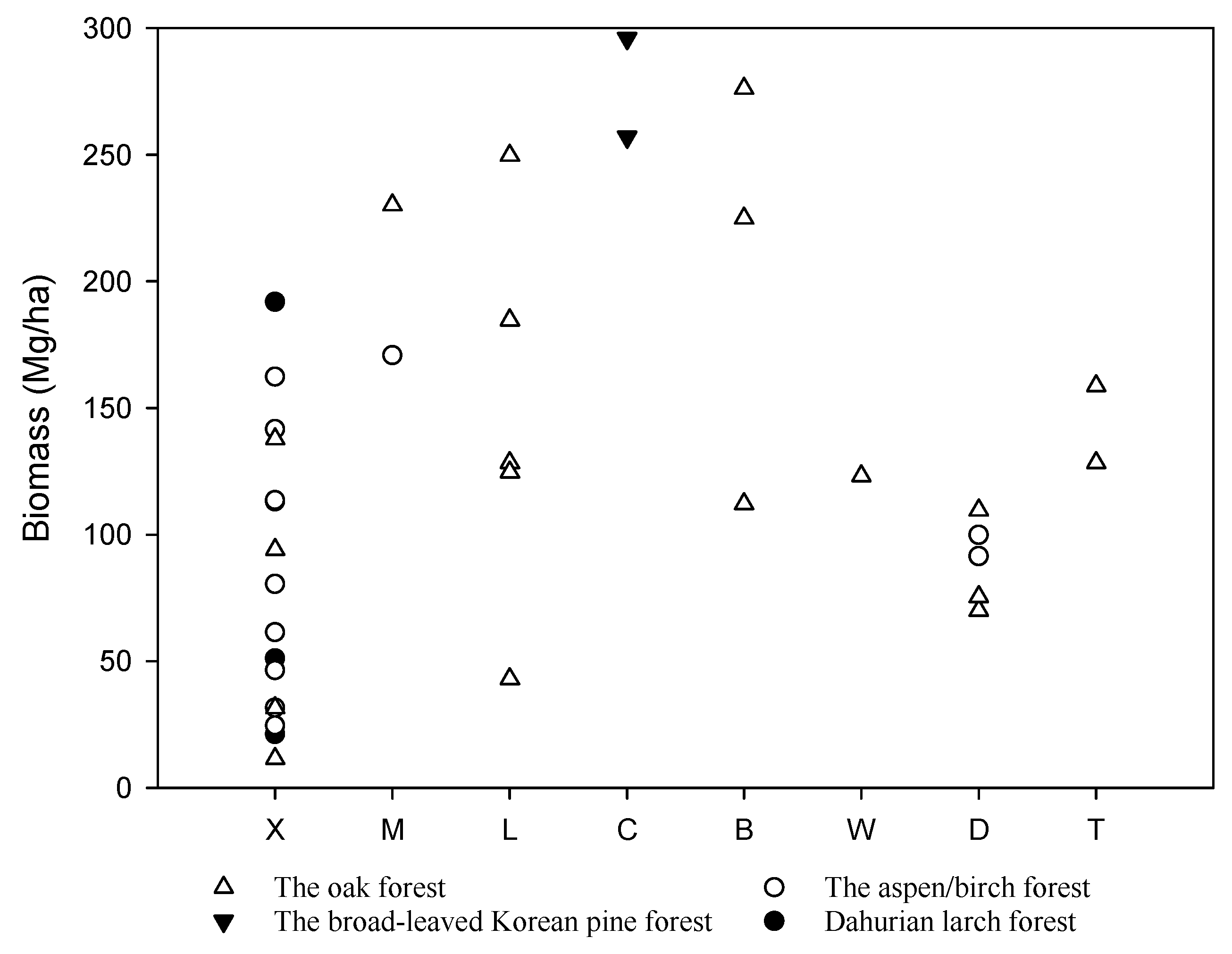
3.3. Habitat-Specific Differentiation in Biomass Contribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Allometric Equations | Sources |
|---|---|---|
| Populus cathayana, Populus davidiana, Salix caprea, Salix phylicifolia, Salix viminalis | B = 0.085 × D2.48 | Wang et al. [77] |
| Corylus mandshurica | B = 0.148 × ((D2) × H)0.86 | Fang et al. [34] |
| Abelia biflora, Lonicera chrysantha, Lonicera hispida, Lonicera japonica, Sambucus williams, Viburnum sargentii | B = 0.00349 × ((D2) × H)1.04 | Fang et al. [34] |
| Deutzia grandiflora, Deutzia parviflora, Hydrangea bretschneideri, Philadelphus pekinensis, Ribes pulchellum | B = 0.014 × ((D2) × H)0.873 | Fang et al. [34] |
| Ulmus laciniata, Ulmus macrocarpa, Ulmus pumila | B = 0.07944 × ((D2) × H)0.9613 | Jiang [78] |
| Acer mono, Malus baccata, Prunus armeniaca, Prunus davidiana, Prunus padus, Sorbus discolor | B = 0.09747 × ((D2) × H)0.9510 | Jiang [78] |
| Quercus mongolica | B = 0.1122 × ((D2) × H)0.8500 | Jiang [78] |
| Juglans mandshurica | B = 0.11554 × ((D2) × H)0.8389 | Jiang [78] |
| Betula chinensis, Betula dahurica, Betula platyphylla | B = 0.16034 × ((D2) × H) 0.8354 | Jiang [78] |
| Fraxinus bungeana, Fraxinus rhynchophylla, Syringa pekinensis, Syringa pubescens | B = 0.19932 × ((D2) × H)0.8553 | Jiang [78] |
| Tilia mandshurica, Tilia mongolica | B = 0.20536 × ((D2) × H)0.7611 | Jiang [78] |
| Larix principis-rupprechtii | B = 0.01758 × ((D2) * H)0.9611 + 0.002 × ((D2) * H)1.1193 + 0.0038 × ((D2) * H)0.8828 + 0.01301 × ((D2) * H)0.8551 | Chen [79] |
| Lespedeza bicolor | B = 0.0202 × ((D2) × H)0.877 | Fang et al. [34] |
| Other species | B = 0.0481 × ((D2) × H)0.837 | Fang et al. [34] |
References
- Dixon, R.K.; Solomon, A.M.; Brown, S.; Houghton, R.A.; Trexier, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Chen, L.; Guan, X.; Li, H.; Wang, Q.; Zhang, W.; Yang, Q.; Wang, S. Spatiotemporal patterns of carbon storage in forest ecosystems in Hunan Province, China. For. Ecol. Manag. 2019, 432, 656–666. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Erb, K.-H.; Kastner, T.; Plutzar, C.; Bais, A.L.S.; Carvalhais, N.; Fetzel, T.; Gingrich, S.; Haberl, H.; Lauk, C.; Niedertscheider, M.; et al. Unexpectedly large impact of forest management and grazing on global vegetation biomass. Nature 2018, 553, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Cramer, W.; Bondeau, A.; Schaphoff, S.; Lucht, W.; Smith, B.; Sitch, S. Tropical forests and the global carbon cycle: Impacts of atmospheric carbon dioxide, climate change and rate of deforestation. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004, 359, 331–343. [Google Scholar] [CrossRef]
- Van der Werf, G.R.; Morton, D.C.; DeFries, R.S.; Olivier, J.G.J.; Kasibhatla, P.S.; Jackson, R.B.; Collatz, G.J.; Randerson, J.T. CO2 emissions from forest loss. Nat. Geosci. 2009, 2, 737–738. [Google Scholar] [CrossRef]
- Woodwell, G.M.; Hobbie, J.E.; Houghton, R.A.; Melillo, J.M.; Moore, B.; Peterson, B.J.; Shaver, G.R. Global deforestation: Contribution to atmospheric carbon dioxide. Science 1983, 222, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Erb, K.H.; Kastner, T.; Luyssaert, S.; Houghton, R.A.; Kuemmerle, T.; Olofsson, P.; Haberl, H. Bias in the attribution of forest carbon sinks. Nat. Clim. Chang. 2013, 3, 854–856. [Google Scholar] [CrossRef] [Green Version]
- Pugh, T.A.M.; Lindeskog, M.; Smith, B.; Poulter, B.; Arneth, A.; Haverd, V.; Calle, L. Role of forest regrowth in global carbon sink dynamics. Proc. Natl. Acad. Sci. USA 2019, 116, 4382–4387. [Google Scholar] [CrossRef] [Green Version]
- Houghton, R.A. Aboveground forest biomass and the global carbon balance. Glob. Chang. Biol. 2005, 11, 945–958. [Google Scholar] [CrossRef]
- McEwan, R.W.; Lin, Y.; Sun, I.F.; Hsieh, C.F.; Su, S.; Chang, L.; Song, G.; Wang, H.; Hwong, J.L.; Lin, K.; et al. Topographic and biotic regulation of aboveground carbon storage in subtropical broad-leaved forests of Taiwan. For. Ecol. Manag. 2011, 262, 1817–1825. [Google Scholar] [CrossRef]
- Lin, D.; Lai, J.; Muller-Landau, H.C.; Mi, X.; Ma, K. Topographic Variation in aboveground biomass in a subtropical evergreen broad-leaved forest in China. PLoS ONE 2012, 7, e48244. [Google Scholar] [CrossRef] [PubMed]
- Paquette, A.; Messier, C. The effect of biodiversity on tree productivity: From temperate to boreal forests. Glob. Ecol. Biogeogr. 2011, 20, 170–180. [Google Scholar] [CrossRef]
- Bolstad, P.V.; Vose, J.M.; McNulty, S.G. Forest productivity, leaf area, and terrain in Southern Appalachian deciduous forests. For. Sci. 2001, 47, 419–427. [Google Scholar]
- Kirby, K.R.; Potvin, C. Variation in carbon storage among tree species: Implications for the management of a small-scale carbon sink project. For. Ecol. Manag. 2007, 246, 208–221. [Google Scholar] [CrossRef]
- Fang, J.; Guo, Z.; Piao, S.; Chen, A. Terrestrial vegetation carbon sinks in China, 1981–2000. Sci. China Ser. D Earth Sci. 2007, 50, 1341–1350. [Google Scholar] [CrossRef]
- Botkin, D.B.; Simpson, L.G. Biomass of the North American boreal forest: A step toward accurate global measures. Biogeochemistry 1990, 9, 161–174. [Google Scholar]
- Goodale, C.L.; Apps, M.J.; Birdsey, R.A.; Field, C.B.; Heath, L.S.; Houghton, R.A.; Jenkins, J.C.; Kohlmaier, G.H.; Kurz, W.; Liu, S.; et al. Forest carbon sinks in the Northern hemisphere. Ecol. Appl. 2002, 12, 891–899. [Google Scholar] [CrossRef]
- Schroeder, P.; Brown, S.; Mo, J.; Birdsey, R.; Cieszewski, C. Biomass estimation for temperate broadleaf forests of the United States using inventory data. For. Sci. 1997, 43, 424–434. [Google Scholar]
- Fang, J.; Chen, A.; Peng, C.; Zhao, S.; Ci, L. Changes in forest biomass carbon storage in China between 1949 and 1998. Science 2001, 292, 2320–2322. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Guo, Q.; Xue, B.; Hu, T.; Alvarez, O.; Tao, S.; Fang, J. Spatial distribution of forest aboveground biomass in China: Estimation through combination of spaceborne lidar, optical imagery, and forest inventory data. Remote Sens. Environ. 2016, 173, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Valencia, R.; Foster, R.B.; Villa, G.; Condit, R.; Svenning, J.C.; Hernandez, C.; Romoleroux, K.; Losos, E.; Magard, E.; Balslev, H. Tree species distributions and local habitat variation in the Amazon: Large forest plot in eastern Ecuador. J. Ecol. 2004, 92, 214–229. [Google Scholar] [CrossRef]
- Chave, J.; Condit, R.; Lao, S.; Caspersen, J.P.; Foster, R.B.; Hubbell, S.P. Spatial and temporal variation of biomass in a tropical forest: Results from a large census plot in Panama. J. Ecol. 2003, 91, 240–252. [Google Scholar] [CrossRef]
- Su, H.; Li, G. Simulating the response of the Quercus mongolica forest ecosystem carbon budget to asymmetric warming. Chin. Sci. Bull. (Chin. Ver.) 2012, 57, 1544–1552. (In Chinese) [Google Scholar]
- Zou, Y.; Sang, W.; Wang, S.; Warrenthomas, E.; Liu, Y.; Yu, Z.; Wang, C.; Axmacher, J.C. Diversity patterns of ground beetles and understory vegetation in mature, secondary, and plantation forest regions of temperate northern China. Ecol. Evol. 2015, 5, 531–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.; Mi, X.; Liu, C.; Ma, K. Tree competition and species coexistence in a Quercus-Betula forest in the Dongling Mountains in northern China. Acta Oecol. 2006, 30, 117–125. [Google Scholar] [CrossRef]
- Liu, H.; Xue, D.; Sang, W. Species diffusion and niche differentiation of the warm temperate deciduous broad-leaved forest in its functional development process. Chin. Sci. Bull. (Chin. Ver.) 2014, 59, 2359–2366. [Google Scholar]
- Gu, H.; Li, J.; Qi, G.; Wang, S. Species spatial distributions in a warm-temperate deciduous broad-leaved forest in China. J. For. Res. 2019. [Google Scholar] [CrossRef]
- Condit, R. Tropical Forest Census Plots: Methods and Results from Barro Colorado Island, Panama and a Comparison with Other Plots; Springer: Berlin/Landes, Germany; Georgetown, TX, USA, 1998. [Google Scholar]
- Lai, J.; Mi, X.; Ren, H.; Ma, K. Species-habitat associations change in a subtropical forest of China. J. Veg. Sci. 2009, 20, 415–423. [Google Scholar] [CrossRef]
- Legendre, P.; Mi, X.; Ren, H.; Ma, K.; Yu, M.; Sun, I.-F.; He, F. Partitioning beta diversity in a subtropical broad-leaved forest of China. Ecology 2009, 90, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011. [Google Scholar]
- Fang, J.; Liu, G.; Zhu, B.; Wang, X.; Liu, S. Study on the carbon cycle of three temperate forest ecological system in Donglingshan in Beijing. Sci. China Ser. D Earth Sci. 2006, 36, 533–543. [Google Scholar]
- Harms, K.; Condit, R.; Hubbell, S.P.; Foster, R.B. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J. Ecol. 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Saatchi, S.; Harris, N.; Brown, S.; Lefsky, M.; Mitchard, E.T.A.; Salas, W.; Zutta, B.R.; Buermann, W.; Lewis, S.L.; Hagen, S.; et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl. Acad. Sci. USA 2011, 108, 9899–9904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, B.T.; Woodall, C.W.; Griffith, D.M. Imputing forest carbon stock estimates from inventory plots to a nationally continuous coverage. Carbon Balance Manag. 2013, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, X.; Dai, H.; Tan, Z.; Zhang, Y.; Guo, X.; Peng, Y.; Dai, L. Research on biological productivity of Korean pine broadleaf forest. In Changbai Mountain in Forest Ecosystem Research; Wang, Z., Ed.; Forestry Press: Beijing, China, 1985; pp. 33–48. [Google Scholar]
- Wang, B.; Yang, X. Comparison of biomass and species diversity of four typical zonal vegetations. J. Fujian Coll For. 2009, 29, 345–350. [Google Scholar]
- Sang, W.; Ma, K.; Chen, L. Primary study on carbon cycling in warm temperate deciduous broad-leaved forest. Acta Phytoecol. Sin. 2002, 26, 543–548. [Google Scholar]
- Hu, H.; Luo, B.; Wei, S.; Wei, S.; Sun, L.; Luo, S.; Ma, H. Biomass carbon density and carbon sequestration capacity in seven typical forest types of the Xiaoxing’an Mountains, China. Chin. J. Plant Ecol. 2015, 39, 140–158. [Google Scholar]
- Brown, S. Measuring carbon in forests: Current status and future challenges. Environ. Pollut. 2002, 116, 363–372. [Google Scholar] [CrossRef]
- Edwards, T.C.; Cutler, D.R.; Zimmermann, N.E.; Geiser, L.; Moisen, G.G. Effects of sample survey design on the accuracy of classification tree models in species distribution models. Ecol. Model. 2006, 199, 132–141. [Google Scholar] [CrossRef]
- Blackard, J.A.; Finco, M.V.; Helmer, E.H.; Holden, G.R.; Hoppus, M.L.; Jacobs, D.M.; Lister, A.J.; Moisen, G.G.; Nelson, M.D.; Riemann, R.; et al. Mapping U.S. forest biomass using nationwide forest inventory data and moderate resolution information. Remote Sens. Environ. 2008, 112, 1658–1677. [Google Scholar] [CrossRef]
- Guitet, S.; Hérault, B.; Molto, Q.; Brunaux, O.; Couteron, P. Spatial structure of above-ground biomass limits accuracy of carbon mapping in rainforest but large scale forest inventories can help to overcome. PLoS ONE 2015, 10, e0138456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, C. Carbon density and distribution of six Chinese temperate forest. Sci. China Life Sci. 2010, 53, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, W.; Liu, W.; Wu, X. Study on the biomass and productivity of Mongolian oak forests in northeast region of China. Chin. J. Eco-Agric. 2006, 14, 21–24. [Google Scholar]
- Huo, C.; You, W.; Zhang, H.; Yan, T.; Wei, W.; Zhao, G.; Guo, J.; Xing, Z. Biomass and net primary productivity of Quecus mongolica plantation in Binglashan Mountains in Liaoning Province. J. Liaoning For. Sci. Technol. 2011, 4, 4–6, 11. [Google Scholar]
- Wang, D.; Cai, W.; Li, D.; Feng, X.; Feng, T.; Li, Y. Study of biomass and production of the forest of Quercus mongolica in Wuling Mountain. Chin. J. Ecol. 1998, 17, 9–15. [Google Scholar]
- Liu, Y.; Wu, M.; Guo, Z.; Jiang, Y.; Liu, S. Biomass and net productivity of Quercus variabilis forest in Baotianman Natural Reserve. Chin. J. Appl. Ecol. 1998, 9, 569–574. [Google Scholar]
- Liu, Y.; Wu, M.; Guo, Z.; Jiang, Y.; Liu, S.; Wang, Z.; Liu, B.; Zhu, X. Studies on biomass and net production of Quercus acutidentata forest in Baotianman Nature Reserve. Acta Ecol. Sin. 2001, 21, 1450–1456. [Google Scholar]
- Iverson, L.R.; Dale, M.E.; Scott, C.T.; Prasad, A. A GIS-derived integrated moisture index to predict forest composition and productivity of Ohio forests (U.S.A.). Landsc. Ecol. 1997, 12, 331–348. [Google Scholar] [CrossRef]
- Western, A.W.; Grayson, R.B.; Blöschl, G.; Willgoose, G.R.; McMahon, T.A. Observed spatial organization of soil moisture and its relation to terrain indices. Water Resour. Res. 1999, 35, 797–810. [Google Scholar] [CrossRef] [Green Version]
- McNab, W.H. A topographic index to quantify the effect of mesoscale landform on site productivity. Can. J. For. Res. 1993, 23, 1100–1107. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; An, S.; Ju, W. Effects of topography on simulated net primary productivity at landscape scale. J. Environ. Manag. 2007, 85, 585–596. [Google Scholar] [CrossRef]
- Ferry, B.; Morneau, F.; Bontemps, J.-D.; Blanc, L.; Freycon, V. Higher treefall rates on slopes and waterlogged soils result in lower stand biomass and productivity in a tropical rain forest. J. Ecol. 2010, 98, 106–116. [Google Scholar] [CrossRef]
- Oliver, C.D. Forest development in North America following major disturbances. For. Ecol. Manag. 1980, 3, 153–168. [Google Scholar] [CrossRef]
- Knapp, B.O.; Pallardy, S.G. Forty-eight years of forest succession: Tree species change across four forest types in Mid-Missouri. Forests 2018, 9, 633. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Jensen, R.; Kabrick, J. Changes in aboveground biomass following alternative harvesting in oak-hickory forests in the eastern USA. iForest-Biogeosci. For. 2015, 8, 652–660. [Google Scholar] [CrossRef] [Green Version]
- Morin, X.; Fahse, L.; Scherer-Lorenzen, M.; Bugmann, H. Tree species richness promotes productivity in temperate forests through strong complementarity between species. Ecol. Lett. 2011, 14, 1211–1219. [Google Scholar] [CrossRef]
- Vilà, M.; Vayreda, J.; Comas, L.; Ibáñez, J.J.; Mata, T.; Obón, B. Species richness and wood production: A positive association in Mediterranean forests. Ecol. Lett. 2007, 10, 241–250. [Google Scholar] [CrossRef]
- Fei, S.; Jo, I.; Guo, Q.; Wardle, D.A.; Fang, J.; Chen, A.; Oswalt, C.M.; Brockerhoff, E.G. Impacts of climate on the biodiversity-productivity relationship in natural forests. Nat. Commun. 2018, 9, 5436. [Google Scholar] [CrossRef]
- Szwagrzyk, J.; Gazda, A. Above-ground standing biomass and tree species diversity in natural stands of Central Europe. J. Veg. Sci. 2007, 18, 555–562. [Google Scholar] [CrossRef]
- Glenn-Lewin, D.C. Species diversity in North American temperate forests. Vegetatio 1977, 33, 153–162. [Google Scholar] [CrossRef]
- Armesto, J.J.; Mitchell, J.D.; Villagran, C. A comparison of spatial patterns of trees in some tropical and temperate Forests. Biotropica 1986, 18, 1–11. [Google Scholar] [CrossRef]
- Hille Ris Lambers, J.; Clark, J.S.; Beckage, B. Density-dependent mortality and the latitudinal gradient in species diversity. Nature 2002, 417, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.J.; Beaulieu, W.T.; Bever, J.D.; Clay, K. Conspecific negative density dependence and forest diversity. Science 2012, 336, 904–907. [Google Scholar] [CrossRef]
- Parker, G.R.; Leopold, D.J.; Eichenberger, J.K. Tree dynamics in an old-growth, deciduous forest. For. Ecol. Manag. 1985, 11, 31–57. [Google Scholar] [CrossRef]
- Larsen, D.R.; Johnson, P.S. Linking the ecology of natural oak regeneration to silviculture. For. Ecol. Manag. 1998, 106, 1–7. [Google Scholar] [CrossRef]
- Nowacki, G.J.; Abrams, M.D. The demise of fire and “Mesophication” of forests in the Eastern United States. BioScience 2008, 58, 123–138. [Google Scholar] [CrossRef]
- Yan, Q.; Zhu, J.; Yu, L. Seed regeneration potential of canopy gaps at early formation stage in temperate secondary forests, Northeast China. PLoS ONE 2012, 7, e39502. [Google Scholar] [CrossRef]
- Suh, M.H.; Lee, D.K. Stand structure and regeneration of Quercus mongolica forests in Korea. For. Ecol. Manag. 1998, 106, 27–34. [Google Scholar] [CrossRef]
- Lieberman, D.; Lieberman, M.; Peralta, R.; Hartshorn, G.S. Mortality patterns and stand turnover rates in a wet tropical forest in Costa Rica. J. Ecol. 1985, 73, 915–924. [Google Scholar] [CrossRef]
- Platt, W.J.; Evans, G.W.; Rathbun, S.L. The population dynamics of a long-lived conifer (Pinus palustris). Am. Nat. 1988, 131, 491–525. [Google Scholar] [CrossRef]
- Condit, R.; Ashton, P.S.; Manokaran, N.; LaFrankie, J.V.; Hubbell, S.P.; Foster, R.B. Dynamics of the forest communities at Pasoh and Barro Colorado: Comparing two 50-ha plots. Philos. Trans. R. Soc. B 1999, 354, 1739–1748. [Google Scholar] [CrossRef]
- Andersonteixeira, K.J.; Davies, S.J.; Bennett, A.C.; Gonzalezakre, E.B.; Mullerlandau, H.C.; Wright, S.J.; Abu Salim, K.; Almeyda Zambrano, A.M.; Alonso, A.; Baltzer, J.L.; et al. CTFS-ForestGEO: A worldwide network monitoring forest in an era of global change. Glob. Chang. Biol. 2015, 21, 528–549. [Google Scholar] [CrossRef]
- Wang, N.; Wang, B.; Wang, R.; Cao, X.; Wang, W.; Chi, L. Biomass allocation patterns and allometric models of Populus davidiaa and Pinus Tabulaeformis Carr. in west Shanxi province. Bull. Soil Water Conserv. 2013, 33, 151–155. [Google Scholar]
- Jiang, H. The Research on Plant Ecology; Institute of Botany: Beijing, China, 1992. [Google Scholar]
- Chen, S. Analyzing Features of Leaf Area Index, Net Primary Productivity and Tree-Ring for Typical Forest Communities in Warm Temperate Zone; Institute of Botany: Beijing, China, 2007. [Google Scholar]
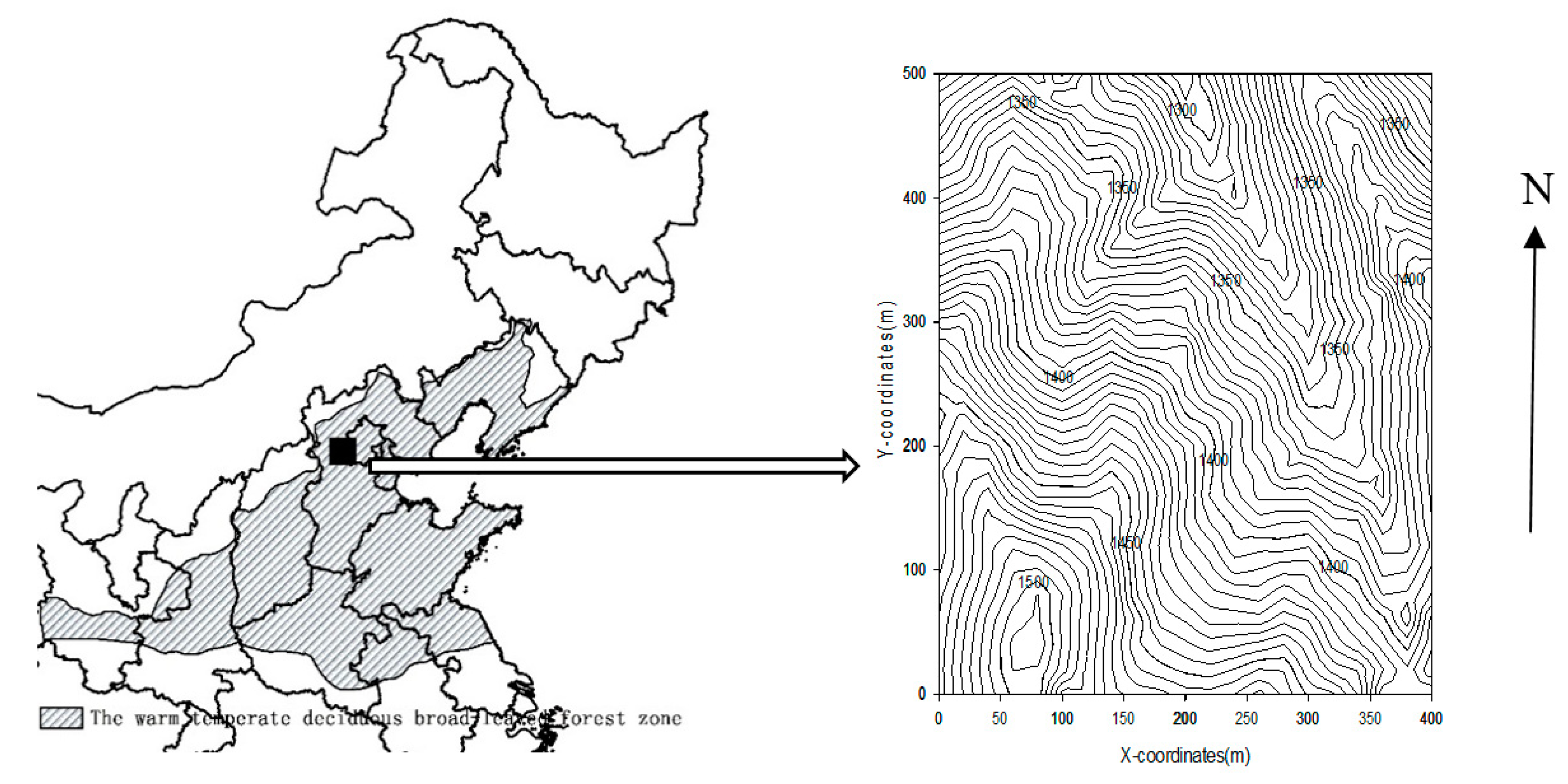
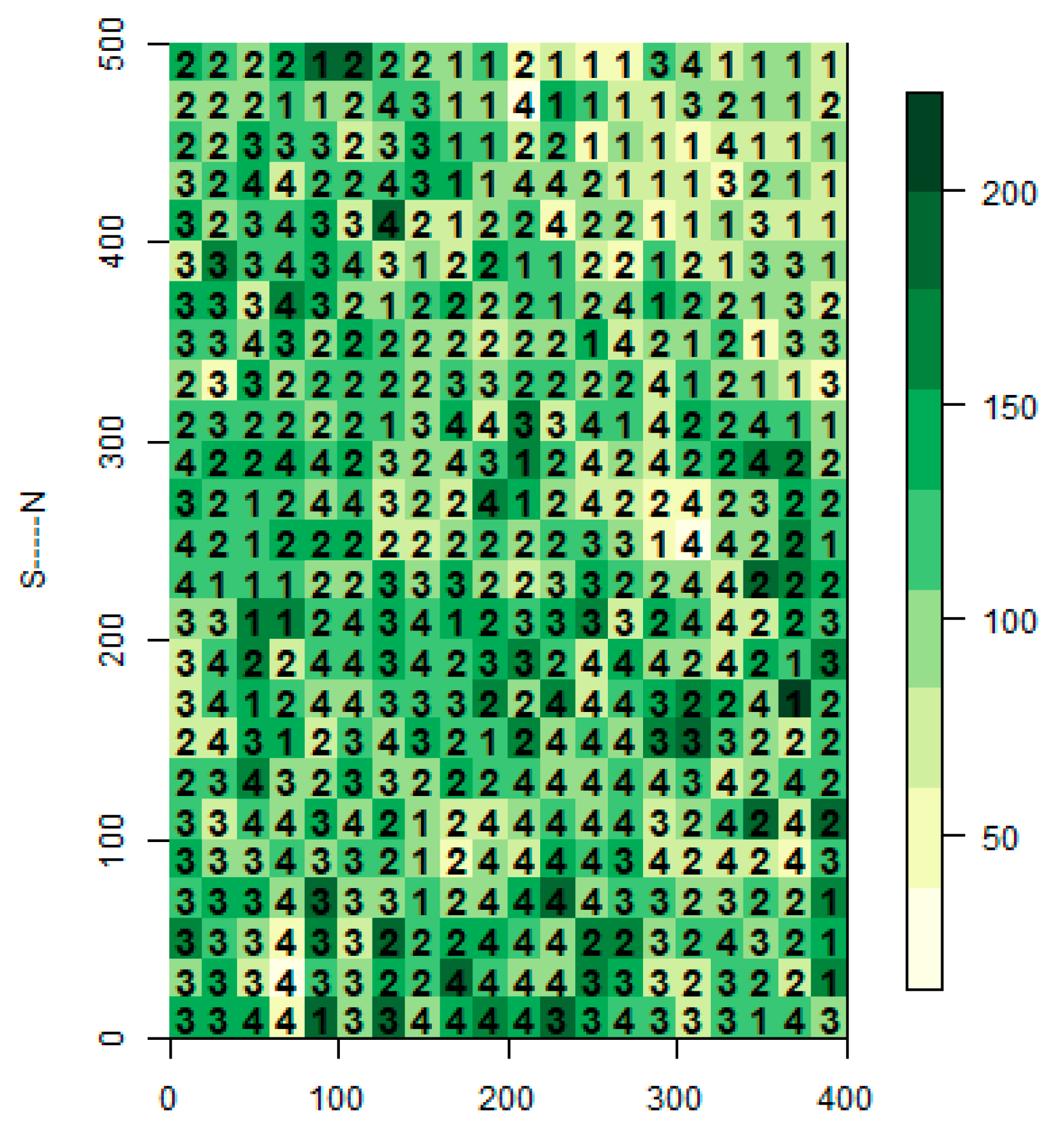
| (A) | |||||
| Habitat Category | Area (ha) | Elevation (m) | Convexity (m) | Slope (°) | Aspect (°) |
| Steep slope | 3.48 | 1367.73 (1359–1378) c | 0.05 (−0.29–0.42) b | 40.75 (40.24–41.40) a | 122.67 (103.6–143.6) a |
| Gentle slope | 4.6 | 1404.59 (1397–1414) b | −0.58 (−1.12–0.05) bc | 23.47 (22.62–24.10) d | 86.63 (77.95–97.76) b |
| Valley | 6.76 | 1381.87 (1376–1388) c | −0.87(−1.22–0.52) c | 34.21 (33.85–34.50) b | 104.97 (91.8–119.0) ab |
| Ridge | 5.16 | 1420.53 (1413–1429) a | 1.72 (1.46–2.06) a | 30.75 (30.39–31.12) c | 113.03 (100.1–129.7) ab |
| Entire plot | 20 | 1394.61 (1390–1399) | 0.03 (−0.18–0.26) | 31.98 (31.42–32.52) | 105.91 (98.9–113.5) |
| (B) | |||||
| Habitat Category | Quadratic Mean Diameter (cm) | Basal Area (m2/ha) | Tree Density (Trees/ha) | Richness (Number of Species/400 m2) | Biomass (Mg/ha) |
| Steep slope | 7.51 (7.08–8.07) b | 22.7(21.2–24.2) bc | 5965(5426–6698) a | 11.6(10.9–12.3) a | 104.8(98.5–111.7) b |
| Gentle slope | 8.27 (7.91–8.70) ab | 21.4(20.3–22.6) c | 4616(4195–5091) b | 11.7(11.1–12.3) a | 103.8(97.9–109.5) b |
| Valley | 8.52 (8.21–8.91) a | 23.4(22.6–24.3) b | 4875(4491–5279) b | 12.1(11.6–12.7) a | 109.9(105.8–114.3) ab |
| Ridge | 8.88 (8.32–9.53) a | 25.8(24.7–26.9) a | 5515(5011–6111) ab | 10.3(9.7–10.9) b | 117.4(112.1–122.9) a |
| Entire plot | 8.38 (8.18–8.60) | 23.4(22.9–24.0) | 5171(4904–5413) | 11.5(11.1–11.8) | 109.6(107.1–112.4) |
| Species Code | Steep Slope | Gentle Slope | Valley | Ridge | Entire Plot |
|---|---|---|---|---|---|
| Acer mono | 14.19 (13.53%) | 10.59 (10.20%) | 15.93 (14.49%) + | 9.15 (7.80%) - | 12.65 (11.55%) |
| Betula dahurica | 11.56 (11.02%) - | 27.25 (26.25%) + | 16.43 (14.94%) | 22.95 (19.55%) | 19.75 (18.03%) |
| Betula platyphylla | 2.88 (2.75%) - | 11.08 (10.67%) + | 5.13 (4.66%) - | 8.53 (7.26%) | 6.98 (6.37%) |
| Fraxinus rhynchophylla | 7.27 (6.94%) + | 1.06 (1.02%) | 1.57 (1.43%) | 1.70 (1.45%) | 2.48 (2.26%) |
| Juglans mandshurica | 3.57 (3.41%) | 6.61 (6.36%) | 5.33 (4.85%) | 0.89 (0.76%)- | 4.17 (3.81%) |
| Populus davidiana | 2.27 (2.16%) | 5.95 (5.73%) | 6.69 (6.08%) + | 3.00 (2.55%) | 4.80 (4.38%) |
| Quercus mongolica | 43.80 (41.78%) | 23.47 (22.61%) - | 40.34 (36.70%) | 58.41 (49.74%) + | 41.72 (38.08%) |
| Sorbus discolor | 0.97 (0.92%) - | 4.52 (4.35%) + | 2.68 (2.44%) | 1.50 (1.28%) | 2.50 (2.28%) |
| Syringa pubescens | 2.69 (2.56%) | 1.70 (1.63%) | 2.63 (2.39%) | 2.72 (2.32%) | 2.45 (2.24%) |
| Tilia mongolica | 3.39 (3.24%) + | 0.71 (0.68%) | 1.59 (1.45%) | 0.82 (0.70%) - | 1.51 (1.37%) |
| Ulmus laciniata | 1.05 (1.00%) | 2.55 (2.46%) + | 2.14 (1.95%) + | 0.01 (0.01%) - | 1.50 (1.37%) |
| Ulmus macrocarpa | 2.03 (1.94%) | 1.43 (1.37%) | 1.96 (1.78%) | 1.23 (1.04%) | 1.66 (1.51%) |
| Ulmus pumila | 3.38 (3.22%) | 1.93 (1.86%) | 1.74 (1.58%) | 2.59 (2.21%) | 2.29 (2.09%) |
| (A) | |||||
| Species | Steep Slope | Gentle Slope | Valley | Ridge | Entire |
| Acer mono | 5.3 (4.83–5.72) ab | 5.56 (5.01–6.26) ab | 6.26 (5.82–6.81) a | 5.00 (4.53–5.63) b | 5.61 (5.37–5.91) |
| Betula dahurica | 7.72 (6.19–9.29) c | 16.12 (14.58–17.45) a | 12.61 (11.27–13.93) b | 13.97 (12.39–15.12) ab | 12.92 (12.22–13.66) |
| Betula platyphylla | 2.93 (1.91–4.32) c | 10.25 (8.47–12.14) a | 7.18 (5.97–8.92) b | 8.70 (7.15–10.24) ab | 7.54 (6.68–8.39) |
| Populus davidiana | 3.20 (2.23–4.36) c | 5.70 (4.36–7.25) ab | 6.46 (5.34–7.61) a | 3.57 (2.71–4.53) bc | 4.97 (4.36–5.62) |
| Quercus mongolica | 15.05 (13.51–16.26) ab | 9.59 (7.68–11.57) c | 14.75 (13.33–16.02) b | 17.98 (16.72–19.13) a | 14.45 (13.67–15.18) |
| (B) | |||||
| Species | Steep Slope | Gentle Slope | Valley | Ridge | Entire |
| Acer mono | 2.25 (1.85–2.80) ab | 1.58 (1.27–2.01) bc | 2.40 (2.13–2.79) a | 1.42 (1.18–1.71) c | 1.93 (1.77–2.11) |
| Betula dahurica | 2.28 (1.73–3.04) b | 5.05 (4.30–5.90) a | 3.09 (2.60–3.67) b | 4.32 (3.58–5.07) a | 3.72 (3.40–4.11) |
| Betula platyphylla | 0.55 (0.32–0.91) c | 2.05 (1.57–2.53) a | 0.96 (0.72–1.32) bc | 1.58 (1.18–2.06) ab | 1.30 (1.12–1.53) |
| Populus davidiana | 0.58 (0.32–0.93) b | 1.31 (0.91–1.78) ab | 1.55 (1.21–1.97) a | 0.73 (0.49–1.08) b | 1.11 (0.93–1.32) |
| Quercus mongolica | 10.32 (8.54–12.25) ab | 5.49 (4.08–7.32) c | 9.36 (7.95–11.00) b | 13.55 (11.81–15.41) a | 9.72 (8.87–10.57) |
| (C) | |||||
| Species | Steep Slope | Gentle Slope | Valley | Ridge | Entire |
| Acer mono | 864.08 (750.2–1014.0) a | 583.91 (504.2–682.0) b | 793.05 (716.4–893.0) a | 612.60 (539.0–713.9) b | 710.75 (660.2–762.3) |
| Betula dahurica | 218.68 (146.6–316.0) a | 186.52 (159.1–211.4) a | 151.78 (128.0–187.5) a | 199.81 (171.6–240.7) a | 183.8 (164.6–206.8) |
| Betula platyphylla | 39.94 (23.56–67.53) b | 82.17 (61.74–109.05) a | 42.90 (32.28–58.35) b | 62.60 (47.87–79.84) ab | 56.5 (48.05–66.00) |
| Populus davidiana | 76.44 (44.32–121.55) a | 82.83 (57.83–128.69) a | 111.98 (88.2–140.8) a | 81.98 (58.18–118.79) a | 91.35 (76.55–109.25) |
| Quercus mongolica | 525.86 (443.9–632.1) a | 206.52 (151.8–277.3) c | 342.31 (292.0–409.4) b | 515.31 (448.4–597.0) a | 387.65 (349.1–422.4) |
| Biomass (Mg/ha) | Steep Slope | Gentle Slope | Valley | Ridge | Entire Plot |
|---|---|---|---|---|---|
| Elevation (m) | −85.13~0.14 (4803.3) | ||||
| Convexity (m) | 148.32~2.15 (4802.5) | ||||
| Slope (°) | 87.68~2.6 (1108.2) | 115.73~1.10 (4788.4) | |||
| aspect (°) | 161.46~15.77 (833.7) | ||||
| Quadratic mean diameter (cm) | −0.22~5.05 (821.7) | 126.53~6.0 (1097.3) | 143.04~6.0 (1578.8) | 138.42~4.93 (1226.2) | 97.44~6.67 (4699.9) |
| Basal area (m2/ha) | −3.61~4.18 (698.8) | 14.51~4.34 (940.5) | 31.85~4.42 (1345.5) | 19.65~4.31 (1037.7) | 34.69~4.41 (3909.3) |
| Tree density (trees/ha) | 140.51~0.001 (4806.5) | ||||
| Species richness (number/400 m2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Qi, G.; Knapp, B.O. Topography Affects Tree Species Distribution and Biomass Variation in a Warm Temperate, Secondary Forest. Forests 2019, 10, 895. https://doi.org/10.3390/f10100895
Wang S, Qi G, Knapp BO. Topography Affects Tree Species Distribution and Biomass Variation in a Warm Temperate, Secondary Forest. Forests. 2019; 10(10):895. https://doi.org/10.3390/f10100895
Chicago/Turabian StyleWang, Shunzhong, Guang Qi, and Benjamin O. Knapp. 2019. "Topography Affects Tree Species Distribution and Biomass Variation in a Warm Temperate, Secondary Forest" Forests 10, no. 10: 895. https://doi.org/10.3390/f10100895




