Intensive Management Increases Phytolith-Occluded Carbon Sequestration in Moso Bamboo Plantations in Subtropical China
Abstract
:1. Introduction
2. Materials and Methods
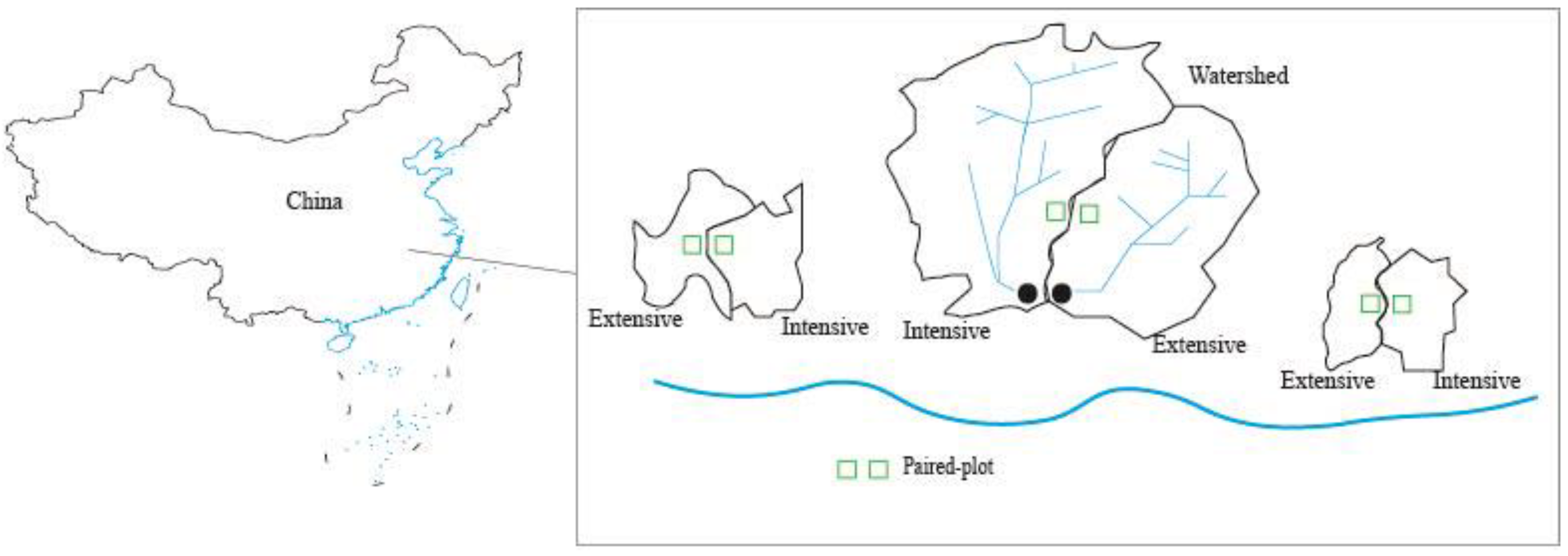
2.1. Study Site
2.2. Bamboo Forest Management Practices
2.3. Plant and Soil Sampling and Preparation
2.4. Plant and Soil Analysis
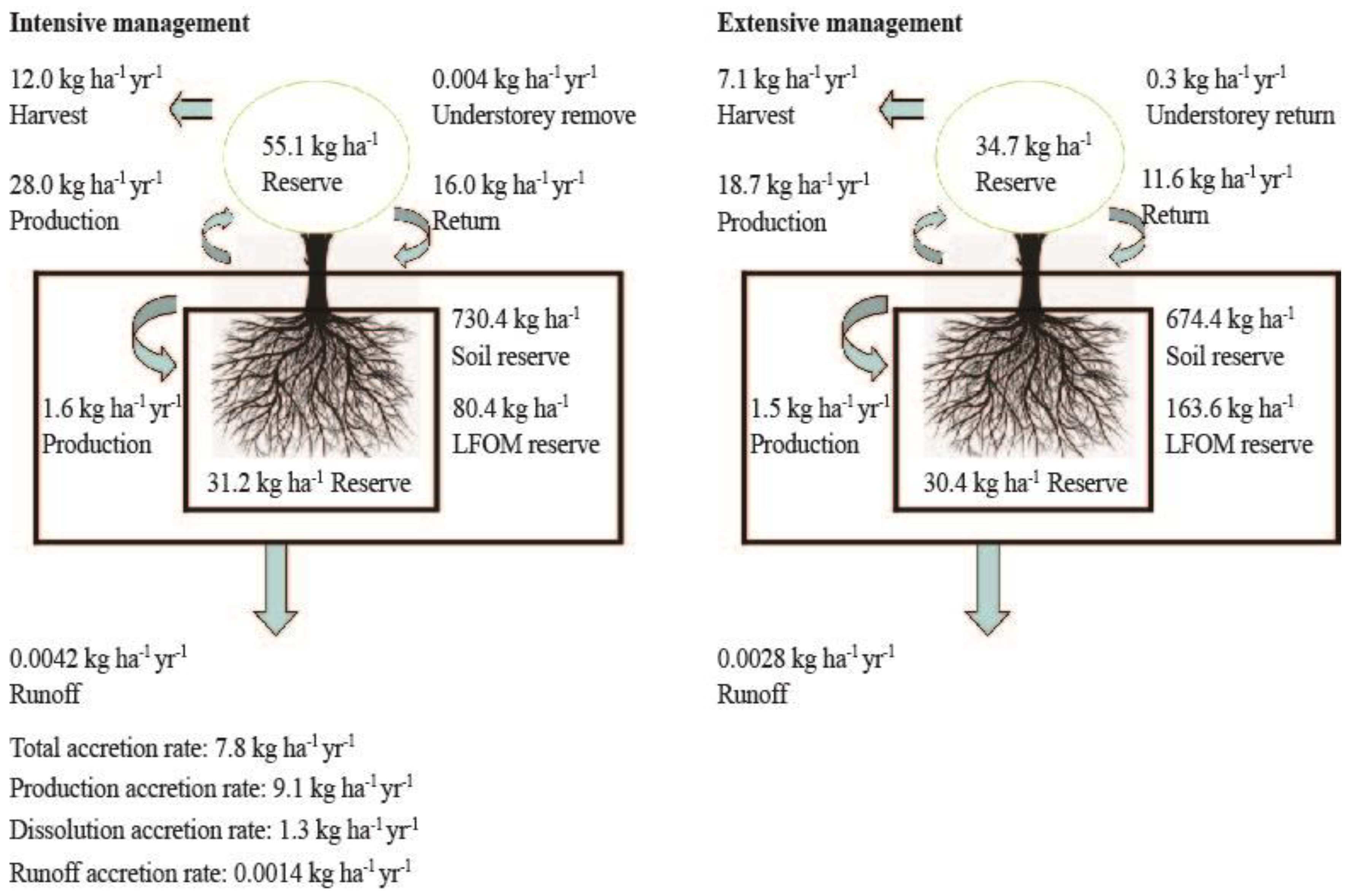
2.5. Calculations and Statistical Analysis
3. Results
3.1. Effects of Plantation Management Intensity on Soil Chemical Properties
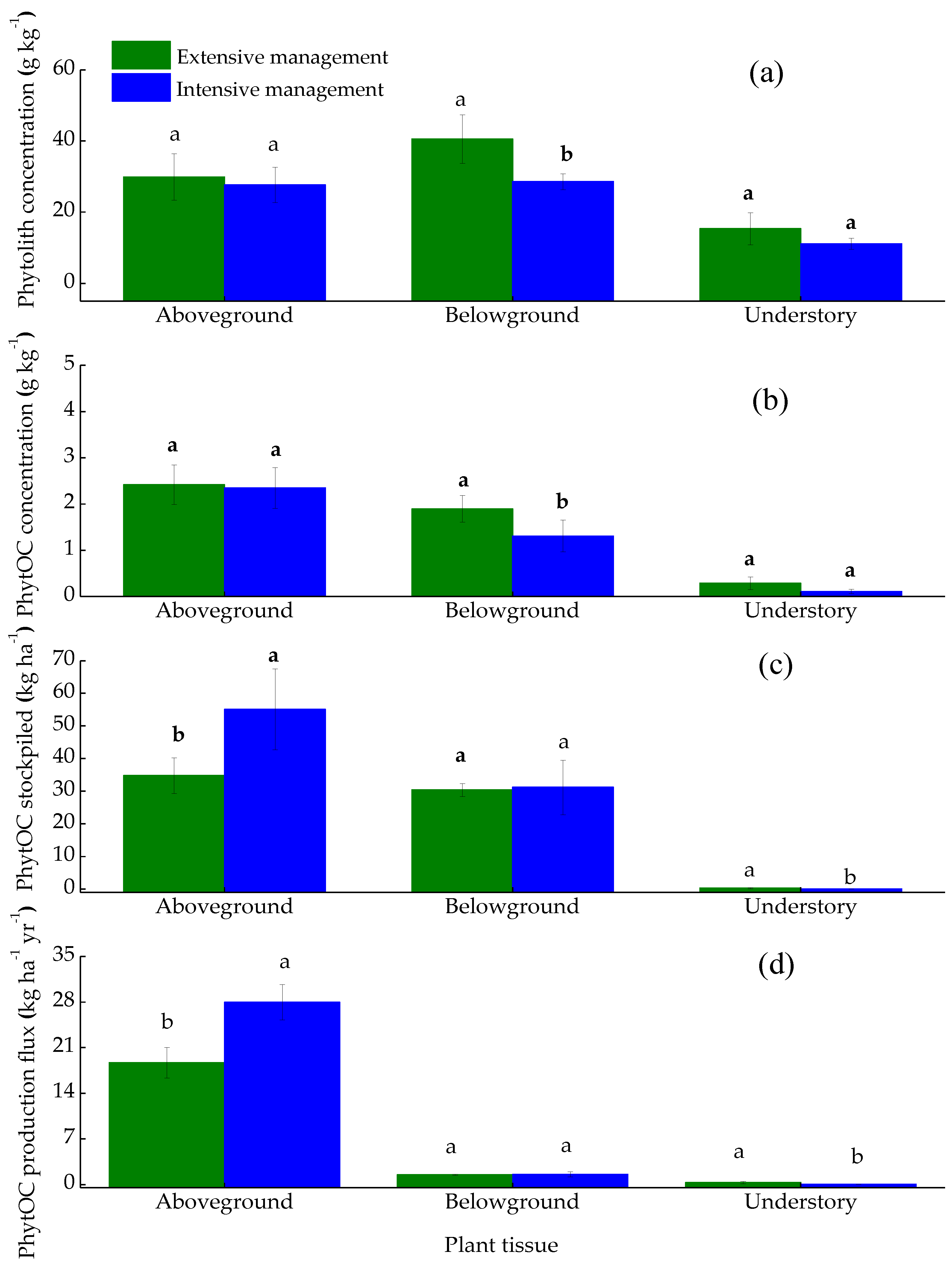
3.2. Effects of Plantation Management Intensity on Plant PhytOC Production
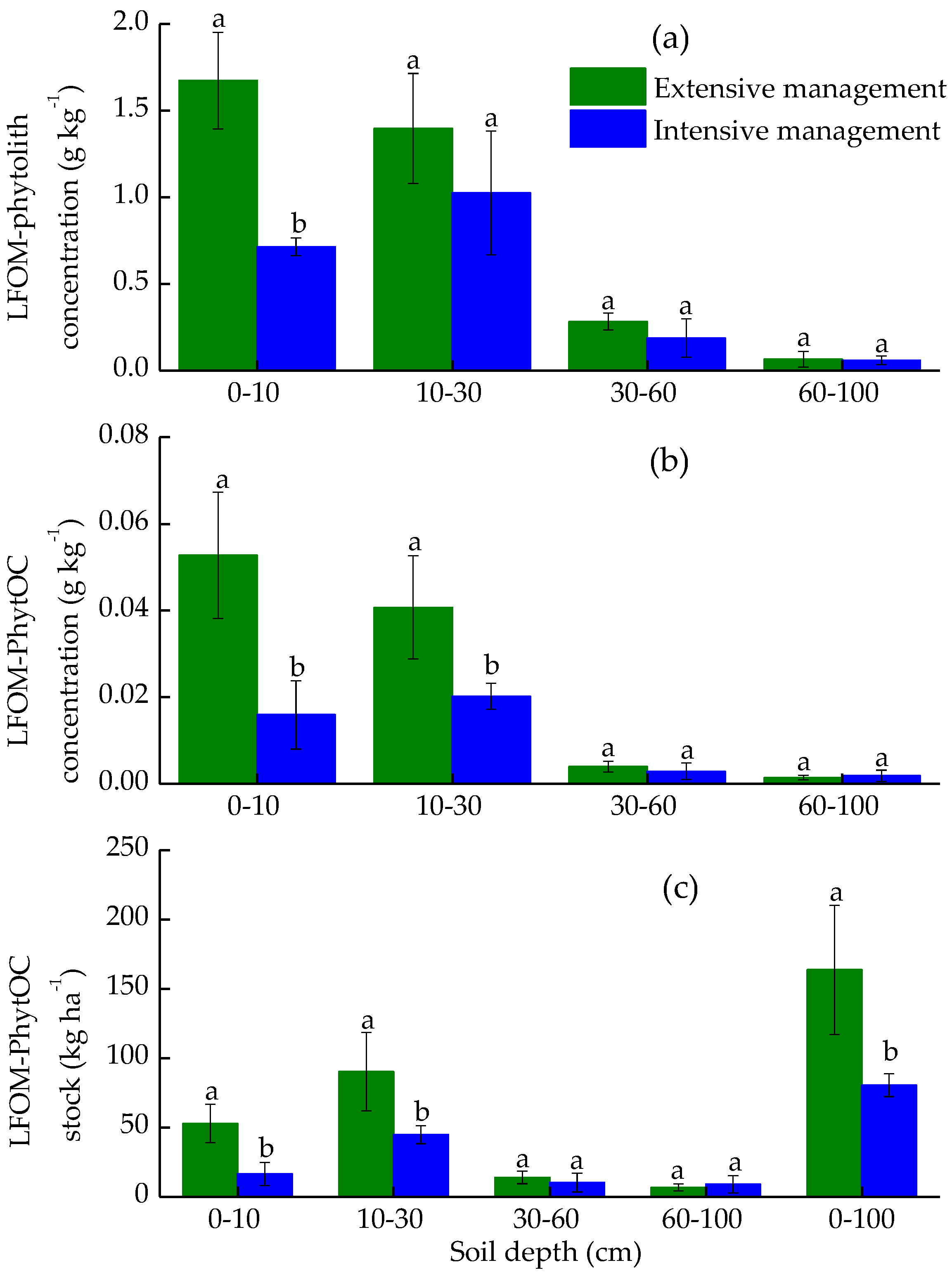
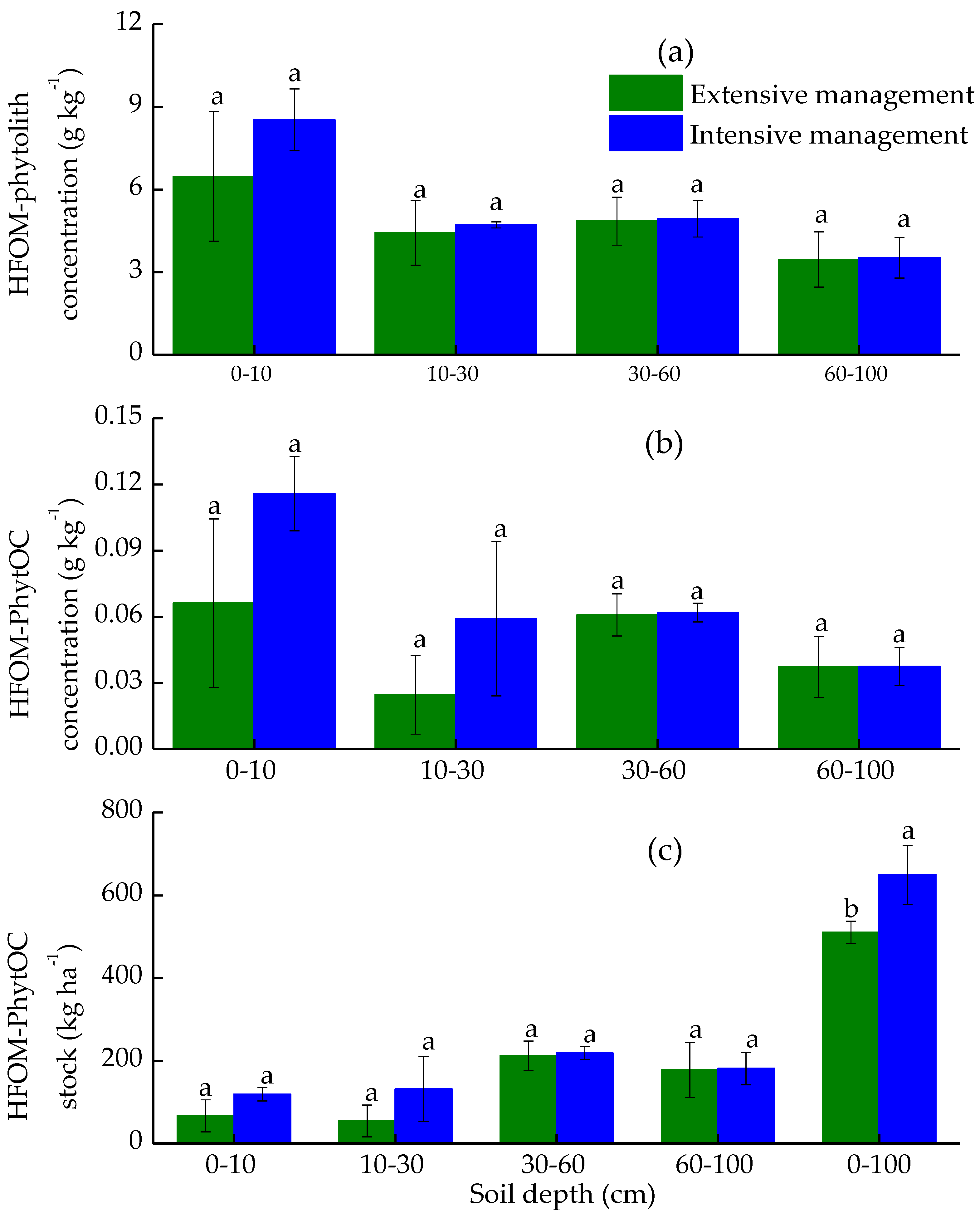
3.3. Effects of Plantation Management Intensity on Soil PhytOC Accumulation and Its Distribution in Physical Fractions
3.4. Effect of Plantation Management Intensity on Soil PhytOC Dissolution and Loss in Runoff
4. Discussion
4.1. Plantation Management Intensity Affected Plant PhytOC Production
4.2. Plantation Management Intensity Affected Soil PhytOC Sequestration
4.3. Management Intensity Effects on Soil PhytOC Dissolution and Loss Through Runoff
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parr, J.F.; Dolic, V.; Lancaster, G.; Boyd, W.E. A microwave digestion method for the extraction of phytoliths from herbarium specimens. Rev. Palaeobot. Palynol. 2001, 116, 203–212. [Google Scholar] [CrossRef]
- Piperno, D.R. Quaternary Environmental History and Agricultural Impact on Vegetation in Central America. Ann. Mo. Bot. Gard. 2006, 93, 274–296. [Google Scholar] [CrossRef]
- Li, Z.; Song, Z.; Parr, J.F.; Wang, H. Occluded C in rice phytoliths: Implications to biogeochemical carbon sequestration. Plant Soil 2013, 370, 615–623. [Google Scholar] [CrossRef]
- Parr, J.; Sullivan, L.; Chen, B.; Ye, G.; Zheng, W. Carbon bio-sequestration within the phytoliths of economic bamboo species. Glob. Chang. Biol. 2010, 16, 2661–2667. [Google Scholar] [CrossRef]
- Blecker, S.W.; McCulley, R.L.; Chadwick, O.A.; Kelly, E.F. Biologic cycling of silica across a grassland bioclimosequence. Glob. Biogeochem. Cycles 2006, 20. [Google Scholar] [CrossRef]
- Parr, J.; Sullivan, L.; Quirk, R. Sugarcane phytoliths: Encapsulation and sequestration of a long-lived carbon fraction. Sugar Tech. 2009, 11, 17–21. [Google Scholar] [CrossRef]
- Parr, J.F.; Sullivan, L.A. Soil carbon sequestration in phytoliths. Soil. Biol. Biochem. 2005, 37, 117–124. [Google Scholar] [CrossRef]
- Drees, L.R. Silica in soils: Quartz and disordered silica polymorphs. In Minerals in Soil Environments; The Alliance of Crop, Soil, and Environmental Science Societies: Madison, WI, USA, 1989. [Google Scholar]
- Song, Z.; Liu, H.; Li, B.; Yang, X. The production of phytolith-occluded carbon in China’s forests: Implications to biogeochemical carbon sequestration. Glob. Chang. Biol. 2013, 19, 2907–2915. [Google Scholar] [CrossRef]
- Song, Z.; Liu, H.; Strömberg, C.A.; Yang, X.; Zhang, X. Phytolith carbon sequestration in global terrestrial biomes. Sci. Total Environ. 2017, 603, 502–509. [Google Scholar] [CrossRef]
- Xiang, T.; Ying, Y.; Teng, J.; Huang, Z.; Wu, J.; Meng, C.; Jiang, P.; Tang, C.; Li, J.; Zheng, R. Sympodial bamboo species differ in carbon bio-sequestration and stocks within phytoliths of leaf litters and living leaves. Environ. Sci. Pollut. Res. 2016, 23, 19257–19265. [Google Scholar] [CrossRef]
- Yang, J.; Wu, J.; Jiang, P.; Xu, Q.; Zhao, P.; He, S. A Study of Phytolith-occluded Carbon Stock in Monopodial Bamboo in China. Sci. Rep. 2015, 5, 13292. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Xiang, T.; Li, Y.; Wu, J.; Jiang, P. Estimation of sequestration potential via phytolith carbon by important forest species in subtropical China. J. Nat. Resour. 2015, 30, 133–140. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Huang, Z.T.; Li, Y.F.; Jiang, P.K.; Chang, S.X.; Song, Z.L.; Liu, J.; Zhou, G.M. Long-term intensive management increased carbon occluded in phytolith (PhytOC) in bamboo forest soils. Sci. Rep. 2014, 4, 3602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, Z.; Xu, X.; Liu, H.; Wu, X.; Li, Z.; Guo, F.; Pan, W. Nitrogen application increases phytolith carbon sequestration in degraded grasslands of North China. Ecol. Res. 2016, 31, 117–123. [Google Scholar] [CrossRef]
- Fishkis, O.; Ingwersen, J.; Streck, T. Phytolith transport in sandy sediment: Experiments and modeling. Geoderma 2009, 151, 168–178. [Google Scholar] [CrossRef]
- Song, Z.; Wang, H.; Strong, P.J.; Guo, F. Phytolith carbon sequestration in China’s croplands. Eur. J. Agron. 2014, 53, 10–15. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.; Wang, J. The effect of chemical fertilizer on soil organic carbon renewal and CO2 emission—A pot experiment with maize. Plant Soil 2012, 353, 85–94. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; Jiang, H.; Yu, S.; Fu, J.; Li, W.; Wang, W.; Ma, Z.; Peng, C. Carbon sequestration by Chinese bamboo forests and their ecological benefits: Assessment of potential, problems, and future challenges. Environ. Rev. 2011, 19, 418–428. [Google Scholar] [CrossRef]
- Zhou, G.; Meng, C.; Jiang, P.; Xu, Q. Review of carbon fixation in bamboo forests in China. Bot. Rev. 2011, 77, 262. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Chang, S.X.; Jiang, P.; Zhou, G.; Fu, S.; Yan, E.; Wu, J.; Lin, L. Long-term intensive management effects on soil organic carbon pools and chemical composition in Moso bamboo (Phyllostachys pubescens) forests in subtropical China. For. Ecol. Manag. 2013, 303, 121–130. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, P.; Wang, H.; Zhou, G.; Wu, J.; Yang, F.; Qian, X. Seasonal soil CO2 efflux dynamics after land use change from a natural forest to Moso bamboo plantations in subtropical China. For. Ecol. Manag. 2011, 262, 1131–1137. [Google Scholar] [CrossRef]
- Fraysse, F.; Pokrovsky, O.S.; Schott, J.; Meunier, J.D. Surface properties, solubility and dissolution kinetics of bamboo phytoliths. Geochim. Cosmochim. Acta 2006, 70, 1939–1951. [Google Scholar] [CrossRef]
- Zaccone, C.; Beneduce, L.; Lotti, C.; Martino, G.; Plaza, C. DNA occurrence in organic matter fractions isolated from amended, agricultural soils. Appl. Soil Ecol. 2018, 130, 134–142. [Google Scholar] [CrossRef]
- Von Lützow, M.; Kögel-Knabner, I.; Ekschmitt, K.; Flessa, H.; Guggenberger, G.; Matzner, E.; Marschner, B. SOM fractionation methods: Relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 2007, 39, 2183–2207. [Google Scholar] [CrossRef]
- Janzen, H. Soil organic matter characteristics after long-term cropping to various spring wheat rotations. Can. J. Soil Sci. 1987, 67, 845–856. [Google Scholar] [CrossRef]
- Janzen, H.; Campbell, C.; Brandt, S.A.; Lafond, G.; Townley-Smith, L. Light-fraction organic matter in soils from long-term crop rotations. Soil Sci. Soc. Am. J. 1992, 56, 1799–1806. [Google Scholar] [CrossRef]
- Malhi, S.; Harapiak, J.; Nyborg, M.; Gill, K.; Monreal, C.; Gregorich, E. Total and light fraction organic C in a thin Black Chernozemic grassland soil as affected by 27 annual applications of six rates of fertilizer N. Nutr. Cycl. Agroecosyst. 2003, 66, 33–41. [Google Scholar] [CrossRef]
- Wang, X.L.; Jia, Y.; Li, X.G.; Long, R.J.; Ma, Q.; Li, F.M.; Song, Y.J. Effects of land use on soil total and light fraction organic, and microbial biomass C and N in a semi-arid ecosystem of northwest China. Geoderma 2009, 153, 285–290. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Dultz, S.; Meharg, A.; Pham, Q.V.; Hoang, A.N.; Dam, T.T.N.; Nguyen, V.T.; Nguyen, K.M.; Nguyen, H.X.; Nguyen, N.T. Phytolith content in Vietnamese paddy soils in relation to soil properties. Geoderma 2019, 333, 200–213. [Google Scholar] [CrossRef]
- Zuo, X.; Lu, H.; Gu, Z. Distribution of soil phytolith-occluded carbon in the Chinese Loess Plateau and its implications for silica–carbon cycles. Plant Soil 2014, 374, 223–232. [Google Scholar] [CrossRef]
- Seta, A.; Blevins, R.; Frye, W.; Barfield, B. Reducing soil erosion and agricultural chemical losses with conservation tillage. J. Environ. Qual. 1993, 22, 661–665. [Google Scholar] [CrossRef]
- Wang, G.; Innes, J.L.; Dai, S.; He, G. Achieving sustainable rural development in Southern China: The contribution of bamboo forestry. Int. J. Sustain. Dev. World Ecol. 2008, 15, 484–495. [Google Scholar] [CrossRef]
- Li, Y.; Liang, X.; Tang, C.; Li, Y.; Chen, Z.; Chang, S.X.; Guo, Z.; Shen, Y.; Xu, Q. Moso bamboo invasion into broadleaf forests is associated with greater abundance and activity of soil autotrophic bacteria. Plant Soil 2018, 428, 163–177. [Google Scholar] [CrossRef]
- Narayanaswamy, C.; Prakash, N. Evaluation of selected extractants for plant-available silicon in rice soils of southern India. Commun. Soil Sci. Plant Anal. 2010, 41, 977–989. [Google Scholar] [CrossRef]
- Yang, Y.S.; Guo, J.F.; Chen, G.S.; Xie, J.S.; Gao, R.; Li, Z.; Jin, Z. Litter production, seasonal pattern and nutrient return in seven natural forests compared with a plantation in southern China. Forestry 2005, 78, 403–415. [Google Scholar] [CrossRef] [Green Version]
- Parr, J.F. A comparison of heavy liquid floatation and microwave digestion techniques for the extraction of fossil phytoliths from sediments. Rev. Palaeobot. Palynol. 2002, 120, 315–336. [Google Scholar] [CrossRef]
- Zuo, X.; Lu, H.; Zhang, J.; Wang, C.; Sun, G.; Zheng, Y. Radiocarbon dating of prehistoric phytoliths: A preliminary study of archaeological sites in China. Sci. Rep. 2016, 6, 26769. [Google Scholar] [CrossRef] [PubMed]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.F.; Huang, Z.T.; Jiang, P.K.; Xiang, T.T.; Ying, Y.Q. Determination of phytolith-occluded carbon content using alkali dissolution-spectrophotometry. Chin. J. Anal. Chem. 2014, 42, 1389–1390. [Google Scholar]
- Guo, F.; Song, Z.; Leigh, S.; Wang, H.; Xu, X.; Wang, D. Enhancing phytolith carbon sequestration in rice ecosystems through basalt powder amendment. Sci. Bull. 2015, 60, 591–597. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Li, F.Y.; Huang, Z.; Jiang, P.; Baoyin, T.; Wang, H. Phytolith-occluded organic carbon as a mechanism for long-term carbon sequestration in a typical steppe: The predominant role of belowground productivity. Sci. Total Environ. 2017, 577, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Lü, H. Carbon sequestration within millet phytoliths from dry-farming of crops in China. Chin. Sci. Bull. 2011, 56, 3451–3456. [Google Scholar] [CrossRef] [Green Version]
- Ngoc Nguyen, M.; Dultz, S.; Guggenberger, G. Effects of pretreatment and solution chemistry on solubility of rice-straw phytoliths. J. Plant Nutr. Soil Sci. 2014, 177, 349–359. [Google Scholar] [CrossRef]
- Cabanes, D.; Weiner, S.; Shahack-Gross, R. Stability of phytoliths in the archaeological record: A dissolution study of modern and fossil phytoliths. J. Archaeol. Sci. 2011, 38, 2480–2490. [Google Scholar] [CrossRef]
- Fraysse, F.; Pokrovsky, O.S.; Schott, J.; Meunier, J.D. Surface chemistry and reactivity of plant phytoliths in aqueous solutions. Chem. Geol. 2009, 258, 197–206. [Google Scholar] [CrossRef]
- Tan, Z.; Lal, R.; Owens, L.; Izaurralde, R. Distribution of light and heavy fractions of soil organic carbon as related to land use and tillage practice. Soil Tillage Res. 2007, 92, 53–59. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Poulton, P.R.; Mcgrath, S.P.; Meunier, J.D. Long-term removal of wheat straw decreases soil amorphous silica at Broadbalk, Rothamsted. Plant Soil 2012, 352, 173–184. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Wu, W.; Lin, H.; Fu, W.; Penttinen, P.; Li, Y.; Jin, J.; Zhao, K.; Wu, J. Soil Organic Carbon Content and Microbial Functional Diversity Were Lower in Monospecific Chinese Hickory Stands than in Natural Chinese Hickory–Broad-Leaved Mixed Forests. Forests 2019, 10, 357. [Google Scholar] [CrossRef]
- Dai, W.; Zhao, K.; Fu, W.; Jiang, P.; Li, Y.; Zhang, C.; Gielen, G.; Gong, X.; Li, Y.; Wang, H. Spatial variation of organic carbon density in topsoils of a typical subtropical forest, southeastern China. Catena 2018, 167, 181–189. [Google Scholar] [CrossRef]
- Li, Z. Current condition and dynamic changes of soil erosion in China. China Water Resour. 2009, 7, 8–11. [Google Scholar]
| Management Intensity | Soil Depth | pH | Soil Organic Carbon | Total Soil SiO2 | Water Soluble Silicon |
|---|---|---|---|---|---|
| Extensive | 0−10 cm | 5.14 ± 0.19a 1 | 23.07 ± 3.83a | 558.2 ± 14.99a | 12.65 ± 4.12a |
| Intensive | 0−10 cm | 4.36 ± 0.13b | 16.13 ± 1.82b | 565.02 ± 14.50a | 6.33 ± 1.85b |
| Extensive | 10−30 cm | 5.23 ± 0.18a | 18.09 ± 0.89a | 561.4 ± 28.54a | 13.52 ± 2.82a |
| Intensive | 10−30 cm | 4.59 ± 0.18b | 12.91 ± 2.25b | 532.11 ± 25.30a | 5.31 ± 1.82b |
| Extensive | 30−60 cm | 5.34 ± 0.29a | 12.94 ± 0.51a | 527.07 ± 50.50a | 9.15 ± 3.51a |
| Intensive | 30−60 cm | 5.05 ± 0.20a | 9.43 ± 1.13b | 507.87 ± 39.10a | 6.24 ± 0.63b |
| Extensive | 60−100 cm | 5.27 ± 0.14a | 5.91 ± 1.38a | 528.52 ± 21.87a | 5.56 ± 2.76a |
| Intensive | 60−100 cm | 5.09 ± 0.09a | 4.85 ± 1.27a | 523.84 ± 29.98a | 6.92 ± 3.52a |
| Management Intensity | Soil Depth | Phytolith Concentration (g kg−1) | PhytOC Concentration (g kg−1) | PhytOC Storage (kg ha−1) |
|---|---|---|---|---|
| Extensive | 0–10 cm | 8.79 ± 3.05a 1 | 0.118 ± 0.047a | 119.56 ± 47.21a |
| Intensive | 0–10 cm | 10.25 ± 1.35a | 0.131 ± 0.010a | 135.27 ± 8.88a |
| Extensive | 10–30 cm | 6.04 ± 0.81a | 0.065 ± 0.013a | 144.65 ± 31.92a |
| Intensive | 10–30 cm | 6.21 ± 0.40a | 0.079 ± 0.036a | 176.43 ± 80.49a |
| Extensive | 30–60 cm | 5.26 ± 0.86a | 0.064 ± 0.011a | 225.97 ± 39.76a |
| Intensive | 30–60 cm | 5.43 ± 0.72a | 0.064 ± 0.004a | 228.68 ± 13.06a |
| Extensive | 60–100 cm | 3.84 ± 1.00a | 0.038 ± 0.014a | 184.22 ± 66.87a |
| Intensive | 60–100 cm | 3.88 ± 0.84a | 0.039 ± 0.010a | 192.07 ± 44.35a |
| Extensive | Total | - | - | 674.42 ± 27.63a |
| Intensive | Total | - | - | 730.36 ± 70.78a |
| Management Intensity | Sediment Loss Rate (kg ha−1 yr−1) | Phytolith Concentration (g kg−1) | PhytOC Concentration (g kg−1) | Phytolith Loss Rate (kg ha−1 yr−1) | PhytOC Loss Rate (kg ha−1 yr−1) |
|---|---|---|---|---|---|
| Extensive | 34.77 | 5.80 ± 0.36 | 0.08 ± 0.01 | 0.20 ± 0.01 | 0.003 ± 0.000 |
| Intensive | 49.36 | 5.99 ± 0.91 | 0.09 ± 0.01 | 0.30 ± 0.05 | 0.004 ± 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Li, Y.; Wu, J.; Huang, Z.; Chang, S.X.; Jiang, P. Intensive Management Increases Phytolith-Occluded Carbon Sequestration in Moso Bamboo Plantations in Subtropical China. Forests 2019, 10, 883. https://doi.org/10.3390/f10100883
Huang C, Li Y, Wu J, Huang Z, Chang SX, Jiang P. Intensive Management Increases Phytolith-Occluded Carbon Sequestration in Moso Bamboo Plantations in Subtropical China. Forests. 2019; 10(10):883. https://doi.org/10.3390/f10100883
Chicago/Turabian StyleHuang, Chengpeng, Yongchun Li, Jiasen Wu, Zhangting Huang, Scott X. Chang, and Peikun Jiang. 2019. "Intensive Management Increases Phytolith-Occluded Carbon Sequestration in Moso Bamboo Plantations in Subtropical China" Forests 10, no. 10: 883. https://doi.org/10.3390/f10100883




