Climate Change Impacts on the Potential Distribution and Range Shift of Dendroctonus ponderosae (Coleoptera: Scolytidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Model and Software
2.1.1. CLIMEX Model
2.1.2. ArcGIS Software
2.2. Data Collection
2.2.1. Climate Data
2.2.2. Known Distribution of D. ponderosae and Host Plants
2.3. Research Methods
2.3.1. Parameter Fitting
Parameters for D. ponderosae
Parameters for Host Plants
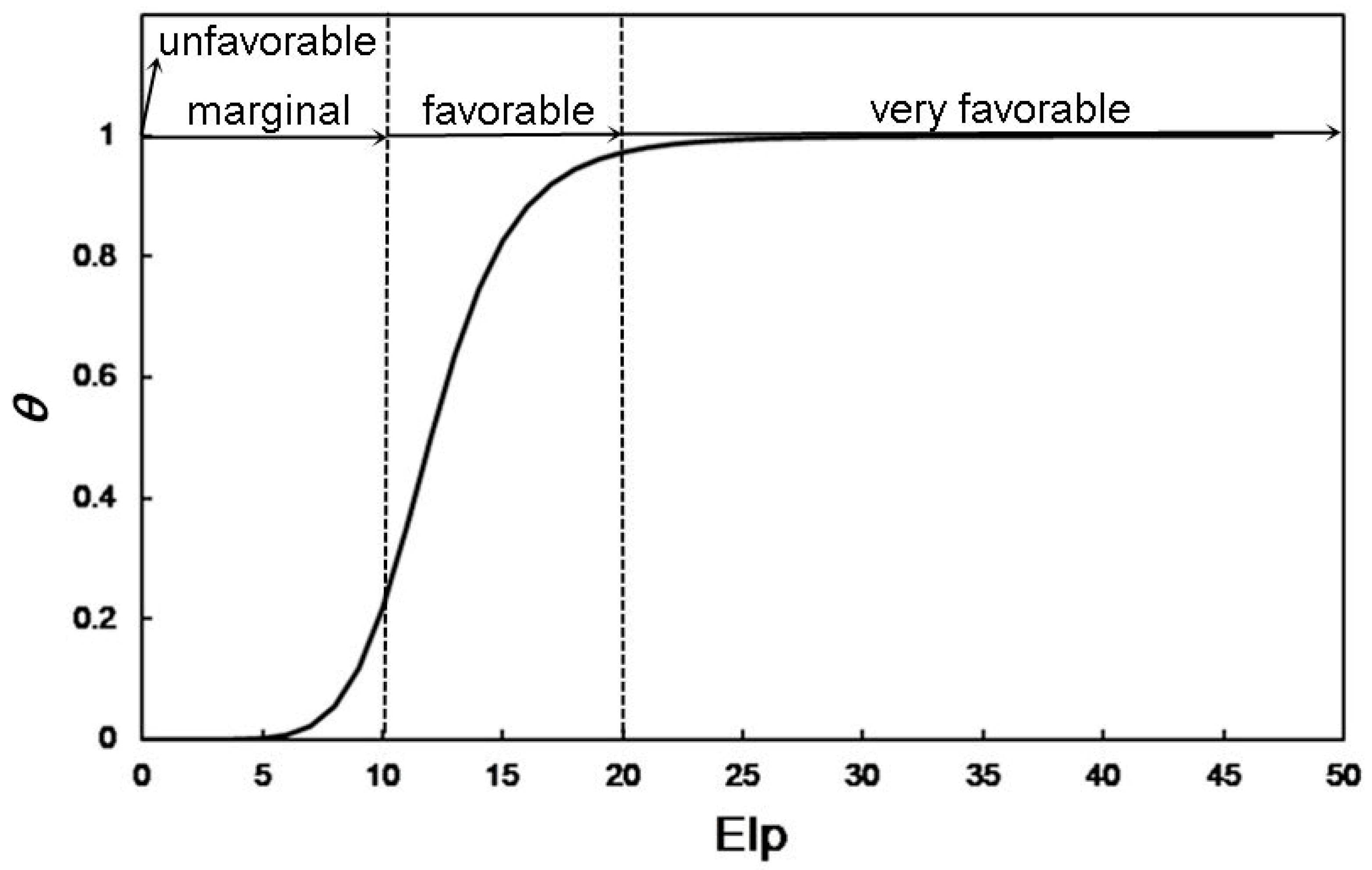
Classification of Ecoclimatic Index (EI) Values
2.3.2. Parameter Verification
2.3.3. Analysis of Results
3. Results
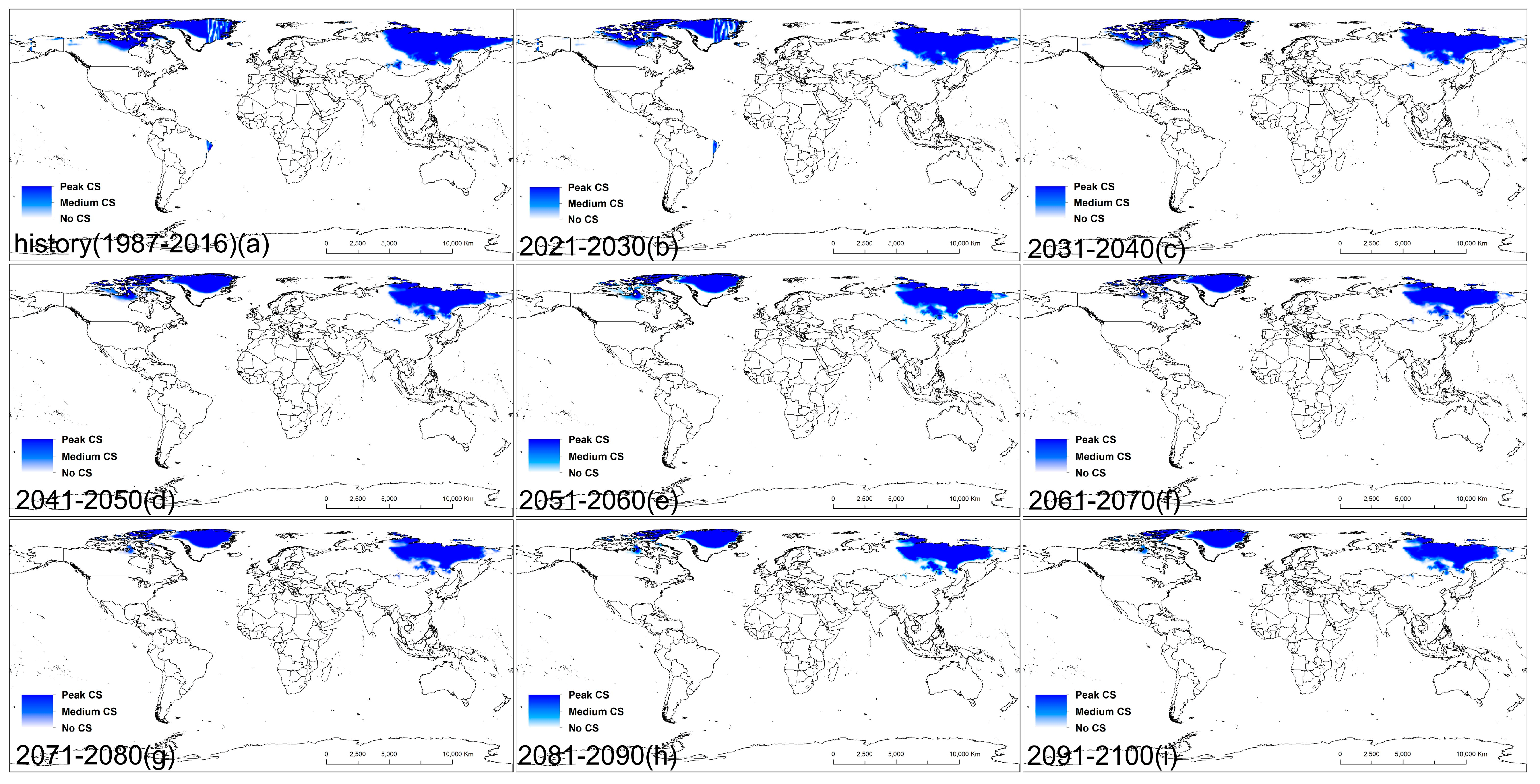
3.1. Potential Distribution of D. ponderosae under Historical Climate Conditions
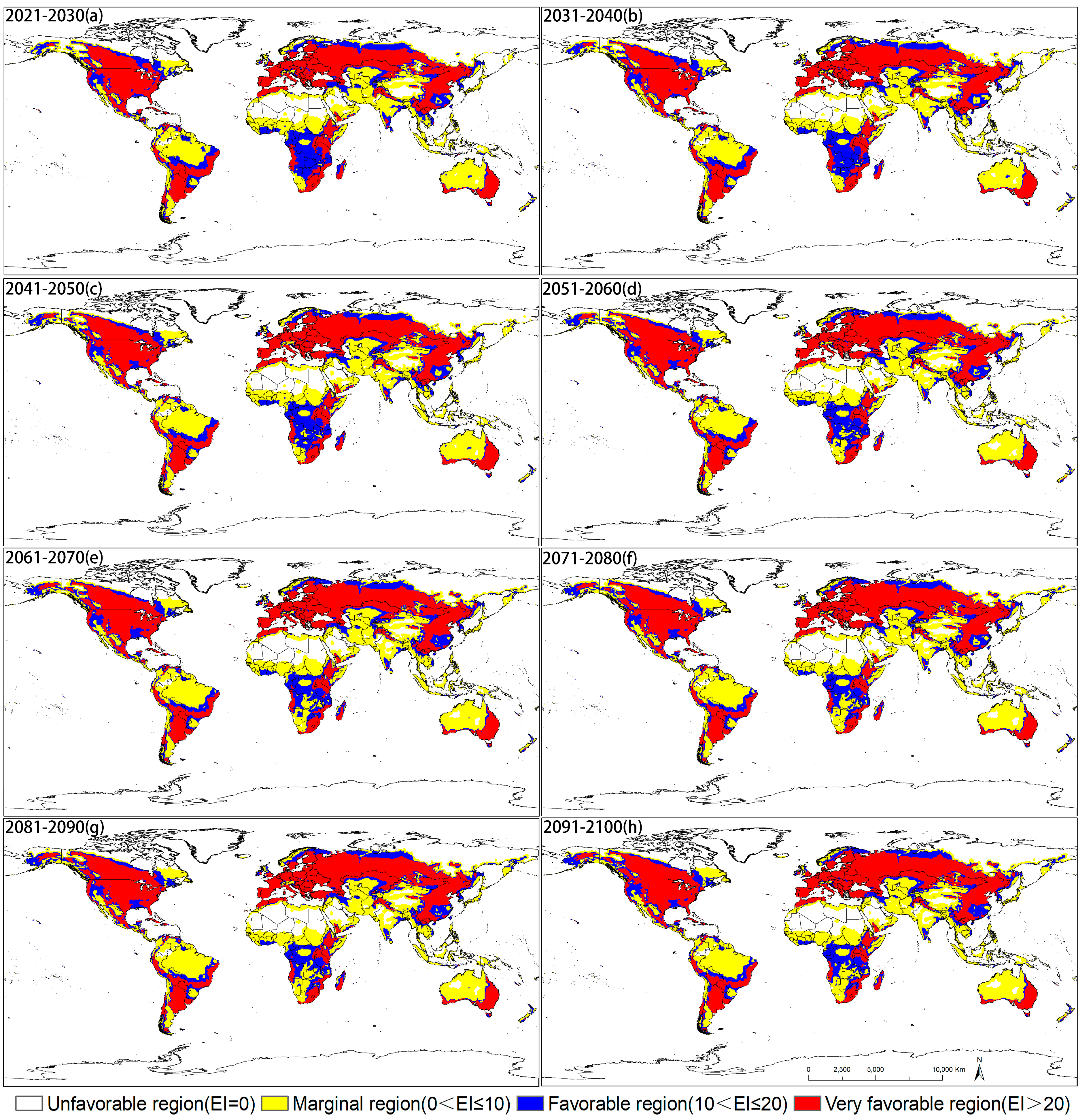
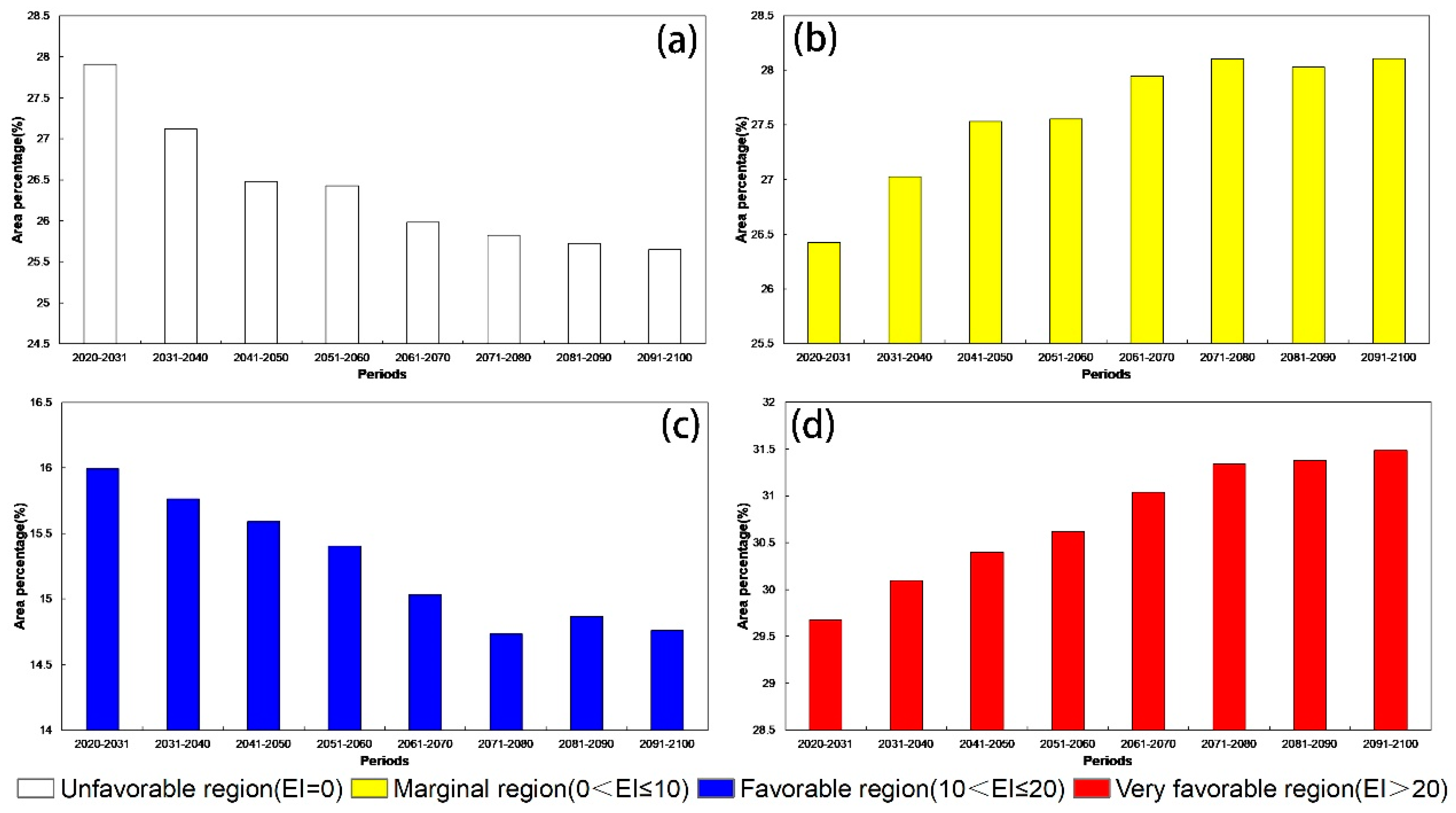
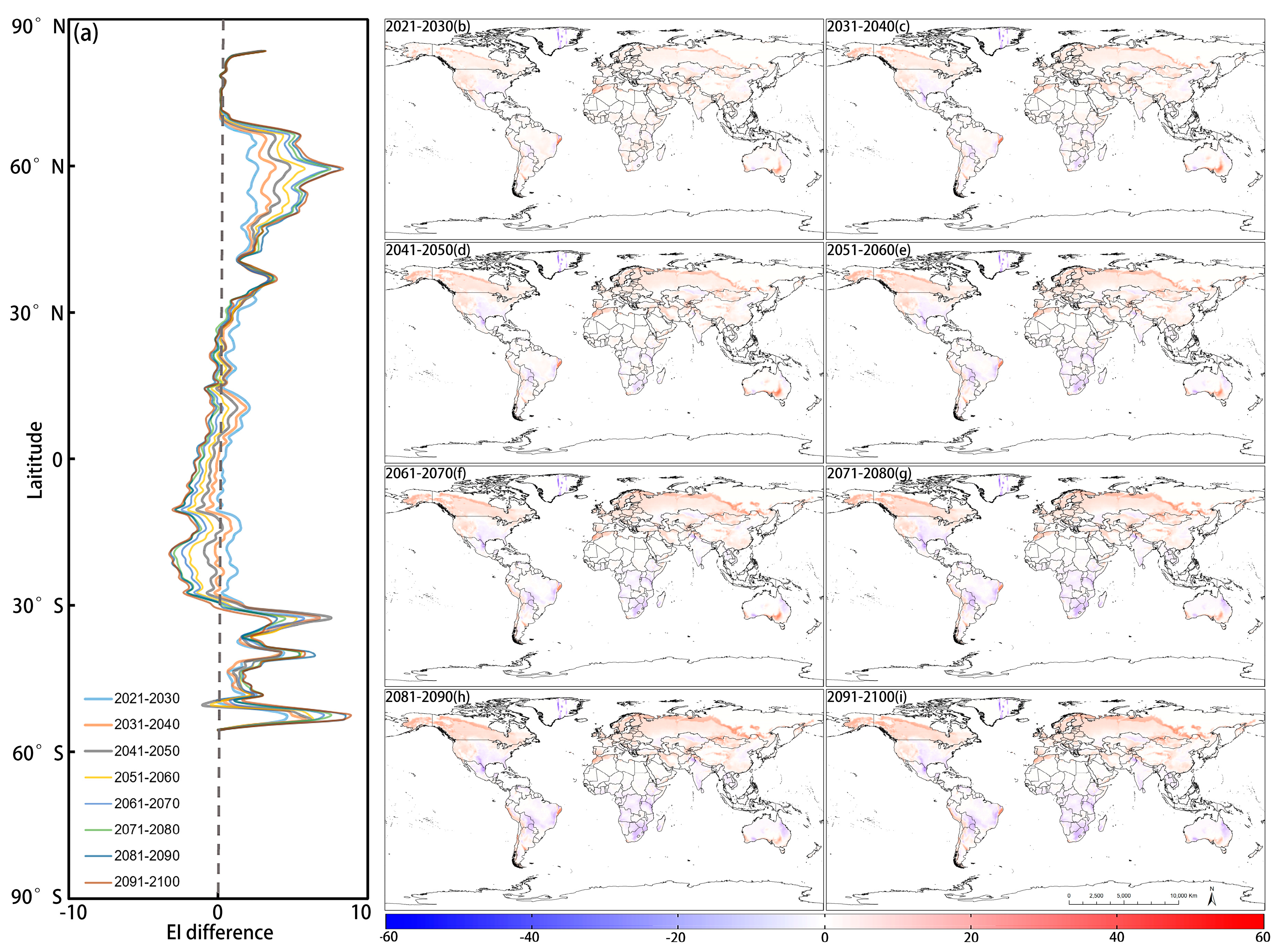
3.2. Potential Distribution of D. ponderosae under Future Climate Conditions
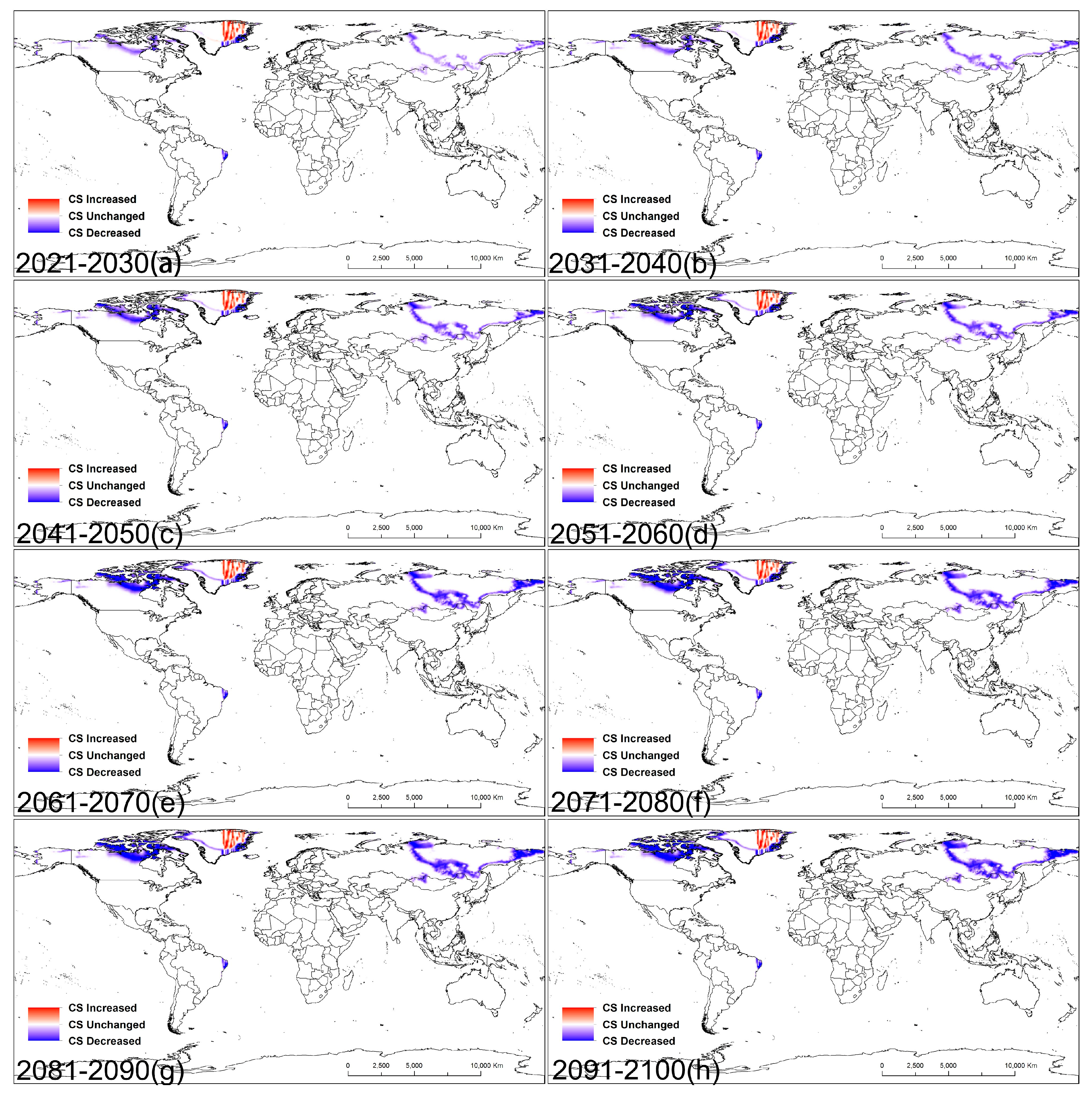
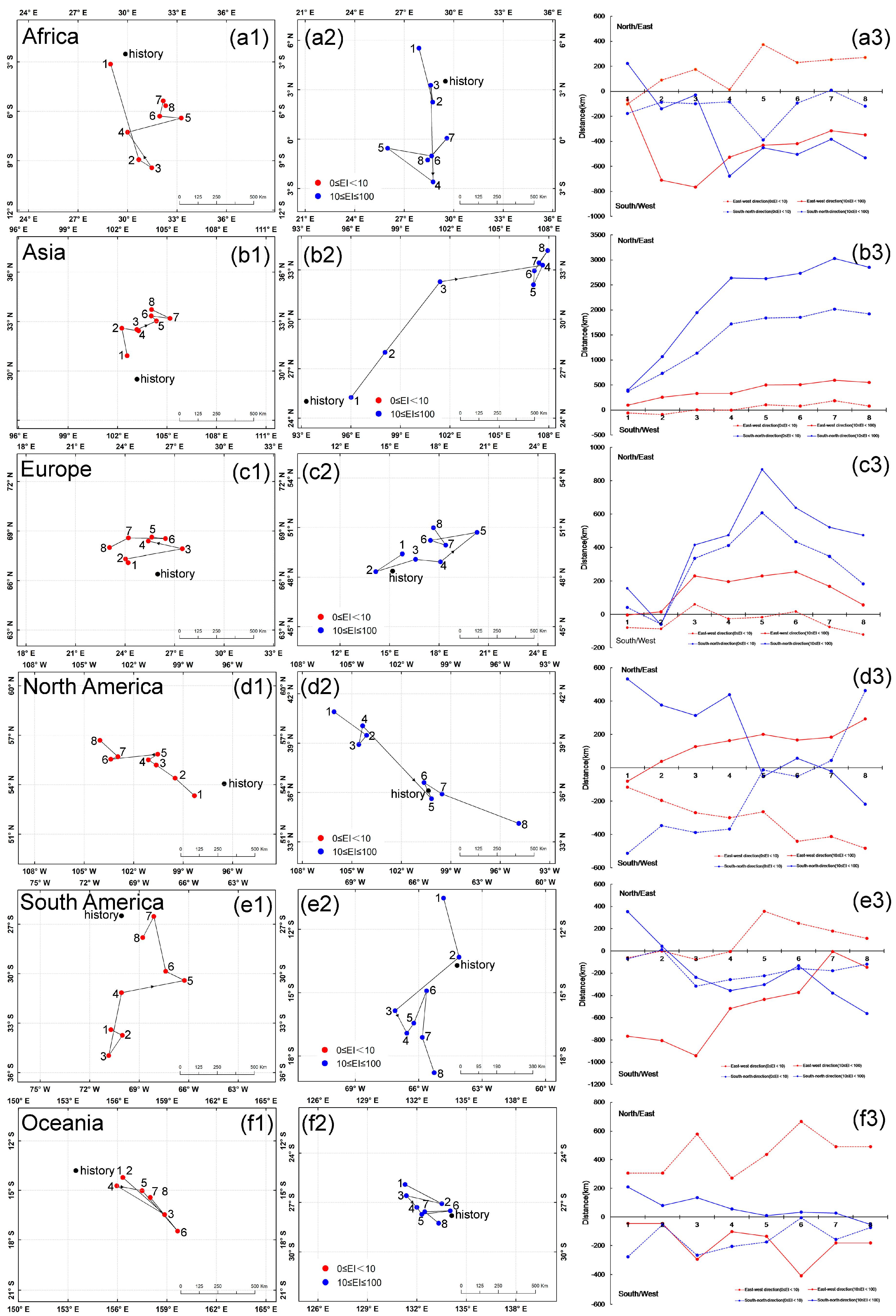
3.3. Central Point Shifts in Potential Distributions under Future Climate Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Stress Parameters | Ranges | Initial Parameter Values | Reasons and References |
|---|---|---|---|
| Cold stress threshold (TTCS; °C) | −25 to −40 | −40 | Temperatures below −25 °C in the fall or −40 °C in the winter can kill the beetles [72] |
| Cold stress accumulation rate (THCS; week−1) | −1 to −0.000001 | −0.01 | Based on temperate template |
| Heat stress threshold (TTHS; °C) | 39 to −44 | 40.5 | Bark temperature above 43.33 °C may lead to death after an exposure of sufficient length, and the bark temperature of logs exposed to sunlight were about 4.44 °C higher than the surrounding air temperature [40]. |
| Heat stress accumulation rate (THHS; week−1) | 0.000001 to 1 | 0.005 | Based on temperate template |
| Dry stress threshold (SMDS) | 0.000001 to −0.01 | 0.001 | Forest become susceptible to fatal attack by D. ponderosae during drought periods, so we referred to semi-arid template [73]. |
| Dry stress accumulation rate (HDS; week−1) | −1 to −0.000001 | −0.005 | Based on temperate template |
| Wet stress threshold (SMWS) | 1.5 to 3 | 2.5 | Based on temperate and desert templates for D. ponderosae primarily inhabiting temperate and tropical climatic conditions and surviving in a tropical desert climate |
| Wet stress accumulation rate (HWS; week−1) | 0.000001 to 0.01 | 0.002 |
| CLIMEX Parameters | Dendroctonus Ponderosae | Pinus spp. | ||
|---|---|---|---|---|
| Semi-Automatic Parameter Fitting Procedure Results | Manual Adjusted Parameter Results | Final Parameters | ||
| Minimum development temperature threshold (DV0; °C) | 5 | 5 | 0 | |
| Lower optimum temperature (DV1; °C) | 18 | 18 | 10 | |
| Upper optimum temperature (DV2;°C) | 25 | 25 | 30 | |
| Maximum development temperature threshold (DV3; °C) | 38 | 38 | 45 | |
| Effective accumulated temperature (PDD; DD) | 833 | 833 | 0 | |
| Lower moisture threshold (SM0) | 0.05 | 0.05 | 0.05 | |
| Upper optimal soil moisture threshold (SM1) | 0.1 | 0.1 | 0.1 | |
| Upper optima soil moisture threshold (SM2) | 0.2 | 0.2 | 0.8 | |
| Limiting high soil moisture threshold (SM3) | 1.5 | 1.5 | 2 | |
| Cold stress threshold (TTCS; °C) | −30.729 | −30.729 | −50 | |
| Cold stress accumulation rate (THCS; week−1) | −0.000001 | −0.1 | −0.1 | −0.1 |
| Heat stress threshold (TTHS; °C) | 38 | 42 | 42 | 50 |
| Heat stress accumulation rate (THHS; week−1) | 0.795 | 0.795 | 0.1 | |
| Dry stress threshold (SMDS) | 0.00993 | 0.00993 | 0.01 | |
| Dry stress accumulation rate (HDS; week−1) | −0.962 | −0.005 | −0.005 | −0.005 |
| Wet stress threshold (SMWS) | 1.5 | 1.5 | 2.5 | |
| Wet stress accumulation rate (HWS; week−1) | 0.00829 | 0.00829 | 0.1 | |
| Daylength at growth rate of zero (LT1) | 10 | |||
| Daylength at maximum growth rate (LT0) | 15 | |||
Appendix B
References
- Pachauri, K.; Meyer, A. Climate Change 2014 Synthesis Report. Environ. Policy Collect. 2014, 27, 408. [Google Scholar]
- Chen, Y.; Ma, C. Effect of global warming on insect: A literature review. Acta Ecol. Sin. 2010, 30, 2159–2172. [Google Scholar]
- Logan, J.A.; Régnière, J.; Powell, J.A. Assessing the impacts of global warming on forest pest dynamics. Front. Ecol. Environ. 2003, 1, 130–137. [Google Scholar] [CrossRef]
- Amman, G.D.; Cole, W.E. Mountain pine beetle dynamics in lodgepole pine forests. Part II: Population dynamics. In General Technical Report, Intermountain Forest and Range Experiment Station, USDA Forest Service; (INT-145); USDA Forest Service: Washington, DC, USA, 1983. [Google Scholar]
- Unger, L.S. Mountain Pine Beetle; Pacific Forestry Centre: Victoria, BC, Canada, 1993; Volume 76. [Google Scholar]
- Coops, N.C.; Waring, R.H.; Wulder, M.A.; White, J.C. Prediction and assessment of bark beetle-induced mortality of lodgepole pine using estimates of stand vigor derived from remotely sensed data. Remote Sens. Environ. 2009, 113, 1058–1066. [Google Scholar] [CrossRef]
- Jenkins, M.J.; Hebertson, E.; Page, W.; Jorgensen, C.A. Bark beetles, fuels, fires and implications for forest management in the Intermountain West. For. Ecol. Manag. 2008, 254, 16–34. [Google Scholar] [CrossRef]
- Pedersen, L. How Serious Is the Mountain Pine Beetle Problem? From a Timber Supply Perspective; Pacific Forestry Centre: Victoria, BC, Canada, 2003; pp. 10–18. [Google Scholar]
- Safranyik, L.; Carroll, A.L.; Régnière, J.; Langor, D.W.; Riel, W.G.; Shore, T.L.; Peter, B.; Cooke, B.J.; Nealis, V.G.; Taylor, S.W. Potential for range expansion of mountain pine beetle into the boreal forest of North America. Can. Entomol. 2010, 142, 415–442. [Google Scholar] [CrossRef]
- Yamaoka, Y. A1 Forest Pests. In Proceedings of the 5th International Congress of Plant Pathology, Kyoto, Japan, 20–27 August 1988. [Google Scholar]
- Charles, P. In the Rockies, Pines Die and Bears Feel It. Available online: https://www.nytimes.com/2007/01/30/science/30bear.html?ref=science (accessed on 1 June 2008).
- Furniss, M.M.; Schenk, J.A. Sustained natural infestations by the mountain pine beetle in seven new Pinus and Picea hosts. J. Econ. Entomol. 1969, 62, 2. [Google Scholar] [CrossRef]
- Cook, S.P.; Martinez, A. Insects emerging from novel species of host trees attacked by mountain pine beetle, Dendroctonus ponderosae Hopkins, 1902 (Coleoptera: Curculionidae: Scolytinae), in the University of Idaho Arboretum. Pan Pac. Entomol. 2018, 94, 75–84. [Google Scholar] [CrossRef]
- Rosenberger, D.W.; Venette, R.C.; Aukema, B.H. Susceptibility of Eurasian Scots pine, Pinus sylvestris L. to the aggressive North American mountain pine beetle, Dendroctonus ponderosae Hopkins. For. Ecol. Manag. 2019, 445, 20–25. [Google Scholar] [CrossRef]
- Kipfmueller, K.F.; Swetnam, T.W.; Morgan, P. Climate and mountain pine beetle-induced tree mortality in the Selway-Bitterroot Wilderness Area; Final Report to the U.S. Department of Agriculture, Forest Service, Research Joint Venture Agreement #RMRS-99611-RJVA; Rocky Mountain Research Station: Fort Collins, CO, USA, 2002.
- Thomson, A.J.; Shrimpton, D.M. Weather associated with the start of mountain pine beetle outbreaks. Can. J. For. Res. 1984, 14, 255–258. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Li, G.; Liu, C.; Liu, Y.; Yang, J.; Zhang, X.; Guo, K. Advances in theotetical issues of species distribution models. Acta Ecol. Sin. 2013, 33, 4827–4835. [Google Scholar]
- Xu, Z.; Peng, H.; Peng, S. The development and evaluation of species distribution models. Acta Ecol. Sin. 2015, 35, 557–567. [Google Scholar]
- Shabani, F.; Kumar, L.; Taylor, S. Climate change impacts on the future distribution of date palms: A modeling exercise using CLIMEX. PLoS ONE 2012, 7, e48021. [Google Scholar] [CrossRef] [PubMed]
- Pattison, R.R.; Mack, R.N. Potential distribution of the invasive tree Triadica sebifera (Euphorbiaceae) in the United States: Evaluating climex predictions with field trials. Glob. Chang. Biol. 2010, 14, 813–826. [Google Scholar] [CrossRef]
- Taylor, S.; Kumar, L. Potential distribution of an invasive species under climate change scenarios using CLIMEX and soil drainage: A case study of Lantana camara L. in Queensland, Australia. J. Environ. Manag. 2013, 114, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zong, S.; He, S.; Liu, Y.; Kong, X. Areas of C hina predicted to have a suitable climate for A noplophora chinensis under a climate-warming scenario. Entomol. Exp. Appl. 2014, 153, 256–265. [Google Scholar] [CrossRef]
- Khormi, H.M.; Kumar, L. Climate change and the potential global distribution of Aedes aegypti: Spatial modelling using GIS and CLIMEX. Geospat. Health 2014, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Jiang, C.; Chen, L.; Qiu, S.; Zhao, Y.; Wang, T.; Zong, S. Predicting the potential distribution in China of Euwallacea fornicates (Eichhoff) under current and future climate conditions. Sci. Rep. 2017, 7, 5772. [Google Scholar] [CrossRef]
- Brasier, C.M.; Scott, J.K. European oak declines and global warming: A theoretical assessment with special reference to the activity of Phytophthora cinnamomi. EPPO Bull. 1994, 24, 221–232. [Google Scholar] [CrossRef]
- Yonow, T.; Hattingh, V.; de Villiers, M. CLIMEX modelling of the potential global distribution of the citrus black spot disease caused by Guignardia citricarpa and the risk posed to Europe. Crop Prot. 2013, 44, 18–28. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Maywald, G.F.; Yonow, T.; Zurcher, E.J.; Herrmann, N.I.; Sutherst, R. Exploring the effects of climate on plants, animals and diseases. CLIMEX Version 2015, 4, 184. [Google Scholar]
- Carroll, A.L.; Regniere, J.; Logan, J.A.; Taylor, S.W.; Bentz, B.J.; Powell, J.A. Impacts of Climate Change on Range Expansion by the Mountain Pine Beetle. Crit. Care Med. 2006, 33, 1185. [Google Scholar]
- Scott, L.M.; Janikas, M.V. Spatial Statistics in ArcGIS; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Zou, Y.; Ge, X.; Guo, S.; Zhou, Y.; Wang, T.; Zong, S. Impacts of climate change and host plant availability on the global distribution of Brontispa longissima (Coleoptera: Chrysomelidae). Pest Manag. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.L.; Taylor, S.W.; Régnière, J.; Safranyik, L. Effect of climate change on range expansion by the mountain pine beetle in British Columbia. In Proceedings of the Mountain Pine Beetle Symposium: Challenges and Solutions, Kelowna, BC, Canada, 30–31 October 2003; Shore, T.L., Brooks, J.E., Stone, J.E., Eds.; Natural Resources Canada, Infromation Report BC-X-399: Victoria, BC, USA; pp. 223–232. [Google Scholar]
- McFarlane, B.L.; Stumpf-Allen, R.C.G.; Watson, D.O. Public perceptions of natural disturbance in Canada’s national parks: The case of the mountain pine beetle (Dendroctonus ponderosae Hopkins). Biol. Conserv. 2006, 130, 340–348. [Google Scholar] [CrossRef]
- Mock, K.E.; Bentz, B.J.; O’neill, E.M.; Chong, J.P.; Orwin, J.; Pfrender, M.E. Landscape-scale genetic variation in a forest outbreak species, the mountain pine beetle (Dendroctonus ponderosae). Mol. Ecol. 2007, 16, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Safranyik, L.; Carroll, A.L. The biology and epidemiology of the mountain pine beetle in lodgepole pine forests. In The Mountain Pine Beetle: A Synthesis of Biology, Management, and Impacts on Lodgepole Pine; Forestry Centre: Victoria, BC, Canada, 2006; pp. 3–66. [Google Scholar]
- EPPO Global Data Base Dendroctonus ponderosae Hopkins. Available online: https://gd.eppo.int/taxon/DENCPO/distribution (accessed on 15 April 2018).
- GBIF Distribution Maps of Dendroctonus ponderosae Hopkins. Available online: https://www.gbif.org/occurrence/search?taxon_key=1228026 (accessed on 15 April 2018).
- GBIF the Global Distribution of Pinus L. Available online: https://www.gbif.org/occurrence/search?taxon_key=2684241&taxon_key=1228026 (accessed on 1 May 2018).
- Safranyik, L.; Barclay, H.; Thomson, A.; Riel, W. A Population Dynamics Model for the Mountain Pine Beetle, Dendroctonus ponderosae Hopk. (Coleoptera: Scolytidae); Pacific Forestry Centre: Victoria, BC, Canada, 1999. [Google Scholar]
- Patterson, J.E. Control of the Mountain Pine Beetle in Lodgepole pine by the Use of Solar Heat; No. 1488-2016-123579; United States Department of Agriculture: Washington, DC, USA, 1930.
- Reid, R.W.; Gates, H. Effect of temperature and resin on hatch of eggs of the mountain pine beetle (Dendroctonus ponderosae). Can. Entomol. 1970, 102, 617–622. [Google Scholar] [CrossRef]
- Bentz, B.J.; Logan, J.A.; Amman, G.D. Temperature-dependent development of the mountain pine beetle (Coleoptera: Scolytidae) and simulation of its phenology. Can. Entomol. 1991, 123, 1083–1094. [Google Scholar] [CrossRef]
- Shepherd, R. Factors influencing the orientation and rates of activity of Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). Can. Entomol. 1966, 98, 507–518. [Google Scholar] [CrossRef]
- McCambridge, W.F. Temperature limits of flight of the mountain pine beetle, Dendroctonus ponderosae. Ann. Entomol. Soc. Am. 1971, 64, 534–535. [Google Scholar] [CrossRef]
- Aukema, B.H.; Carroll, A.L.; Zheng, Y.; Zhu, J.; Raffa, K.F.; Dan Moore, R.; Stahl, K.; Taylor, S.W. Movement of outbreak populations of mountain pine beetle: Influences of spatiotemporal patterns and climate. Ecography 2008, 31, 348–358. [Google Scholar] [CrossRef]
- Reid, R.W. Biology of the mountain pine beetle, Dendroctonus monticolae Hopkins, in the east Kootenay region of British Columbia I. Life cycle, brood development, and flight periods. Can. Entomol. 1962, 94, 531–538. [Google Scholar] [CrossRef]
- Safranyik, L.; Shrimpton, D.M.; Whitney, H.S. An interpretation of the interaction between lodgepole pine, the mountain pine beetle and its associated blue stain fungi in western Canada. Manag. Lodg. Pine Ecosyst. 1975, 1, 406–428. [Google Scholar]
- Creeden, E.P.; Hicke, J.A.; Buotte, P.C. Climate, weather, and recent mountain pine beetle outbreaks in the western United States. For. Ecol. Manag. 2014, 312, 239–251. [Google Scholar] [CrossRef]
- Zhou, Y.; Gong, Y.; Fan, J. Comparison on Cold Resistance of 4 Pine Conifers. J. Northwest For. Univ. 2011, 26, 69–71. [Google Scholar]
- Kishchenko, I.T. Effect of climatic factors on the growth of representatives of the genus Pinus (Pinaceae) under conditions of introduction. Russ. J. Ecol. 2004, 35, 214–219. [Google Scholar] [CrossRef]
- Gong, Y.H.; Fan, J.F.; Zhou, Y.X.; Sun, Q.; Yang, J.F. Research on the cold resistance of Pinus nigra var.austriaca and Pseudotsuga menziesii var.glauca. J. Northwest Sci. Tech. Univ. Agric. For. 2006, 34, 105–109. [Google Scholar]
- Acevedo, P.; Real, R. Favourability: Concept, distinctive characteristics and potential usefulness. Naturwissenschaften 2012, 99, 515–522. [Google Scholar] [CrossRef]
- Farfán, L. Comparative Population Genetic Analysis of Fungal Associates of the Mountain Pine Beetle (Dendroctonus ponderosae); University of British Columbia: Vancouver, BC, Canada, 2014. [Google Scholar]
- De la Giroday, H.M.C.; Carroll, A.L.; Aukema, B.H. Breach of the northern Rocky Mountain geoclimatic barrier: Initiation of range expansion by the mountain pine beetle. J. Biogeogr. 2012, 39, 1112–1123. [Google Scholar] [CrossRef]
- Sutherst, R.W. Prediction of species geographical ranges. J. Biogeogr. 2003, 30, 805–816. [Google Scholar] [CrossRef]
- Bentz, B.; Vandygriff, J.; Jensen, C.; Coleman, T.; Maloney, P.; Smith, S.; Grady, A.; Schen-Langenheim, G. Mountain pine beetle voltinism and life history characteristics across latitudinal and elevational gradients in the western United States. For. Sci. 2013, 60, 434–449. [Google Scholar] [CrossRef]
- Berzitis, E.A.; Minigan, J.N.; Hallett, R.H.; Newman, J.A. Climate and host plant availability impact the future distribution of the bean leaf beetle (Cerotoma trifurcata). Glob. Chang. Biol. 2014, 20, 2778–2792. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; He, S.; Zhu, C.; Wang, T.; Xu, Z.; Zong, S. Projecting the current and future potential global distribution of Hyphantria cunea (Lepidoptera: Arctiidae) using CLIMEX. Pest Manag. Sci. 2019, 75, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Flato, G.M.; Boer, G.J.; Lee, W.G.; McFarlane, N.A.; Ramsden, D.; Reader, M.C.; Weaver, A.J. The Canadian Centre for Climate Modelling and Analysis global coupled model and its climate. Clim. Dyn. 2000, 16, 451–467. [Google Scholar] [CrossRef]
- Bentz, B.J.; Mullins, D.E. Ecology of mountain pine beetle (Coleoptera: Scolytidae) cold hardening in the intermountain west. Environ. Entomol. 1999, 28, 577–587. [Google Scholar] [CrossRef]
- Régnière, J.; Bentz, B. Modeling cold tolerance in the mountain pine beetle, Dendroctonus ponderosae. J. Insect Physiol. 2007, 53, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, D.W.; Aukema, B.H.; Venette, R.C. Cold tolerance of mountain pine beetle among novel eastern pines: A potential for trade-offs in an invaded range? For. Ecol. Manag. 2017, 400, 28–37. [Google Scholar] [CrossRef]
- Sambaraju, K.R.; Carroll, A.L.; Zhu, J.; Stahl, K.; Moore, R.D.; Aukema, B.H. Climate change could alter the distribution of mountain pine beetle outbreaks in western Canada. Ecography 2012, 35, 211–223. [Google Scholar] [CrossRef]
- Dormann, C.F. Promising the future? Global change projections of species distributions. Basic Appl. Ecol. 2007, 8, 387–397. [Google Scholar] [CrossRef]
- Midgley, G.F.; Hughes, G.O.; Thuiller, W.; Rebelo, A.G. Migration rate limitations on climate change-induced range shifts in Cape Proteaceae. Divers Distribut. Divers. Distrib. 2010, 12, 555–562. [Google Scholar] [CrossRef]
- Paulo, D.M.; Luis Mauricio, B. Spatial analysis improves species distribution modelling during range expansion. Biol. Lett. 2008, 4, 577–580. [Google Scholar]
- Bentz, B.J.; Logan, J.A.; Vandygriff, J.C. Latitudinal variation in Dendroctonus ponderosae (Coleoptera: Scolytidae) development time and adult size. Can. Entomol. 2001, 133, 375–387. [Google Scholar] [CrossRef]
- Trewin, B.J. Assessing the Risk of Establishment by the Dengue Vector, Aedes Aegypti (L.)(Diptera: Culicidae), Through Rainwater Tanks in Queensland: Back to the Future; University of Queensland: Queensland, Australia, 2018. [Google Scholar]
- Bentz, B.J.; Hood, S.M.; Hansen, E.M.; Vandygriff, J.C.; Mock, K.E. Defense traits in the long-lived Great Basin bristlecone pine and resistance to the native herbivore mountain pine beetle. New Phytol 2017, 213, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Eidson, E.L.; Mock, K.E.; Bentz, B.J. Mountain pine beetle host selection behavior confirms high resistance in Great Basin bristlecone pine. For. Ecol. Manag. 2017, 402, 12–20. [Google Scholar] [CrossRef]
- Desurmont, G.A.; Donoghue, M.J.; Clement, W.L.; Agrawal, A.A. Evolutionary history predicts plant defense against an invasive pest. Proc. Natl. Acad. Sci. USA 2011, 108, 7070–7074. [Google Scholar] [CrossRef] [PubMed]
- Dordel, J.; Feller, M.C.; Simard, S.W. Effects of mountain pine beetle (Dendroctonus ponderosae Hopkins) infestations on forest stand structure in the southern Canadian Rocky Mountains. For. Ecol. Manag. 2008, 255, 3563–3570. [Google Scholar] [CrossRef]
- Beal, J.A. Relation Between Tree Growth and Outbreaks of the Black Hills Beetle. J. For. 1943, 41, 359–366. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Ge, X.; Zou, Y.; Guo, S.; Wang, T.; Zong, S. Climate Change Impacts on the Potential Distribution and Range Shift of Dendroctonus ponderosae (Coleoptera: Scolytidae). Forests 2019, 10, 860. https://doi.org/10.3390/f10100860
Zhou Y, Ge X, Zou Y, Guo S, Wang T, Zong S. Climate Change Impacts on the Potential Distribution and Range Shift of Dendroctonus ponderosae (Coleoptera: Scolytidae). Forests. 2019; 10(10):860. https://doi.org/10.3390/f10100860
Chicago/Turabian StyleZhou, Yuting, Xuezhen Ge, Ya Zou, Siwei Guo, Tao Wang, and Shixiang Zong. 2019. "Climate Change Impacts on the Potential Distribution and Range Shift of Dendroctonus ponderosae (Coleoptera: Scolytidae)" Forests 10, no. 10: 860. https://doi.org/10.3390/f10100860
APA StyleZhou, Y., Ge, X., Zou, Y., Guo, S., Wang, T., & Zong, S. (2019). Climate Change Impacts on the Potential Distribution and Range Shift of Dendroctonus ponderosae (Coleoptera: Scolytidae). Forests, 10(10), 860. https://doi.org/10.3390/f10100860




