Enrichment of Logging Gaps with High-Value Timber Species: How Far Fertilizer, Biochar and Mammal Predation Affect Performances of Cylicodiscus gabunensis Harms Seedlings
Abstract
1. Introduction
2. Materials and Methods
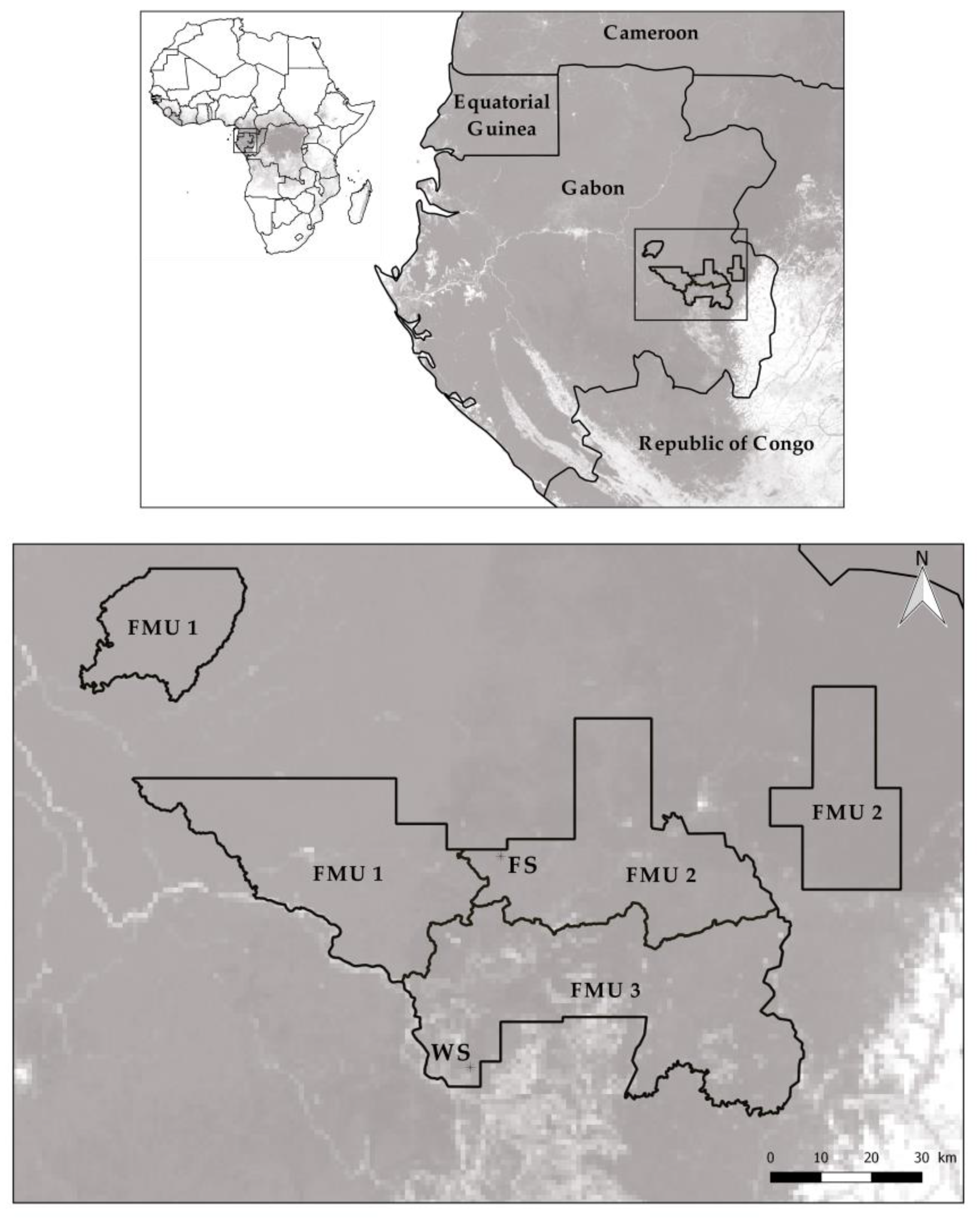
2.1. Study Area
2.2. Study Species
2.3. Silvicultural Experiments
2.3.1. Performance of C. gabunensis in Logging Gaps and Effects of Fertilizers
2.3.2. Wildlife Damage in Planted Logging Gaps
2.4. Data Analysis
2.4.1. Assessing Effect of Fertility on Seedling Growth
2.4.2. Identification of Consumers of C. gabunensis Seedlings in Logging Gaps
3. Results
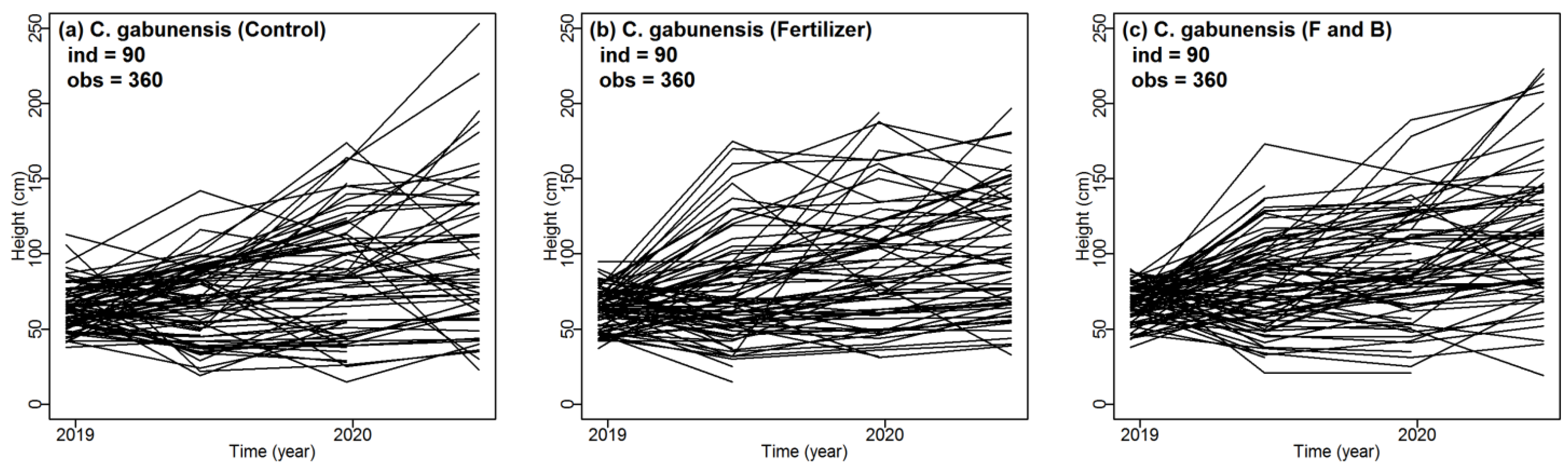
3.1. Performance of C. gabunensis in Logging Gaps and Impact of Fertilizer
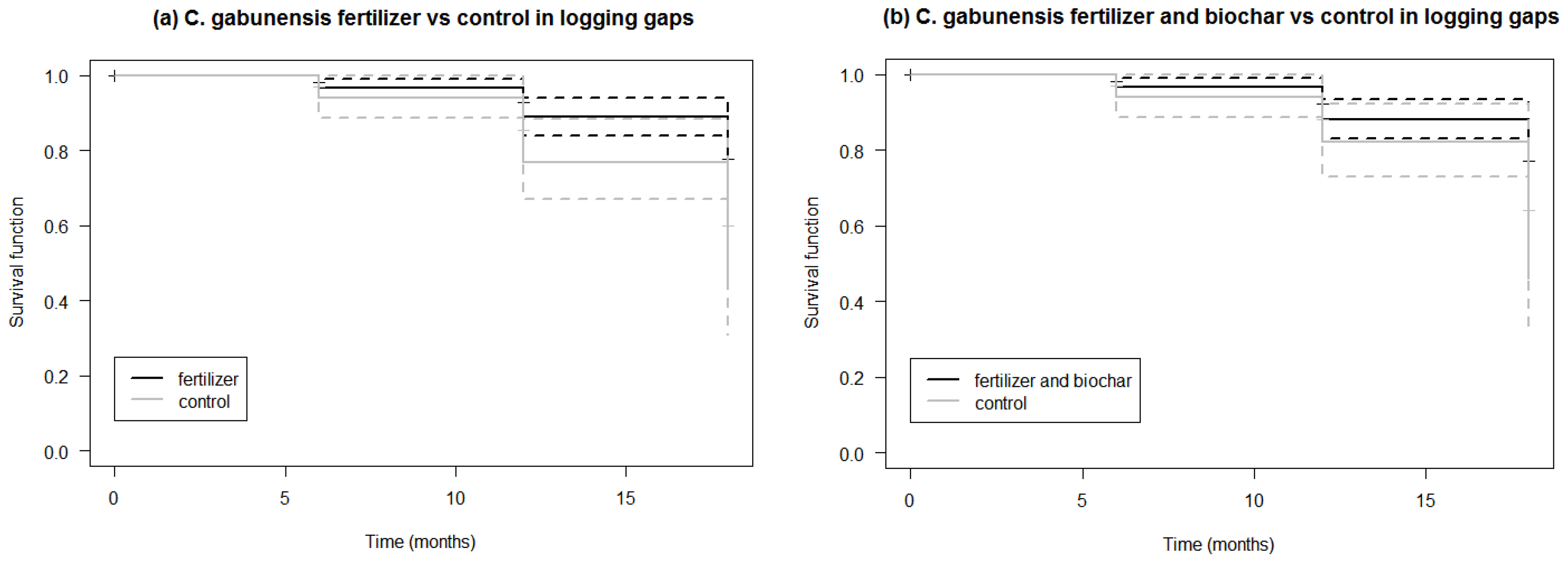
3.2. Survival of C. gabunensis Seedlings in Logging Gaps
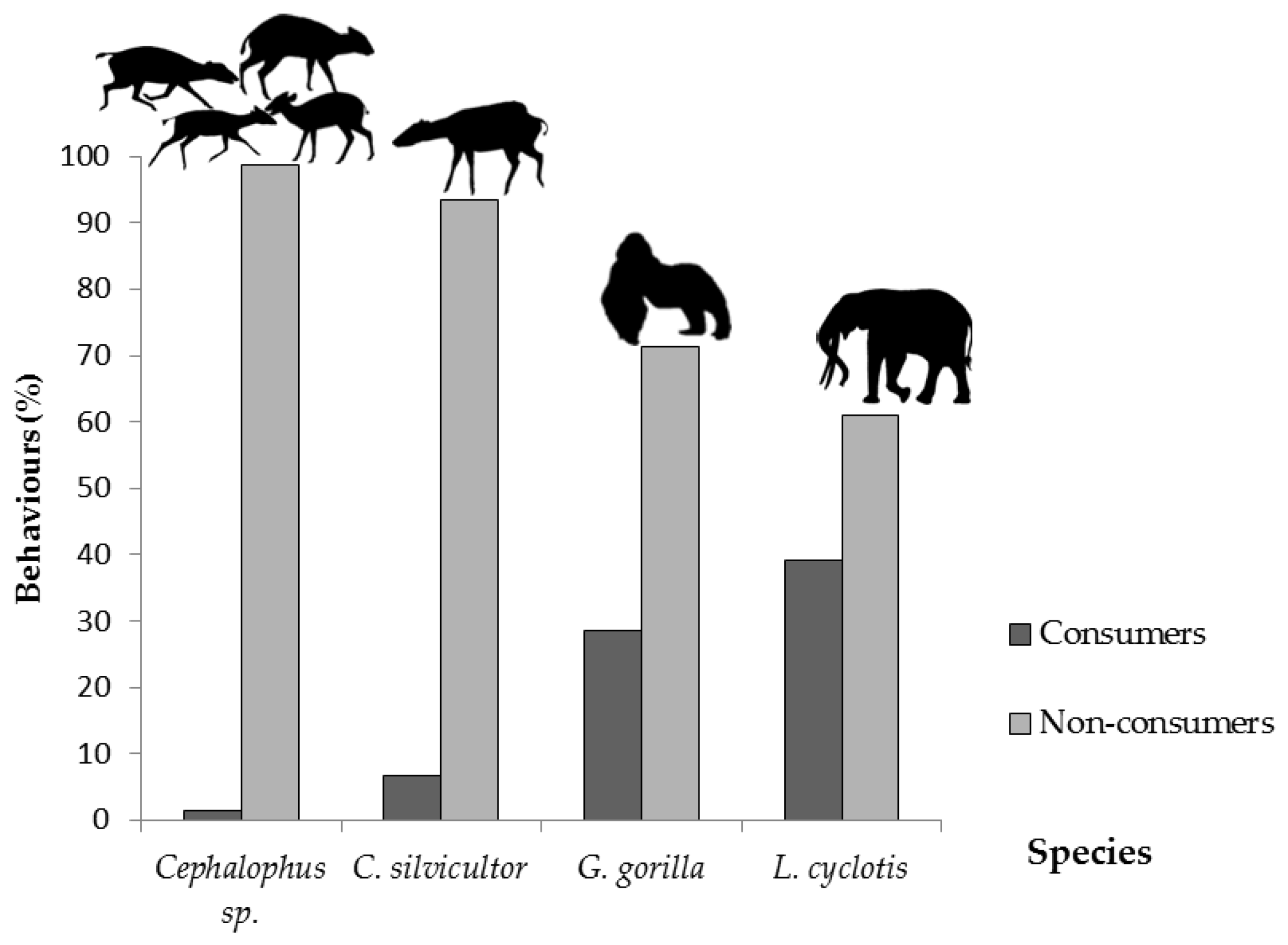
3.3. Predation of C. gabunensis Seedlings in Logging Gaps
4. Discussion
4.1. Growth and Mortality of C. gabunensis Seedlings in Logging Gaps
4.2. Predators of C. gabunensis Seedlings in Logging Gaps
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Treatment | Time (Months) | Mean Height (cm) | Standard Error | Mean Diameter (cm) | Standard Error |
|---|---|---|---|---|---|
| Control | 0 | 64.04 | 1.65 | 0.49 | 0.01 |
| Fertilizer | 0 | 63.00 | 1.40 | 0.49 | 0.01 |
| F and C | 0 | 66.17 | 1.37 | 0.51 | 0.01 |
| Control | 6 | 69.18 | 2.83 | 0.64 | 0.02 |
| Fertilizer | 6 | 77.19 | 3.95 | 0.75 | 0.03 |
| F and C | 6 | 81.44 | 3.43 | 0.81 | 0.03 |
| Control | 12 | 84.48 | 4.75 | 0.78 | 0.02 |
| Fertilizer | 12 | 91.31 | 4.69 | 0.91 | 0.04 |
| F and C | 12 | 92.65 | 4.26 | 0.92 | 0.03 |
| Control | 18 | 99.05 | 6.52 | 0.85 | 0.03 |
| Fertilizer | 18 | 100.87 | 5.22 | 0.96 | 0.04 |
| F and C | 18 | 113.84 | 5.76 | 0.97 | 0.04 |
| npar | AIC | BIC | logLik | Deviance | Chisq | Df | Pr(>Chisq) | |
|---|---|---|---|---|---|---|---|---|
| m0 | 6 | 8645.6 | 8674.3 | −4316.8 | 8633.6 | / | / | / |
| m1 | 8 | 8645.4 | 8683.8 | −4314.7 | 8629.4 | 4.1841 | 2 | 0.1234 |
| npar | AIC | BIC | logLik | Deviance | Chisq | Df | Pr(>Chisq) | |
|---|---|---|---|---|---|---|---|---|
| m2 | 6 | −334.86 | −306.07 | 173.43 | −346.86 | / | / | / |
| m3 | 8 | −342.73 | −304.34 | 179.36 | −358.73 | 11.87 | 2 | 0.002645 |
| npar | AIC | BIC | logLik | Deviance | Chisq | Df | Pr(>Chisq) | |
|---|---|---|---|---|---|---|---|---|
| m4 | 7 | 8647.0 | 8680.6 | −4316.5 | 8633.0 | / | / | / |
| m5 | 8 | 8648.8 | 8687.2 | −4316.4 | 8632.8 | 0.183 | 1 | 0.6688 |
| npar | AIC | BIC | logLik | Deviance | Chisq | Df | Pr(>Chisq) | |
|---|---|---|---|---|---|---|---|---|
| m6 | 7 | −346.23 | −312.64 | 180.11 | −360.23 | / | / | / |
| m7 | 8 | −369.25 | −330.87 | 192.63 | −385.25 | 25.027 | 1 | 5.652 × 10 −7 |
| Species | IUCN Status | No. of Detections |
|---|---|---|
| Philantomba monticola simpsoni Thunberg, 1789 | LC | 4 |
| Cephalophus ogilbyi crusalbum Grubb, 1978 | NT | 9 |
| Cephalophus silvicultor Afzelius, 1815 | NT | 76 |
| Cephalophus sp. | / | 146 |
| Pan troglodytes Btroglodytes Blumenbash, 1775 | NT | 47 |
| Civettictis civetta Schreber, 1776 | LC | 20 |
| Loxodonta cyclotis Matschie, 1900 | CR | 64 |
| Genetta sp. | / | 15 |
| Gorilla gorilla gorilla Savage, 1847 | CR | 14 |
| Unidentified bird species | / | 4 |
| Panthera pardus pardus Linnaeus, 1758 | VU | 2 |
| Potamochoerus porcus Linnaeus, 1758 | LC | 4 |
| Syncerus caffer nanus Boddaert, 1785 | NT | 13 |
| Behaviours | C. silvicultor | Cephalophus sp. | L. cyclotis | G. gorilla |
|---|---|---|---|---|
| Pulling up a C. gabunensis plant and eat their leaves | - | - | 1 | - |
| Circulating | 24 | 27 | 9 | 8 |
| Interacting with camera | 3 | 8 | 2 | - |
| Playing | - | - | 1 | - |
| Eating other vegetation | 33 | 71 | 20 | 1 |
| Eating C. gabunensis leaves and breaking the plant | - | - | 5 | - |
| Eating C. gabunensis leaves without breaking the plant | 5 | 2 | 13 | 4 |
| Sniffing other plants and eating their leaves | - | 1 | - | - |
| Sniffing other plants without eating | - | 1 | 2 | - |
| Sniffing C. gabunensis plants and eating their leaves | - | - | 6 | - |
| Sniffing C. gabunensis plants without eating their leaves | 3 | 6 | 3 | - |
| Sniffing vegetation on the ground | 8 | 30 | 1 | - |
| Staying static | - | - | 1 | 1 |
References
- Meunier, Q.; Moumbogou, C.; Doucet, J.-L. Les Arbres Utiles Du Gabon; Les Presses agronomiques de Gembloux: Gembloux, Belgique, 2015. [Google Scholar]
- Ndonda Makemba, R.; Moupela, C.; Tosso, F.; Brostaux, Y.; Drouet, T.; Oslisly, R.; Freycon, V.; Doucet, J.-L. New Evidence on the Role of Past Human Activities and Edaphic Factors on the Fine-Scale Distribution of an Important Timber Species: Cylicodiscus Gabunensis Harms. For. Ecol. Manag. 2022, 521, 120440. [Google Scholar] [CrossRef]
- Ndonda Makemba, R.; Tosso, D.F.; Moupela, C.; Daïnou, K.; Doucet, J.-L. Cylicodiscus gabunensis Harms: Une espèce prisée dans le commerce international (synthèse bibliographique). Biotechnol. Agron. Société Environ. 2019, 23, 188–202. [Google Scholar] [CrossRef]
- Daïnou, K.; Tosso, D.F.; Bracke, C.; Bourland, N.; Forni, E.; Hubert, D.; Kankolongo, M.; Loumeto, J.J.; Louppe, D.; Ngomanda, A. Guide Pratique Des Plantations d’arbres Des Forêts Denses Humides d’Afrique; Les Presses Universitaires de Liège: Agronomie Gembloux, Belgique, 2021. [Google Scholar]
- Eba’a Atyi, R.; Hiol Hiol, F.; Lescuyer, G.; Mayaux, P.; Defourny, P.; Bayol, N.; Saracco, F.; Pokem, D.; Sufo Kankeu, R.; Nasi, R. Les forêts du bassin du Congo: État des Forêts 2021; Center for International Forestry Research (CIFOR): Bogor Regency, Indonesia, 2022. [Google Scholar]
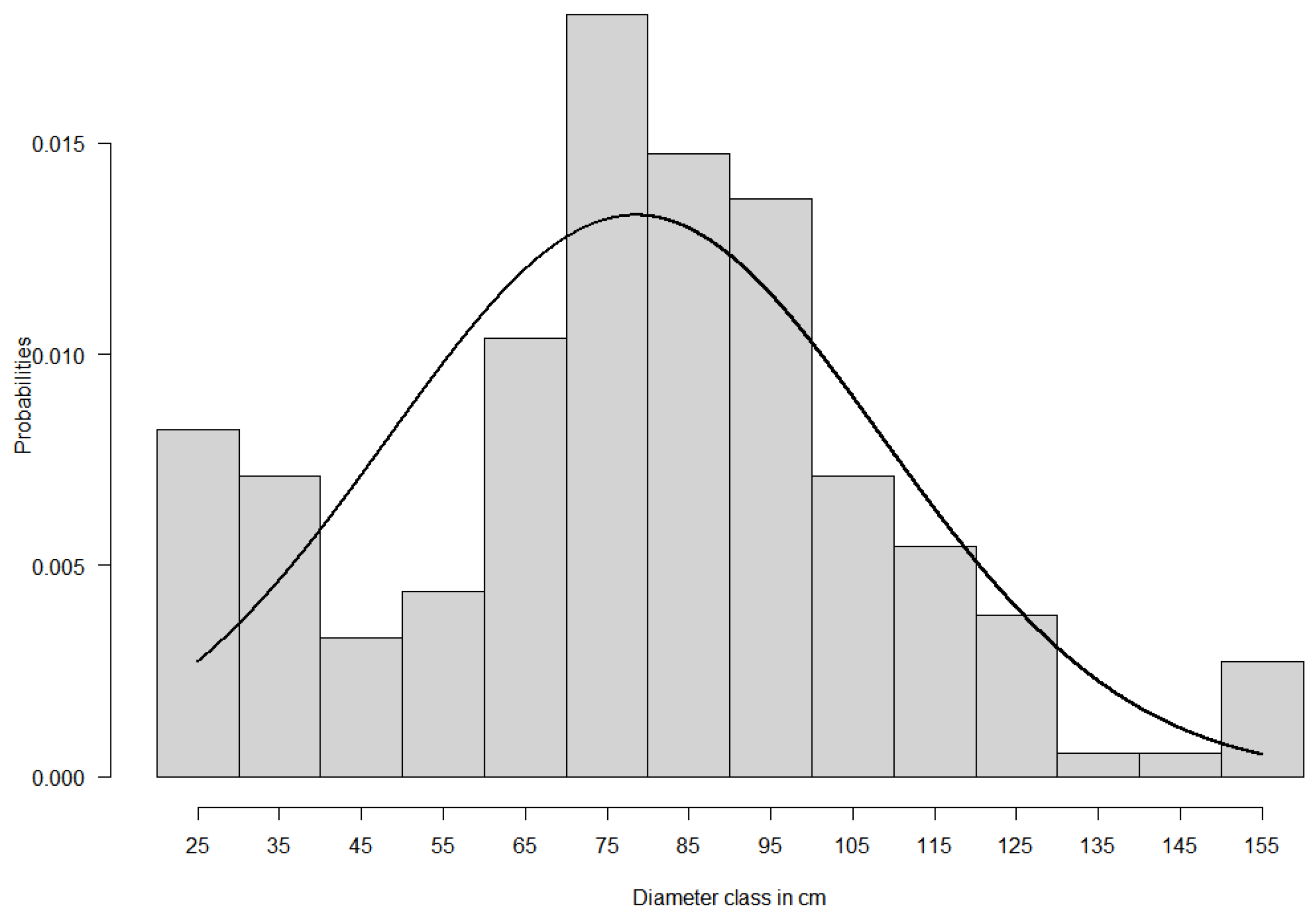
- Obiang, N.L.E.; Ngomanda, A.; Hymas, O.; Chézeauxl, É.; Picard, N. Diagnosing the Demographic Balance of Two Light-Demanding Tree Species Populations in Central Africa from Their Diameter Distribution. For. Ecol. Manag. 2014, 313, 55–62. [Google Scholar] [CrossRef]
- Morin-Rivat, J.; Fayolle, A.; Favier, C.; Bremond, L.; Gourlet-Fleury, S.; Bayol, N.; Lejeune, P.; Beeckman, H.; Doucet, J.-L. Present-Day Central African Forest Is a Legacy of the 19th Century Human History. Elife 2017, 6, e20343. [Google Scholar] [CrossRef]
- Doucet, J.-L.; Kouadio, Y.L. Le Moabi, Une Espèce «phare» de l’exploitation Forestière En Afrique Centrale. Parcs Réserves 2007, 62, 25–31. [Google Scholar]
- FRMi. Vision Stratégique et Industrialisation de La Filière Bois Dans Les 6 Pays Du Bassin Du Congo, Horizon 2030; Rapport Stratégique Régional; FRMi: Montpellier, France, 2018. [Google Scholar]
- Doucet, J.-L.; Kouadio, Y.L.; Monticelli, D.; Lejeune, P. Enrichment of Logging Gaps with Moabi (Baillonella Toxisperma Pierre) in a Central African Rain Forest. For. Ecol. Manag. 2009, 258, 2407–2415. [Google Scholar] [CrossRef]
- Grogan, J.; Landis, R.M.; Free, C.M.; Schulze, M.D.; Lentini, M.; Ashton, M.S. Big-Leaf Mahogany S Wietenia Macrophylla Population Dynamics and Implications for Sustainable Management. J. Appl. Ecol. 2014, 51, 664–674. [Google Scholar] [CrossRef]
- Marien, J.-N.; Mallet, B. Nouvelles Perspectives Pour Les Plantations Forestières En Afrique Centrale. 2004. Available online: https://agritrop.cirad.fr/522570/ (accessed on 29 September 2022).
- Doucet, J.-L.; Daïnou, K.; Ligot, G.; Ouédraogo, D.-Y.; Bourland, N.; Ward, S.E.; Tekam, P.; Lagoute, P.; Fayolle, A. Enrichment of Central African Logged Forests with High-Value Tree Species: Testing a New Approach to Regenerating Degraded Forests. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2016, 12, 83–95. [Google Scholar] [CrossRef]
- Ouédraogo, D.-Y.; Fayolle, A.; Daïnou, K.; Demaret, C.; Bourland, N.; Lagoute, P.; Doucet, J.-L. Enrichment of Logging Gaps with a High Conservation Value Species (Pericopsis Elata) in a Central African Moist Forest. Forests 2014, 5, 3031–3047. [Google Scholar] [CrossRef]
- Brunck, F. Parasites Des Plantations Forestières d’Afrique Tropicale et de Madagascar et Mesures de Protection. BOIS FORETS Trop. 1965, 103, 17–25. [Google Scholar]
- Brunck, F.; Grison, F.; Maître, H.-F. L’okoumé Aucoumea klaineana Pierre: Monographie; JOUVE; CIRAD-CTFT: Nogent-sur-Marne Cedex, France, 1990; ISBN 978-2-85411-015-9. [Google Scholar]
- Fuhr, M.; Delègue, M.-A.; Nasi, R.; Minkoué, J.-M. Dynamique et Croissance de l’Okoumé En Zone Côtière Du Gabon; CIRAD-Forêt: Montpellier, France, 1998; Volume 16. [Google Scholar]
- TEREA. Plan d’aménagement de La Concession Forestière Sous Aménagement Durable de La Société Precious Woods -CEB 2000-2024. CEB; TEREA: Libreville, Gabon, 2015. [Google Scholar]
- Moupela, C.; Doucet, J.-L.; Daïnou, K.; Tagg, N.; Bourland, N.; Vermeulen, C. Dispersal and predation of diaspores of Coula edulis Baill. in an evergreen forest of Gabon. Afr. J. Ecol. 2014, 52, 88–96. [Google Scholar] [CrossRef]
- Cabaillé, G.; Fontès, J. Les Inventaires Forestiers Au Gabon: Applications à La Phytogéographie. BOIS FORETS Trop. 1978, 177, 15–33. [Google Scholar]
- Noce, S.; Caporaso, L.; Santini, M. A New Global Dataset of Bioclimatic Indicators. Sci. Data 2020, 7, 398. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Chatelin, Y.; Collinet, J.; Guichard, E.; Sala, G.-H.; Le Rouget, G. Les Sols Du Gabon: Pedogenese, Repartition et Aptitudes: Cartes a 1: 2.000. 000; ORSTOM: Paris, France, 1981. [Google Scholar]
- Jones., A.; Breuning-Madsen, H.; Brossard, M.; Dampha, A.; Deckers, J.; Dewitte, O.; Gallali, T.; Hallett, S.; Jones, R.; Kilasara, M.; et al. Soil Atlas of Africa; European Commission: Luxembourg, 2013; ISBN 978-92-79-26715-4. [Google Scholar]
- Réjou-Méchain, M.; Mortier, F.; Bastin, J.-F.; Cornu, G.; Barbier, N.; Bayol, N.; Bénédet, F.; Bry, X.; Dauby, G.; Deblauwe, V. Unveiling African Rainforest Composition and Vulnerability to Global Change. Nature 2021, 593, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-Resolution Global Maps of 21st-Century Forest Cover Change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [PubMed]
- De La Mensbruge, G. La Germination et Les Plantules Des Essences Arborées de La Forêt Dense Humide de La Côte d’Ivoire. Publication N°26.; Centre Technique Forestier Tropical: Nogent-sur-Marne, France , 1966. [Google Scholar]
- Onguene, N.A.; Kuyper, T.W. Mycorrhizal Associations in the Rain Forest of South Cameroon. For. Ecol. Manag. 2001, 140, 277–287. [Google Scholar] [CrossRef]
- Tedersoo, L.; Laanisto, L.; Rahimlou, S.; Toussaint, A.; Hallikma, T.; Pärtel, M. Global Database of Plants with Root-Symbiotic Nitrogen Fixation: NodDB. J. Veg. Sci. 2018, 29, 560–568. [Google Scholar] [CrossRef]
- Duah-Gyamfi, A.; Kyereh, B.; Adam, K.A.; Agyeman, V.K.; Swaine, M.D. Natural Regeneration Dynamics of Tree Seedlings on Skid Trails and Tree Gaps Following Selective Logging in a Tropical Moist Semi-Deciduous Forest in Ghana. Open J. For. 2014, 04, 49–57. [Google Scholar] [CrossRef]
- Lefebvre, D.; Román-Dañobeytia, F.; Soete, J.; Cabanillas, F.; Corvera, R.; Ascorra, C.; Fernandez, L.E.; Silman, M. Biochar Effects on Two Tropical Tree Species and Its Potential as a Tool for Reforestation. Forests 2019, 10, 678. [Google Scholar] [CrossRef]
- Ligot, G.; Mackels, B. Photographies Hémisphériques; Université de Liège: Gembloux, Belgique, 2011; p. 27. [Google Scholar]
- Adam, B.; Benoit, J.C.; Sinoquet, H.; Balandier, P.; Marquier, A. PiafPHOTHEM: Un Logiciel de Seuillage de Photographies Hémisphériques. Centre Technique Forestier Tropical: Nogent-sur-Vernisson, France, 2013; p. 7. [Google Scholar]
- Frazer, G.W.; Canham, C.D.; Lertzman, K.P. Gap Light Analyzer (GLA): Imaging Software to Extract Canopy Structure and Gap Light Transmission Indices from True-Colour Fisheye Photographs, Users Manual and Program Documentation. 1999. Available online: https://www.scienceopen.com/document?vid=4956bf51-5c3a-45ce-bf58-7d9e0f903afb (accessed on 29 September 2022).
- Caravaggi, A.; Gatta, M.; Vallely, M.-C.; Hogg, K.; Freeman, M.; Fadaei, E.; Dick, J.T.; Montgomery, W.I.; Reid, N.; Tosh, D.G. Seasonal and Predator-Prey Effects on Circadian Activity of Free-Ranging Mammals Revealed by Camera Traps. PeerJ 2018, 6, e5827. [Google Scholar] [CrossRef]
- O’Brien, T.G.; Kinnaird, M.F.; Wibisono, H.T. Crouching Tigers, Hidden Prey: Sumatran Tiger and Prey Populations in a Tropical Forest Landscape. In Proceedings of the Animal Conservation Forum; Cambridge University Press: Cambridge, UK, 2003; pp. 131–139. [Google Scholar]
- Tobler, M. Camera Base Version 1.7, User Guide. 2015. Available online: https://Www.Atrium-Biodiversity.Org/Tools/Camerabase/ (accessed on 13 May 2022).
- Greenberg, S. Timelapse Quick Start Guide. A Minimalist Guide to Downloading and Using Timelapse to Tag Image and Video Files. 20 May. Available online: https://Saul.Cpsc.Ucalgary.ca/Timelapse/Pmwiki.Php?N=Main.Download2 (accessed on 20 May 2022).
- Meek, P.D.; Ballard, G.; Claridge, A.; Kays, R.; Moseby, K.; O’brien, T.; O’connell, A.; Sanderson, J.; Swann, D.E.; Tobler, M. Recommended Guiding Principles for Reporting on Camera Trapping Research. Biodivers. Conserv. 2014, 23, 2321–2343. [Google Scholar] [CrossRef]
- Kingdon, J. The Kingdon Field Guide to African Mammals, 2nd ed.; Bloomsbury Publishing: London, UK, 2015. [Google Scholar]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: Berlin/Heidelberg, Germany, 2001; Volume 608. [Google Scholar]
- Goel, M.K.; Khanna, P.; Kishore, J. Understanding Survival Analysis: Kaplan-Meier Estimate. Int. J. Ayurveda Res. 2010, 1, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Tosso, F.; Cherchye, G.; Hardy, O.J.; Daïnou, K.; Lognay, G.; Tagg, N.; Haurez, B.; Souza, A.; Heuskin, S.; Doucet, J.-L. Characterization of Animal Communities Involved in Seed Dispersal and Predation of Guibourtia Tessmannii (Harms) J.Léonard, a Species Newly Listed on Appendix II of CITES. Afr. J. Ecol. 2018, 56, 468–476. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. arXiv 2014, arXiv:Prepr. ArXiv14065823. [Google Scholar]
- Zhang, H.; Lu, N.; Feng, C.; Thurston, S.W.; Xia, Y.; Zhu, L.; Tu, X.M. On Fitting Generalized Linear Mixed-Effects Models for Binary Responses Using Different Statistical Packages. Stat. Med. 2011, 30, 2562–2572. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Lenth, R.V.; Buerkner, P.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Riebl, H.; Singmann, H. Package ‘Emmeans’:Estimated Marginal Means, Aka Least-Squares Means. Version 1.8.1-1. Available online: https://www.google.com/search?q=emmeans+package+r&oq=emmeans+package+r&aqs=chrome.0.69i59j0i22i30j69i60l3.8130j0j7&sourceid=chrome&ie=UTF-8 (accessed on 13 September 2022).
- Opuni-Frimpong, E.; Opoku, S.M.; Opoku, E.M.; Storer, A.J. Silvicultural Systems for Plantation Mahogany in Africa: Effect of Mixed Species Stands on Growth and Hypsipyla Attack of African Mahogany (Khaya Anthotheca and Khaya Ivorensis). Ghana J. For. 2020, 36, 72–88. [Google Scholar]
- Bourland, N.; Cerisier, F.; Daïnou, K.; Livingstone Smith, A.; Hubau, W.; Beeckman, H.; Brostaux, Y.; Fayolle, A.; Biwolé, A.B.; Fétéké, F. How Tightly Linked Are Pericopsis Elata (Fabaceae) Patches to Anthropogenic Disturbances in Southeastern Cameroon? Forests 2015, 6, 293–310. [Google Scholar] [CrossRef]
- Sileshi, G.; Akinnifesi, F.K.; Mkonda, A.; Ajayi, O.C. Effect of Growth Media and Fertilizer Application on Biomass Allocation and Survival of Uapaca Kirkiana Müell Arg Seedlings. Sci Res Essays 2007, 2, 408–415. [Google Scholar]
- Román-Dañobeytia, F.; Huayllani, M.; Michi, A.; Ibarra, F.; Loayza-Muro, R.; Vázquez, T.; Rodríguez, L.; García, M. Reforestation with Four Native Tree Species after Abandoned Gold Mining in the Peruvian Amazon. Ecol. Eng. 2015, 85, 39–46. [Google Scholar] [CrossRef]
- Schwartz, G.; Lopes, J.C.A.; Mohren, G.M.J.; Peña-Claros, M. Post-Harvesting Silvicultural Treatments in Logging Gaps: A Comparison between Enrichment Planting and Tending of Natural Regeneration. For. Ecol. Manag. 2013, 293, 57–64. [Google Scholar] [CrossRef]
- Leroy-Deval, J. La Sylviculture de l’Okoumé (Maladies et Défauts de l’Okoumé). CENTRE TECHNIQUE FORESTIER TROPICAL 1976, Tome 2, 75. [Google Scholar]
- Fayolle, A.; Ouedraogo, D.-Y.; Ligot, G.; Daïnou, K.; Bourland, N.; Tekam, P.; Doucet, J.-L. Differential Performance between Two Timber Species in Forest Logging Gaps and in Plantations in Central Africa. Forests 2015, 6, 380–394. [Google Scholar] [CrossRef]
- Poulsen, J.R.; Koerner, S.E.; Moore, S.; Medjibe, V.P.; Blake, S.; Clark, C.J.; Akou, M.E.; Fay, M.; Meier, A.; Okouyi, J. Poaching Empties Critical Central African Wilderness of Forest Elephants. Curr. Biol. 2017, 27, R134–R135. [Google Scholar] [CrossRef]
- Short, J. Diet and Feeding Behaviour of the Forest Elephant. 1981. Available online: https://www.degruyter.com/document/doi/10.1515/mamm.1981.45.2.177/html (accessed on 29 September 2022).
- Scalbert, M.; Vermeulen, C.; Breuer, T.; Doucet, J.-L. The Challenging Coexistence of Forest Elephants Loxodonta Cyclotis and Timber Concessions in Central Africa. Mammal Rev. 2022. [Google Scholar] [CrossRef]
- Holvoet, J. Ecorcement des espèces ligneuses par l’éléphant de forêt (Loxodonta cyclotis Matschie, 1900) dans le Parc National de la Lopé (Gabon); Mémoire; Gembloux Agro-Bio Tech (Université de Liège): Gembloux, Belgium, 2021. [Google Scholar]
- Ngama, S. Introduction to Elephant Ecophysiology: Principles, Methods and Case Studies on Forest Elephant (Loxodonta Africana Cyclotis) Crop Raiders in Gabon. Ph.D. Thesis, Gembloux Agro-Bio Tech (University of Liège), Gembloux, Belgium, 2018. [Google Scholar]
- Chapman, C.A.; Chapman, L.J. Forest Regeneration in Logged and Unlogged Forests of Kibale National Park, Uganda. Biotropica 1997, 29, 396–412. [Google Scholar] [CrossRef]
- Omeja, P.A.; Lawes, M.J.; Corriveau, A.; Valenta, K.; Sarkar, D.; Paim, F.P.; Chapman, C.A. Recovery of Tree and Mammal Communities during Large-Scale Forest Regeneration in Kibale National Park, Uganda. Biotropica 2016, 48, 770–779. [Google Scholar] [CrossRef]
- Lawes, M.J.; Chapman, C.A. Does the Herb Acanthus Pubescens and/or Elephants Suppress Tree Regeneration in Disturbed Afrotropical Forest? For. Ecol. Manag. 2006, 221, 278–284. [Google Scholar] [CrossRef]
- Piiroinen, T.; Valtonen, A.; Roininen, H. Vertebrate Herbivores Are the Main Cause of Seedling Mortality in a Logged African Rainforest—Implications for Forest Restoration. Restor. Ecol. 2017, 25, 442–452. [Google Scholar] [CrossRef]
- Rosin, C.; Beals, K.K.; Belovitch, M.W.; Harrison, R.E.; Pendred, M.; Sullivan, M.K.; Yao, N.; Poulsen, J.R. Assessing the Effects of Elephant Foraging on the Structure and Diversity of an Afrotropical Forest. Biotropica 2020, 52, 502–508. [Google Scholar] [CrossRef]
- Wing, L.D.; Buss, I.O. Elephants and Forests. Wildl. Monogr. 1970, 3–92. [Google Scholar]
- Gill, R.M.A.; Webber, J.; Peace, A. The Economic Implications of Deer Damage: A Review of Current Evidence. For. Res. Agency Wrecclesham Surrey 2000, 1–49. [Google Scholar]
- Bernes, C.; Macura, B.; Jonsson, B.G.; Junninen, K.; Müller, J.; Sandström, J.; Lõhmus, A.; Macdonald, E. Manipulating Ungulate Herbivory in Temperate and Boreal Forests: Effects on Vegetation and Invertebrates. A Systematic Review. Environ. Evid. 2018, 7, 13. [Google Scholar] [CrossRef]
- Ramirez, J.I.; Jansen, P.A.; den Ouden, J.; Goudzwaard, L.; Poorter, L. Long-Term Effects of Wild Ungulates on the Structure, Composition and Succession of Temperate Forests. For. Ecol. Manag. 2019, 432, 478–488. [Google Scholar] [CrossRef]
- Ramirez, J.I.; Jansen, P.A.; Poorter, L. Effects of Wild Ungulates on the Regeneration, Structure and Functioning of Temperate Forests: A Semi-Quantitative Review. For. Ecol. Manag. 2018, 424, 406–419. [Google Scholar] [CrossRef]

| Species | Total Number of Detections | Average Number of Visits | Average Duration of Visits (min) |
|---|---|---|---|
| Cephalophus sp. | 146 | 4.87 ± 4.39 | 1.5 ± 0.71 |
| Cephalophus silvicultor | 76 | 2.53 ± 3.18 | 3.4 ± 2.88 |
| Gorilla gorilla | 14 | 0.47 ± 0.73 | 1.25 ± 0.5 |
| Loxodonta cyclotis | 64 | 2.13 ± 1.57 | 5.8 ± 4.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makemba, R.N.; Tosso, F.; Moupela, C.; Ligot, G.; Brostaux, Y.; Doucet, J.-L. Enrichment of Logging Gaps with High-Value Timber Species: How Far Fertilizer, Biochar and Mammal Predation Affect Performances of Cylicodiscus gabunensis Harms Seedlings. Forests 2022, 13, 1937. https://doi.org/10.3390/f13111937
Makemba RN, Tosso F, Moupela C, Ligot G, Brostaux Y, Doucet J-L. Enrichment of Logging Gaps with High-Value Timber Species: How Far Fertilizer, Biochar and Mammal Predation Affect Performances of Cylicodiscus gabunensis Harms Seedlings. Forests. 2022; 13(11):1937. https://doi.org/10.3390/f13111937
Chicago/Turabian StyleMakemba, Romaric Ndonda, Félicien Tosso, Christian Moupela, Gauthier Ligot, Yves Brostaux, and Jean-Louis Doucet. 2022. "Enrichment of Logging Gaps with High-Value Timber Species: How Far Fertilizer, Biochar and Mammal Predation Affect Performances of Cylicodiscus gabunensis Harms Seedlings" Forests 13, no. 11: 1937. https://doi.org/10.3390/f13111937
APA StyleMakemba, R. N., Tosso, F., Moupela, C., Ligot, G., Brostaux, Y., & Doucet, J.-L. (2022). Enrichment of Logging Gaps with High-Value Timber Species: How Far Fertilizer, Biochar and Mammal Predation Affect Performances of Cylicodiscus gabunensis Harms Seedlings. Forests, 13(11), 1937. https://doi.org/10.3390/f13111937






