Branch Occlusion and Discoloration under the Natural Pruning of Mytilaria laosensis
Abstract
:1. Introduction
2. Material and Methods
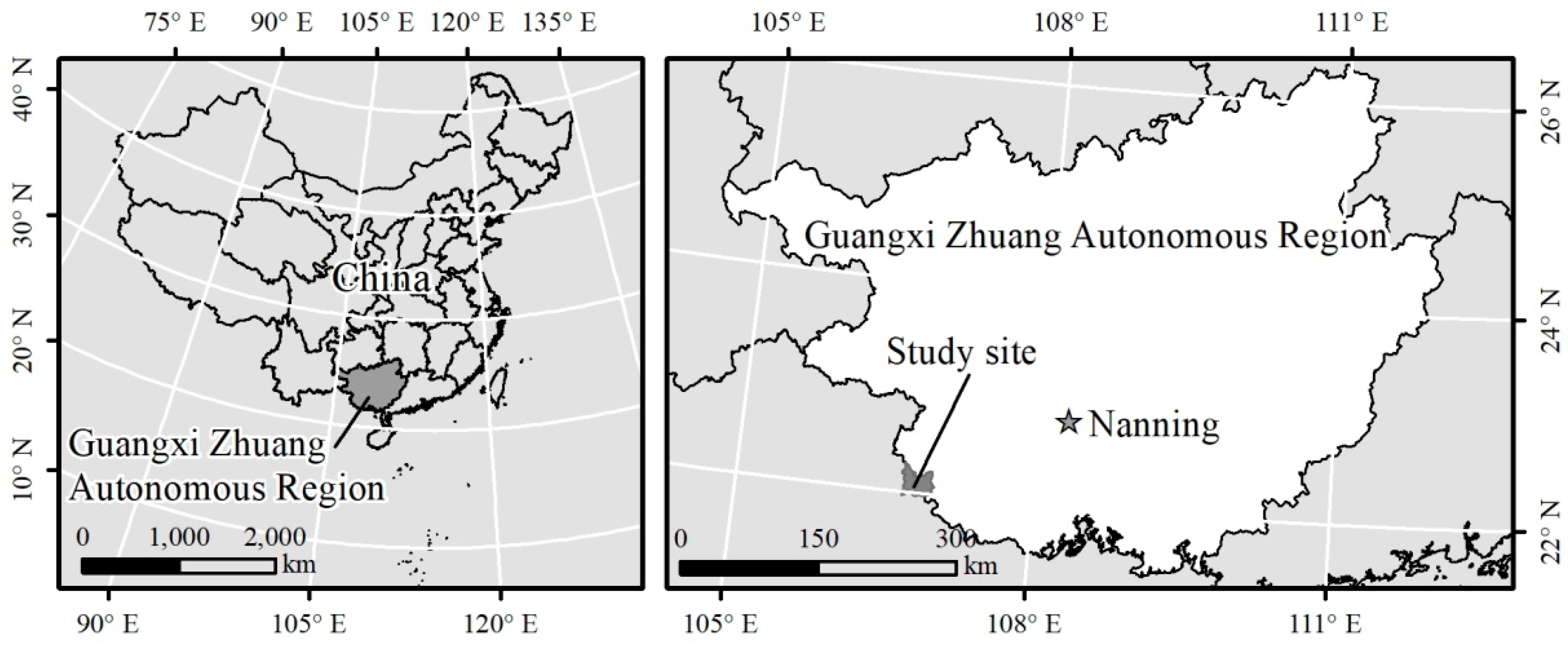
2.1. Experimental Site
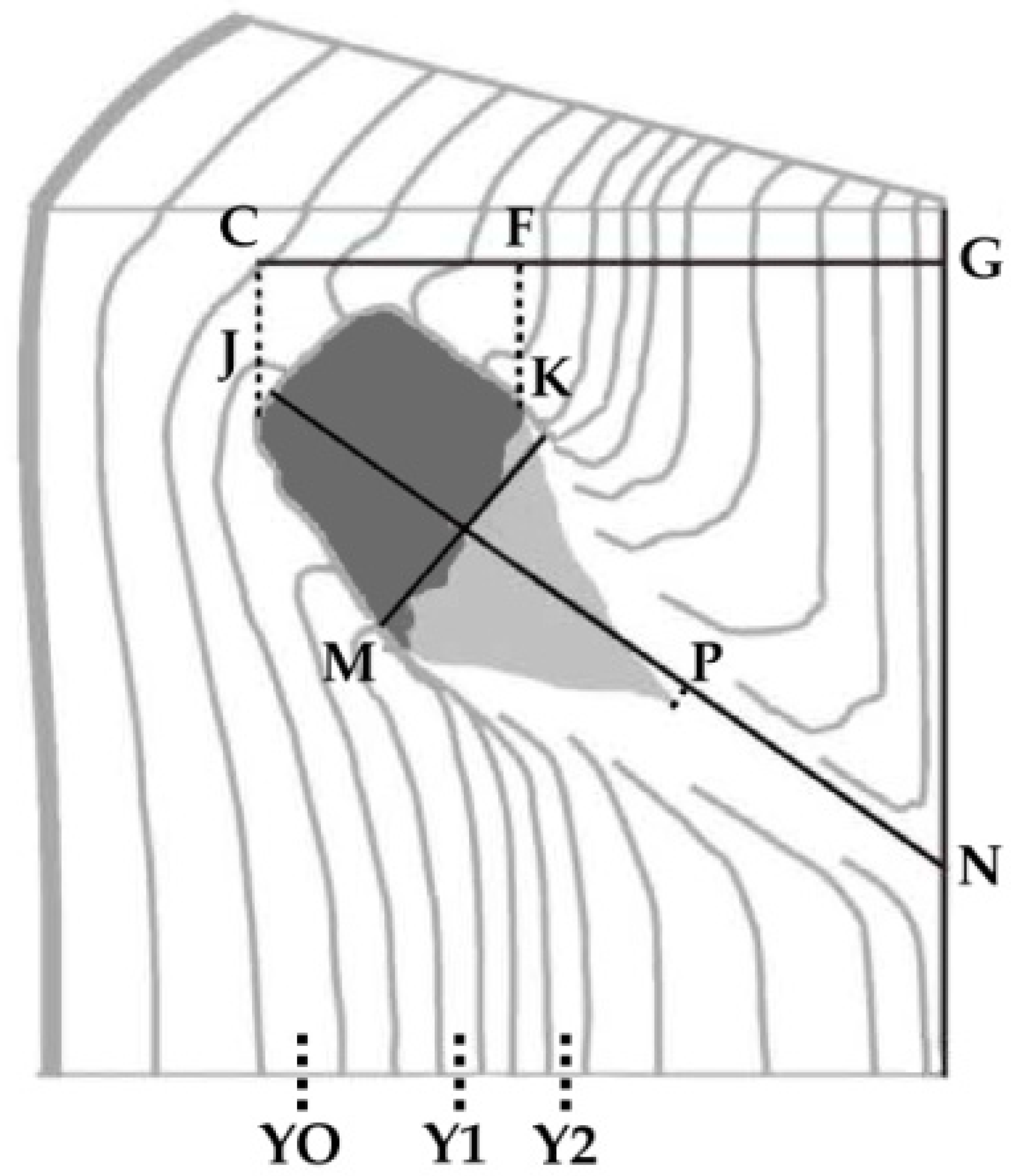
2.2. Measurements
2.3. Statistical Analysis
3. Results
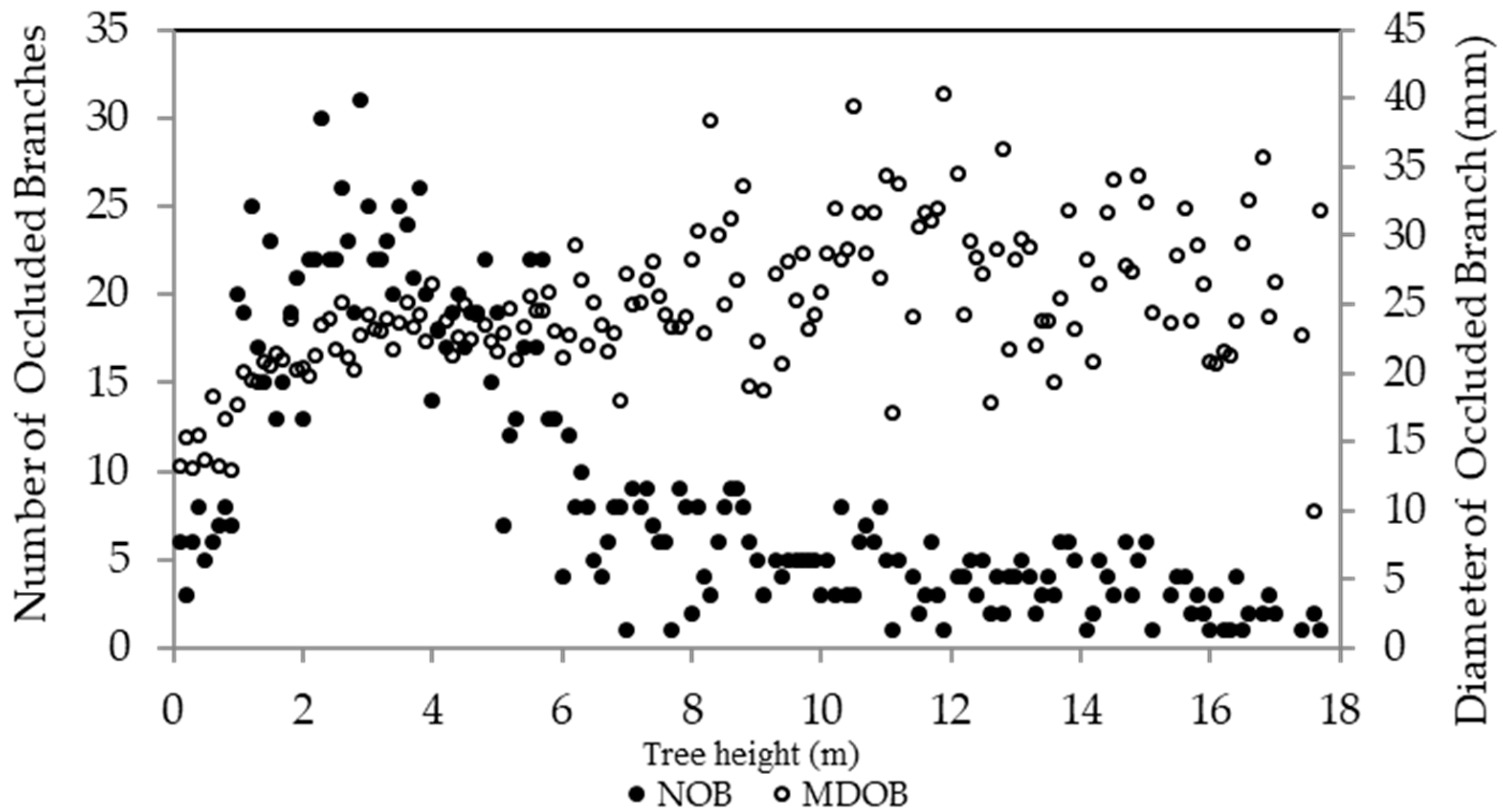
3.1. Spatial Distribution of Branch Occlusions
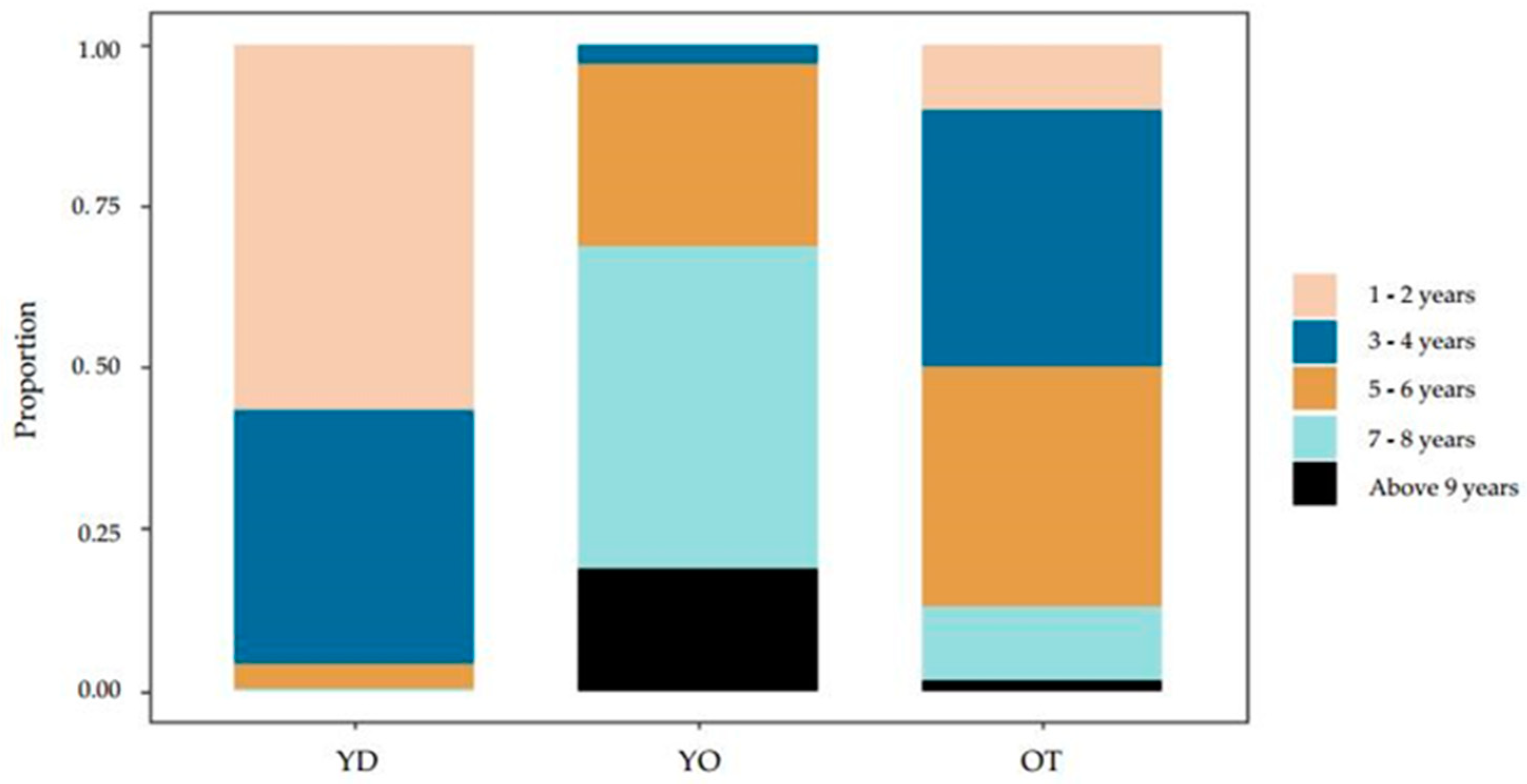
3.2. Temporal Distribution of Branch Occlusion
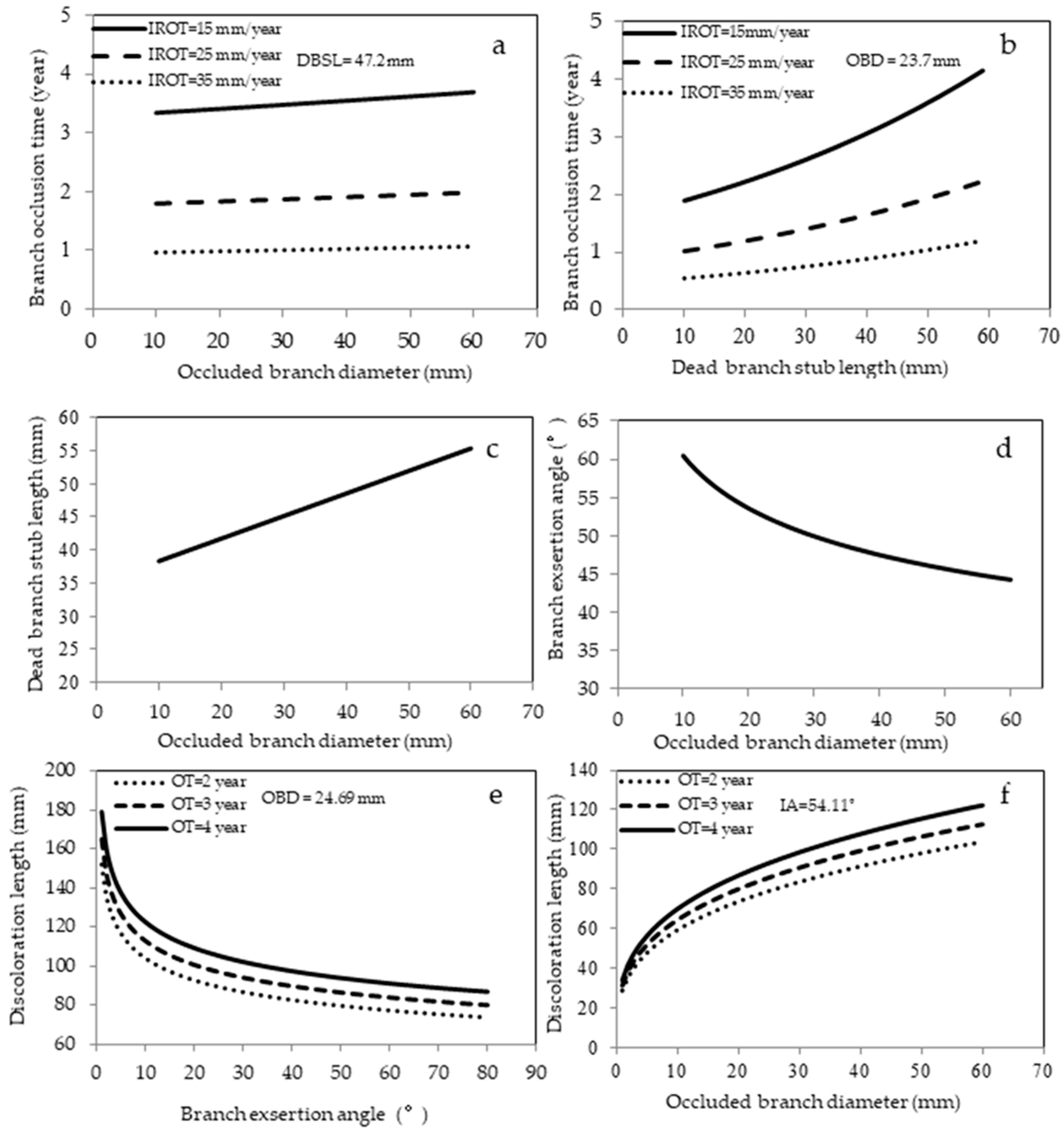
3.3. Models for Branch Occlusion
3.3.1. Branch Occlusion Time
3.3.2. Dead Branch Stub Length
3.3.3. Branch Exsertion Angle
3.3.4. Branch Discoloration Length
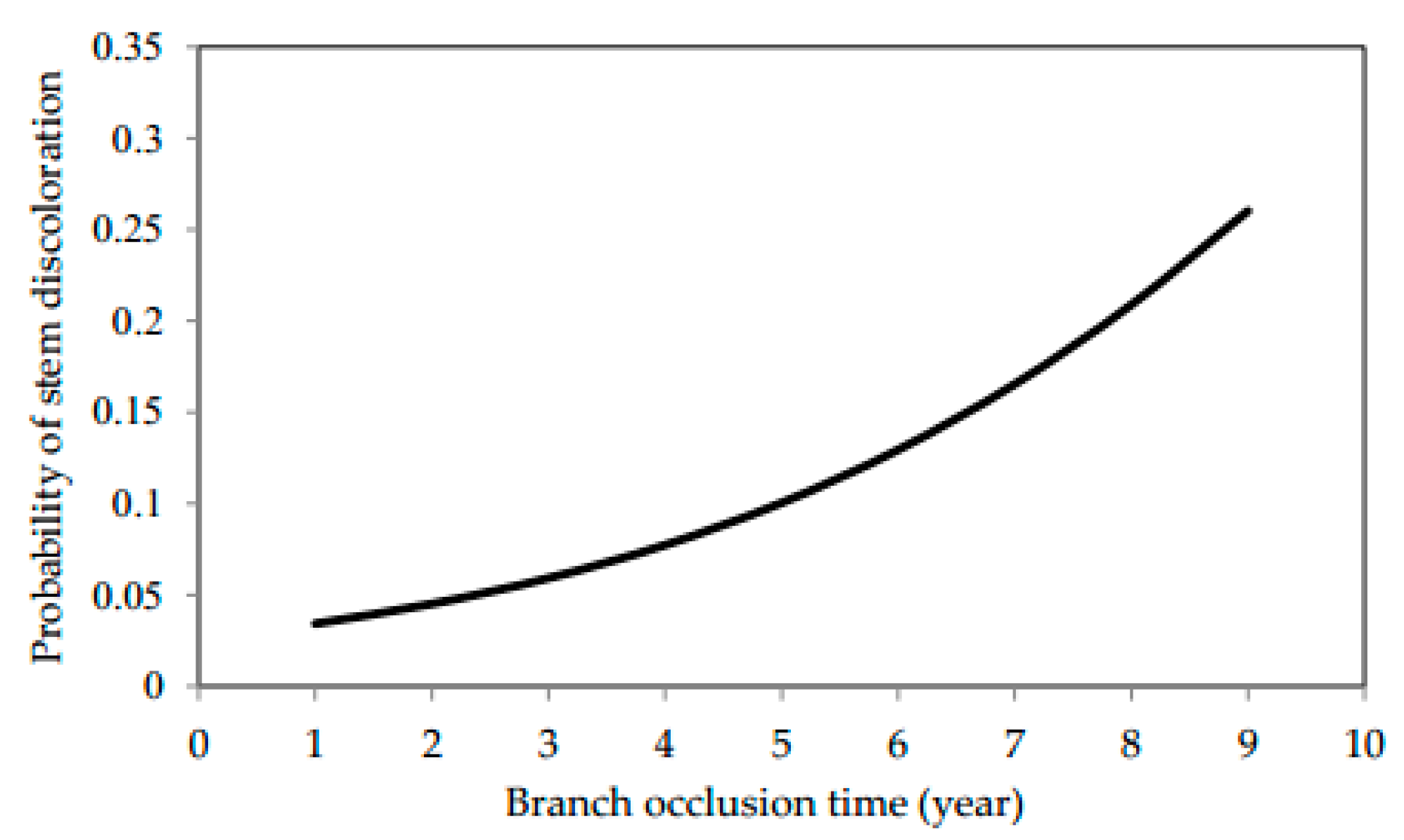
3.4. Wood Discoloration
4. Discussion
4.1. Branch Distribution
4.2. Branch Occlusion Time
4.3. Branch Exsertion Angle
4.4. Discoloration
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hein, S. Knot attributes and occlusion of naturally pruned branches of Fagus sylvatica. For. Ecol. Manag. 2008, 256, 2046–2057. [Google Scholar] [CrossRef]
- Montagu, K.D.; Kearney, D.E.; Smith, R.G.B. The biology and silviculture of pruning planted eucalypts for clear wood production—A review. For. Ecol. Manag. 2003, 179, 1–13. [Google Scholar] [CrossRef]
- Maguire, D.A.; Kershaw, J.R.; Hann, D.W. Predicting the effects of silvicultural regime on branch size and crown wood core in Douglas-fir. For. Sci. 1991, 37, 1409–1428. [Google Scholar]
- Roeh, R.L.; Maguire, D.A. Crown profile models based on branch attributes in coastal Douglas-fir. For. Ecol. Manag. 1997, 96, 77–100. [Google Scholar] [CrossRef]
- Karaszewski, Z.; Bembenek, M.; Mederski, P.S.; Szczepanska-Alvarez, A.; Byczkowski, R.; Kozlowska, A.; Michnowicz, K.; Przytula, W.; Giefing, D.F. Identifying beech round wood quality-distribution of beech timber qualities and influencing defects. Drewno. Pr. Nauk. Donies. Komunik. 2013, 56, 39–54. [Google Scholar]
- Macdonald, E.; Hubert, J. A review of the effects of silviculture on timber quality of Sitka spruce. Forestry 2002, 75, 107–138. [Google Scholar] [CrossRef]
- Shigo, A.L. Compartmentalization: A conceptual framework for understanding how trees grow and defend themselves. Annu. Rev. Phytopathol. 1984, 22, 189–214. [Google Scholar] [CrossRef]
- O’Hara, K.L. Pruning wounds and occlusion: A long-standing conundrum in forestry. J. For. 2007, 105, 131–138. [Google Scholar]
- Kicki, J. Effects of initial spacing on the stem and branch properties and graded quality of Picea abies (L.) karst. Scand. J. For. Res. 1992, 7, 12. [Google Scholar]
- Mäkinen, H.; Song, T. Evaluation of models for branch characteristics of Scots pine in Finland. For. Ecol. Manag. 2002, 158, 25–39. [Google Scholar] [CrossRef]
- Beaulieu, E.; Schneider, R.; Berninger, F.; Ung, C.H.; Swift, D.E. Modeling jack pine branch characteristics in Eastern Canada. For. Ecol. Manag. 2011, 262, 1748–1757. [Google Scholar] [CrossRef]
- Hein, S.; Mäkinen, H.; Yue, C.; Kohnle, U. Modelling branch characteristics of Norway spruce from wide spacing in Germany. For. Ecol. Manag. 2007, 242, 155–164. [Google Scholar] [CrossRef]
- Smith, R.G.B.; Dingle, J.; Kearney, D.; Montagu, K. Branch occlusion after pruning in four contrasting sub-tropical eucalypt species. J. Trop. For. Sci. 2006, 18, 117–123. [Google Scholar]
- Wang, C.S.; Hein, S.; Zhao, Z.G.; Guo, J.J.; Zeng, J. Branch occlusion and discoloration of Betula alnoides under artificial and natural pruning. For. Ecol. Manag. 2016, 375, 200–210. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, J.; Jia, H.Y.; Zeng, J.; Guo, W.F.; Cai, D.X. Growth and nutrient uptake dynamics of Mytilaria laosensis seedlings under exponential and conventional fertilizations. Soil Sci. Plant Nutr. 2012, 58, 618–626. [Google Scholar] [CrossRef]
- Guo, W.F.; Cai, D.X.; Jia, H.Y.; LI, Y.X.; Lu, Z.F. Growth laws of Mytilaria laosensis plantation. For. Res. 2006, 19, 585. [Google Scholar]
- Huang, Z.T.; Wang, S.F.; Jiang, Y.M.; Mo, J.X. Exploitation and utilization prospects of eximious native tree species Mytilaria laosensis. J. Guangxi Agric. Sci. 2009, 40, 1220–1223. [Google Scholar]
- Wang, Z.H.; Yin, G.T.; Yang, J.C.; Qin, G.M. Effects of different density on branch development of Mytilaria laosensis. For. Res. 2019, 32, 78–86. [Google Scholar]
- Dănescu, A.; Ehring, A.; Bauhus, J.; Albrecht, A.; Hein, S. Modelling discoloration and duration of branch occlusion following green pruning in Acer pseudoplatanus and Fraxinus excelsior. For. Ecol. Manag. 2015, 335, 87–98. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D. Mixed-Effects Models in S and S-PLUS; Springer Science and Business Media: Berlin, Germany, 2006. [Google Scholar]
- Stewart, G.W. Collinearity and least squares regression. Stat. Sci. 1987, 2, 68–84. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Pepe, M.S. Receiver operating characteristic methodology. J. Am. Stat. Assoc. 2000, 95, 308–311. [Google Scholar] [CrossRef]
- Zhang, S.; Chauret, G.; Duchesne, I.; Schnelder, R. Maximizing the Value of Jack Pine Resource; Technical Report; Forintek Canada Corp: Quebec, QC, Canada, 2005. [Google Scholar]
- Wang, C.; Zhao, Z.; Hein, S.; Zeng, J.; Schuler, J.; Guo, J.J.; Zeng, J. Effect of planting density on knot attributes and branch occlusion of Betula alnoides under natural pruning in southern China. Forests 2015, 6, 1343–1361. [Google Scholar] [CrossRef]
- Moberg, L. Models of internal knot diameter for Pinus sylvestris. Scand. J. For. Res. 2000, 15, 177–187. [Google Scholar] [CrossRef]
- Moberg, L. Models of internal knot properties for Picea abies. For. Ecol. Manag. 2001, 147, 123–138. [Google Scholar] [CrossRef]
- Trincado, G.; Burkhart, H.E. A framework for modeling the dynamics of first-order branches and spatial distribution of knots in Loblolly pine trees. Can. J. For. Res. 2009, 39, 566–579. [Google Scholar] [CrossRef]
- Mäkinen, H. Effect of intertree competition on branch characteristics of Pinus sylvestris families. Scand. J. For. Res. 1996, 11, 129–136. [Google Scholar] [CrossRef]
- Sandi, M.; Sandi, W.; Nicolescu, V.N. Wood discoloration in relation to wound size in northern red oak (Quercus rubra L.) trees subject to artificial pruning. Span. J. Rural Dev. 2012, 3, 53–60. [Google Scholar] [CrossRef]
- Maurin, V.; DesRochers, A. Physiological and growth responses to pruning season and intensity of hybrid poplar. For. Ecol. Manag. 2013, 304, 399–406. [Google Scholar] [CrossRef]
- Hevia, A.; Álvarez-González, J.G.; Majada, J. Comparison of pruning effects on tree growth, productivity and dominance of two major timber conifer species. For. Ecol. Manag. 2016, 374, 82–92. [Google Scholar] [CrossRef]
- Hein, S.; Spiecker, H. Comparative analysis of occluded branch characteristics for Fraxinus excelsior and Acer pseudoplatanus with natural and artificial pruning. Can. J. For. Res. 2007, 37, 1414–1426. [Google Scholar] [CrossRef]
- Nicolescu, V.N.; Sandi, M.; Păun, M. Occlusion of pruning wounds on northern red oak (Quercus rubra) trees in Romania. Scand. J. For. Res. 2013, 28, 340–345. [Google Scholar] [CrossRef]
- O’Hara, K.L.; Buckland, P.A. Prediction of pruning wound occlusion and defect core size in ponderosa pine. West. J. Appl. For. 1996, 11, 40–43. [Google Scholar] [CrossRef]
- Petruncio, M.; Briggs, D.; Barbour, R.J. Predicting pruned branch stub occlusion in young, coastal Douglas-fir. Can. J. For. Res. 1997, 27, 1074–1082. [Google Scholar] [CrossRef]
- Forrester, D.I.; Baker, T.G. Growth responses to thinning and pruning in Eucalyptus globulus, Eucalyptus nitens, and Eucalyptus grandis plantations in southeastern Australia. Can. J. For. Res. 2011, 42, 75–87. [Google Scholar] [CrossRef]
- Forrester, D.I.; Collopy, J.J.; Beadle, C.L.; Warren, C.R.; Baker, T.G. Effect of thinning, pruning and nitrogen fertiliser application on transpiration, photosynthesis and water-use efficiency in a young Eucalyptus nitens plantation. For. Ecol. Manag. 2012, 266, 286–300. [Google Scholar] [CrossRef]
- Hein, S.; Collet, C.; Ammer, C.; Goff, N.L.; Skovsgaard, J.P.; Savill, P. A review of growth and stand dynamics of Acer pseudoplatanus L. in Europe: Implications for silviculture. Forestry 2008, 82, 361–385. [Google Scholar] [CrossRef]
- Day, M.E.; Greenwood, M.S.; Diaz-Sala, C. Age-and size-related trends in woody plant shoot development: Regulatory pathways and evidence for genetic control. Tree Physiol. 2002, 22, 507–513. [Google Scholar] [CrossRef]
- Schwarze, F.; Engels, J.; Mattheck, C. Holzzersetzende Pilze in Bäumen. Strategien der Holzzersetzung Rombach Ökologie 1999, 5. [Google Scholar]
- Wardlaw, T.J.; Neilsen, W.A. Decay and other defects associated with pruned branches of Eucalyptus nitens. Tasforests Hobartt 1999, 11, 49–58. [Google Scholar]
- Beadle, C.; Barry, K.; Hardiyanto, E.; Irianto, R.; Mohammed, C.; Rimbawanto, A. Effect of pruning Acacia mangium on growth, form and heart rot. For. Ecol. Manag. 2007, 238, 261–267. [Google Scholar] [CrossRef]
- Barry, K.M.; Pearce, R.B.; Mohammed, C.M. Properties of reaction zones associated with decay from pruning wounds in plantation grown Eucalyptus nitens. For. Pathol. 2000, 30, 233–245. [Google Scholar] [CrossRef]
- Lonsdale, D.; Pautasso, M.; Holdenrieder, O. Wood-decaying fungi in the forest: Conservation needs and management options. Eur. J. For. Res. 2008, 127, 1–22. [Google Scholar] [CrossRef]
- Barry, K.M.; Irianto, R.S.B.; Tjahjono, B.; Tarigan, M.; Agustini, L.; Hardiyanto, E.B.; Mohammed, C.L. Variation of heart rot, sap wood infection and polyphenol extractives with provenance of Acacia mangium. For. Pathol. 2006, 36, 183–197. [Google Scholar] [CrossRef]

| Variables | Definition | Unit |
|---|---|---|
| Tree-level variables | ||
| H | Tree total height | (m) |
| DBH | Diameter at breast height | (cm) |
| HCB | Height of the crown base | (m) |
| CD | Diameter of the crown | (m) |
| Branch-level variables | ||
| EA | Branch exsertion angle | (°) |
| BH | Branch height on stem | (m) |
| YD | Year of branch death | (year) |
| YO | Year of branch complete occlusion | (year) |
| OT | Branch occlusion time | (year) |
| IROT | Mean stem radial increment during | (mm/year) |
| the branch occlusion | ||
| OB | Occluded branch | |
| OBD CT | Occluded branch diameter Cork tissue (0 = absent, 1 = present) | (mm) |
| DBSL | Dead branch stub length | (mm) |
| DL | Discoloration length | (mm) |
| DSW | Discoloration in stem wood | |
| (0 = absent, 1 = present) | ||
| Model descriptors | ||
| a, b, c | Coefficient of fixed effect | |
| t, tb | Subscripts for tree, branch | |
| α, β, γ | Coefficient of random effect | |
| Ln ( ) | Natural log-link | |
| RMSE | Root mean squared error | |
| R2(c). R2(m) | Conditional and marginal R2 |
| Variables | Unit | Minimum–Maximum | Mean |
|---|---|---|---|
| Tree total height | m | 16.4–22.8 | 19.3 |
| Diameter at breast height | cm | 17.2–25.3 | 21.1 |
| Height of the live crown base | m | 4.2–8.8 | 6.8 |
| Diameter of the crown | m | 6.5–10.4 | 8.6 |
| Variables (Abbreviation) | Unit | Minimum–Maximum | Mean |
|---|---|---|---|
| Branch exsertion angle (EA) | (°) | 3.9–98.5 | 54.1 |
| Branch height on the stem (BH) | (m) | 0.1–18.8 | 9.5 |
| Year of branch death (YD) | (year) | 2–8 | 2.6 |
| Branch occlusion time (OT) | (year) | 1–10 | 4.6 |
| Mean stem radial increment during | (mm/year) | 1.3–55 | 11.1 |
| branch occlusion (IROT) | |||
| Occluded branch diameter (OBD) | (mm) | 4.7–59.1 | 23.7 |
| Dead branch stub length (DBSL) | (mm) | 6.1–148.5 | 47.2 |
| Discoloration length (DL) | (mm) | 17.2–193.3 | 100.3 |
| Discoloration in stem wood (DSW) | 0–1 | 0.09 |
| Equation | Response Variable | Parameters | Predictor Variable | Estimate | SE | R2(m) | R2(c) |
|---|---|---|---|---|---|---|---|
| Equation (1) | Branch occlusion time (OT) * | a0 | Intercept | 1.361 | 0.037 | 56.29% | - |
| a1 | OBD | 0.022 | 0.003 | ||||
| a2 | DBSL | 0.016 | 0.001 | ||||
| a3 | IROT | −0.062 | 0.004 | ||||
| Equation (2) | Dead branch stub length (DBSL) * | a0 | Intercept | 2.935 | 0.133 | 9.56% | - |
| a1 | OBD * | 0.226 | 0.039 | ||||
| Equation (3) | Branch exsertion angle (EA) * | a0 | Intercept | 4.49 | 0.052 | 12.51% | 16.94% |
| a1 | OBD * | −0.174 | 0.015 | ||||
| γpt | 0.013 | 0.005 | |||||
| Equation (4) | Branch discoloration length (DL) * | a0 | Intercept | 3.846 | 0.129 | 17.64% | 26.17% |
| a1 | OT | 0.082 | 0.003 | ||||
| a2 | OBD * | 0.312 | 0.016 | ||||
| a3 | EA * | −0.165 | 0.026 | ||||
| δpt | 0.013 | 0.004 | |||||
| Equation (5) | Probability of wood discoloration (DSW) | a0 | Intercept | −3.645 | 0.282 | 57.18% | 81.54% |
| a1 | OT | 0.282 | 0.051 | ||||
| εptb | 0.012 | 0.004 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, G.; Hao, J.; Yang, J.; Li, R.; Yin, G. Branch Occlusion and Discoloration under the Natural Pruning of Mytilaria laosensis. Forests 2019, 10, 892. https://doi.org/10.3390/f10100892
Qin G, Hao J, Yang J, Li R, Yin G. Branch Occlusion and Discoloration under the Natural Pruning of Mytilaria laosensis. Forests. 2019; 10(10):892. https://doi.org/10.3390/f10100892
Chicago/Turabian StyleQin, Guoming, Jian Hao, Jinchang Yang, Rongsheng Li, and Guangtian Yin. 2019. "Branch Occlusion and Discoloration under the Natural Pruning of Mytilaria laosensis" Forests 10, no. 10: 892. https://doi.org/10.3390/f10100892




