Pre-Carpels from the Middle Triassic of Spain
Abstract
:1. Introduction
2. Results
2.1. Systematic Palaeontology
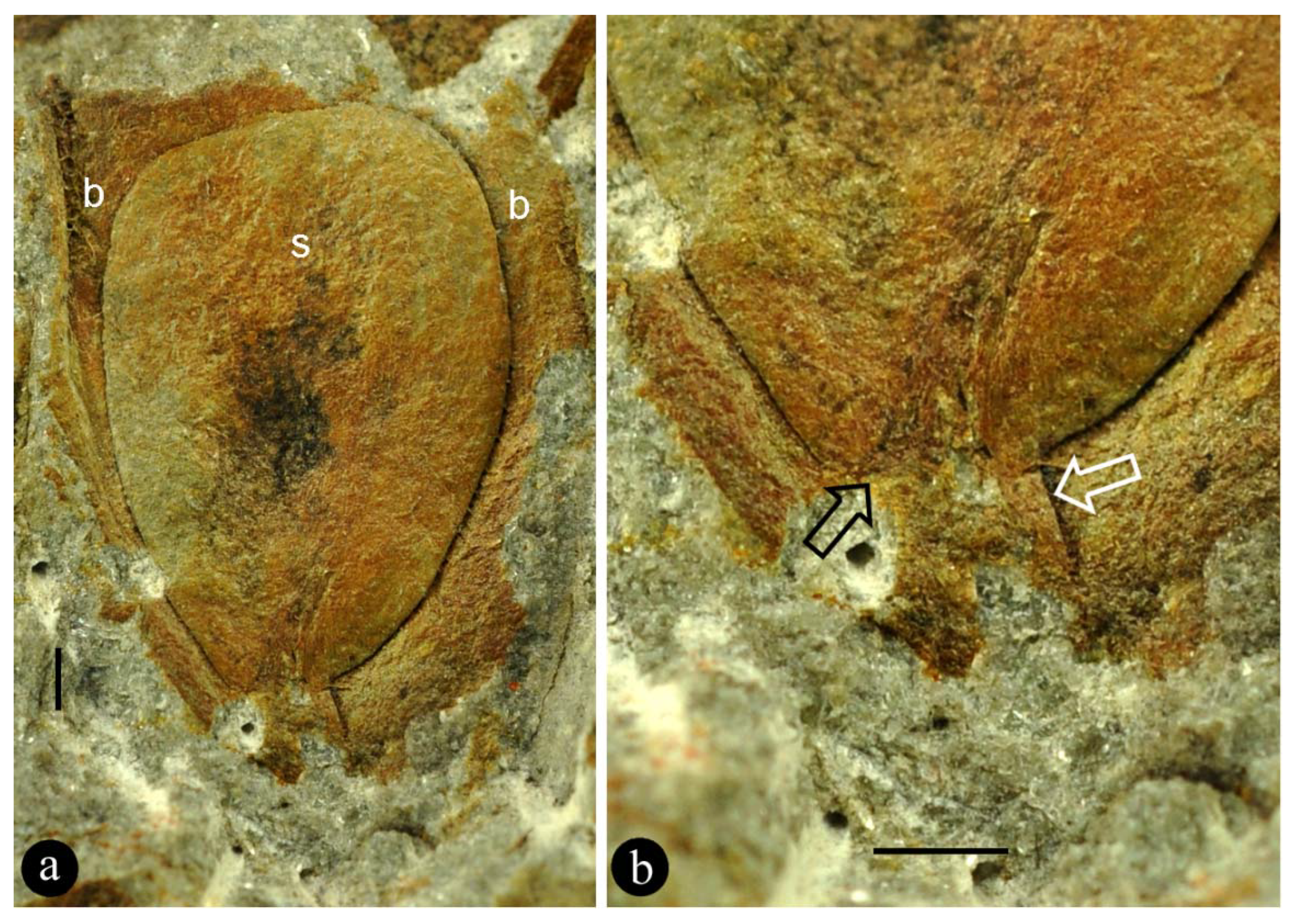
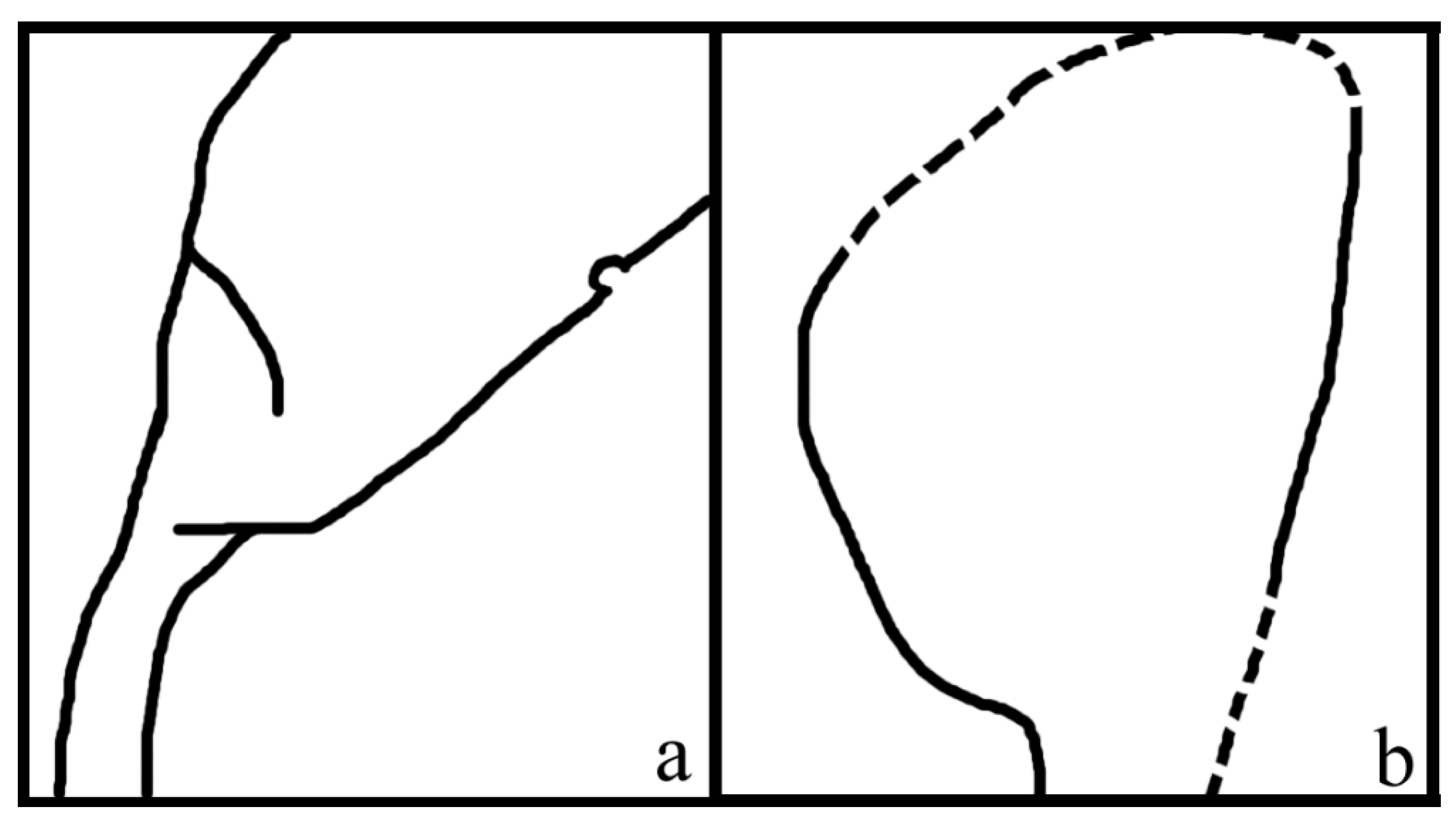
2.1.1. Combina gen. nov.
2.1.2. Combina triassica gen. et sp. nov.
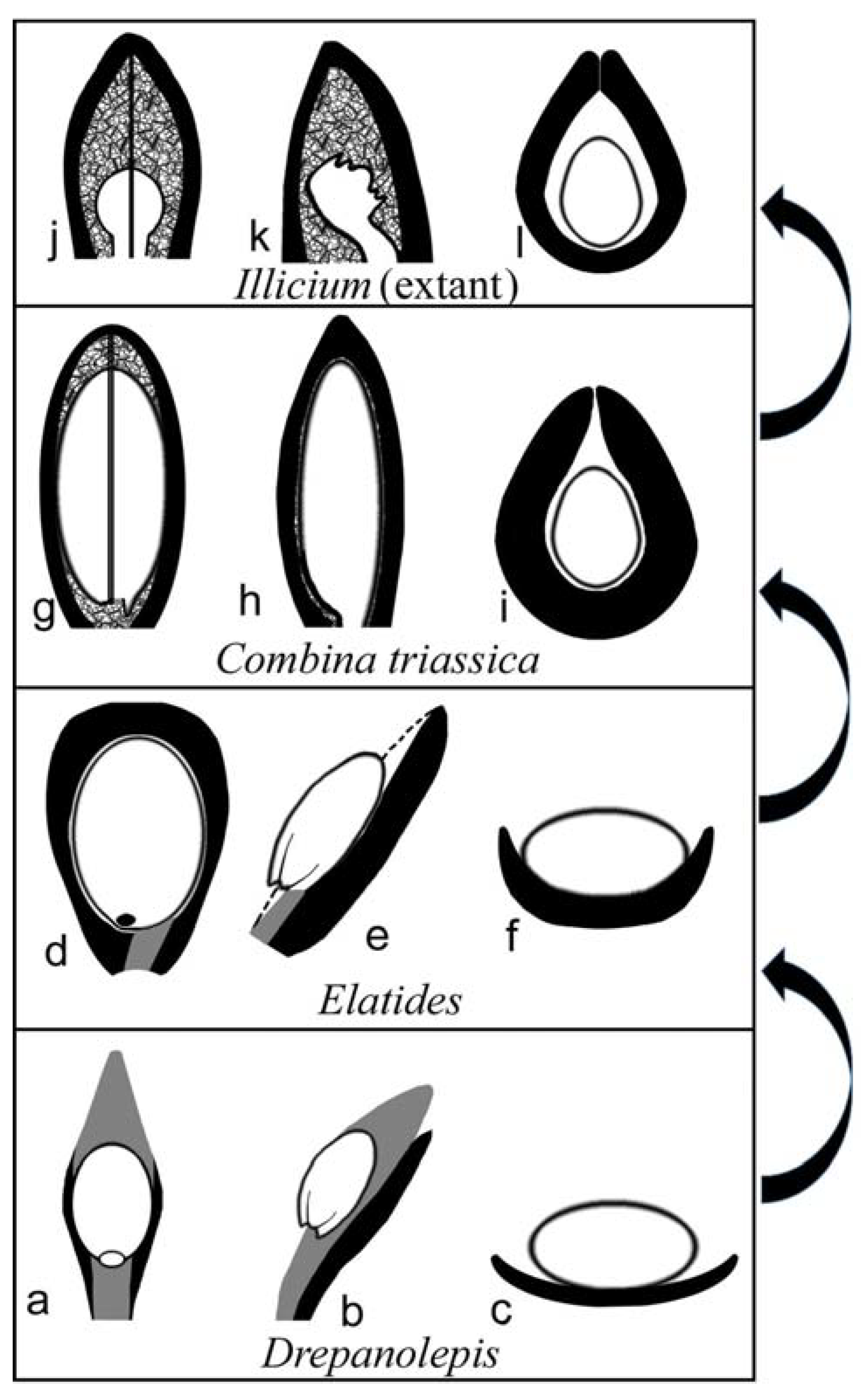
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sauquet, H.; von Balthazar, M.; Magallón, S.; Doyle, J.A.; Endress, P.K.; Bailes, E.J.; Barroso de Morais, E.; Bull-Hereñu, K.; Carrive, L.; Chartier, M.; et al. The ancestral flower of angiosperms and its early diversification. Nat. Commun. 2017, 8, 16047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.-T.; Yi, T.-S.; Gao, L.-M.; Ma, P.-F.; Zhang, T.; Yang, J.-B.; Gitzendanner, M.A.; Fritsch, P.W.; Cai, J.; Luo, Y.; et al. Origin of angiosperms and the puzzle of the Jurassic gap. Nat. Plants 2019, 5, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.W.; Kirchner, G. The origin and evolution of the angiosperm carpel. In Flowering Plant Origin, Evolution and Phylogeny; Taylor, D.W., Hickey, L.J., Eds.; Chapman AND Hall: New York, NY, USA, 1996; pp. 116–140. [Google Scholar]
- Arber, E.A.N.; Parkin, J. On the origin of angiosperms. J. Linn. Soc. Lond. Bot. 1907, 38, 29–80. [Google Scholar] [CrossRef] [Green Version]
- Eames, A.J. Morphology of the Angiosperms; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1961; p. 518. [Google Scholar]
- Cronquist, A. The Evolution and Classification of Flowering Plants, 2nd ed.; New York Botanical Garden: New York, NY, USA, 1988; p. 555. [Google Scholar]
- Takhtajan, A. Flowering Plants, Origin and Dispersal; Oliver AND Boyd Ltd.: Edinburgh, UK, 1969; p. 310. [Google Scholar]
- Qiu, Y.-L.; Lee, J.; Bernasconi-Quadroni, F.; Soltis, D.E.; Soltis, P.S.; Zanis, M.; Zimmer, E.A.; Chen, Z.; Savolainen, V.; Chase, M.W. The earliest angiosperms: Evidence from mitochondrial, plastid and nuclear genomes. Nature 1999, 402, 404–407. [Google Scholar] [CrossRef]
- Endress, P.K.; Doyle, J.A. Reconstructing the ancestral angiosperm flower and its initial specializations. Am. J. Bot. 2009, 96, 22–66. [Google Scholar] [CrossRef]
- Sokoloff, D.D.; Remizowa, M.V.; El, E.S.; Rudall, P.J.; Bateman, R.M. Supposed Jurassic angiosperms lack pentamery, an important angiosperm-specific feature. New Phytol. 2020, 228, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Coiro, M.; Doyle, J.A.; Hilton, J. How deep is the conflict between molecular and fossil evidence on the age of angiosperms? New Phytol. 2019, 223, 83–99. [Google Scholar] [CrossRef] [Green Version]
- Bateman, R.M. Hunting the snark: The flawed search for mythical Jurassic angiosperms. J. Exp. Bot. 2020, 71, 22–35. [Google Scholar] [CrossRef]
- Fu, Q.; Diez, J.B.; Pole, M.; García-Ávila, M.; Wang, X. Nanjinganthus is an angiosperm, isn’t it? China Geol. 2020, 3, 359–361. [Google Scholar] [CrossRef]
- Fu, Q.; Diez, J.B.; Pole, M.; Garcia-Avila, M.; Liu, Z.-J.; Chu, H.; Hou, Y.; Yin, P.; Zhang, G.-Q.; Du, K.; et al. An unexpected noncarpellate epigynous flower from the Jurassic of China. eLife 2018, 7, e38827. [Google Scholar] [CrossRef]
- Taylor, D.W.; Li, H. Paleobotany: Did flowering plants exist in the Jurassic period? eLife 2018, 7, e43421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Floral ontogeny of Illicium lanceolatum (Schisandraceae) and its implications on carpel homology. Phytotaxa 2019, 416, 200–210. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Wang, X. How the ovules get enclosed in magnoliaceous carpels. PLoS ONE 2017, 12, e0174955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diez, J.B.; Broutin, J.; Grauvogel-Stamm, L.; Bourquin, S.; Bercovici, A.; Ferrer, J. Anisian floras from the NE Iberian Peninsula and Balearic Islands: A synthesis. Rev. Palaeobot. Palynol. 2010, 162, 522–542. [Google Scholar] [CrossRef]
- Schweitzer, H.-J.; Kirchner, M. Die Rhaeto-Jurassischen Floren des Iran und Afghanistans: 9. Coniferophyta. Paläontographica B 1996, 238, 77–139. [Google Scholar]
- Rao, A.R.; Bose, M.N. Further observations on Ontheodendron florini Sahni & Rao. Palaeobotanist 1958, 7, 29–31. [Google Scholar]
- Bose, M.N.; Maheshwari, H.K. The genus Ontheodendron Sahni and Rao. Palaeobotanist 1974, 23, 231–232. [Google Scholar]
- Fu, Q.; Liu, J.; Wang, X. Offspring development conditioning (ODC): A universal evolutionary trend in sexual reproduction of organisms. J. Northwest Univ. (Nat. Sci. Ed.) 2021, 51, 163–172. [Google Scholar]
- Canright, J.E. The comparative morphology and relationships of the Magnoliaceae. III. Carpels. Am. J. Bot. 1960, 47, 145–155. [Google Scholar] [CrossRef]
- Wang, X. The Dawn Angiosperms; Springer: Heidelberg, Germany, 2010. [Google Scholar]
- Wang, X. The Dawn Angiosperms, 2nd ed.; Springer: Cham, Switzerland, 2018; p. 407. [Google Scholar]
- Wang, X.; Liu, Z.-J.; Liu, W.; Liao, W.; Zhang, X.; Liu, Z.; Hu, G.; Guo, X.; Wang, Y. Stepping out of the shadow of Goethe: For a more scientific plant systematics. Chin. Bull. Bot. 2020, 55, 505–512. [Google Scholar]
- Roe, J.L.; Nemhauser, J.L.; Zambryski, P.C. TOUSLED participates in apical tissue formation during gynoecium development in Arabidopsis. Plant Cell 1997, 9, 335–353. [Google Scholar] [PubMed] [Green Version]
- Rounsley, S.D.; Ditta, G.S.; Yanofsky, M.F. Diverse roles for MADS box genes in Arabidopsis development. Plant Cell 1995, 7, 1259–1269. [Google Scholar] [PubMed] [Green Version]
- Skinner, D.J.; Hill, T.A.; Gasser, C.S. Regulation of ovule development. Plant Cell 2004, 16 (Suppl. 1), S32–S45. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.; Kramer, E.M. The evolution of reproductive structures in seed plants: A re-examination based on insights from developmental genetics. New Phytol. 2012, 194, 910–923. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.-Z.; Hilu, K.; Wang, Y.-L. From leaf and branch into a flower: Magnolia tells the story. Bot. Stud. 2014, 55, 28. [Google Scholar] [CrossRef]
- Wang, X.; Luo, B. Mechanical pressure, not genes, makes ovulate parts leaf-like in Cycas. Am. J. Plant Sci. 2013, 4, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Liu, Z.J.; Wang, M.; Wang, X. Fossil and living cycads say “No more megasporophylls”. J. Morphol. Anat. 2017, 1, 107. [Google Scholar]
- Melville, R. A new theory of the angiosperm flower: I. The gynoecium. Kew Bull. 1962, 16, 1–50. [Google Scholar] [CrossRef]
- Taylor, D.W. Angiosperm ovule and carpels: Their characters and polarities, distribution in basal clades, and structural evolution. Postilla 1991, 208, 1–40. [Google Scholar]
- Rümpler, F.; Theißen, G. Reconstructing the ancestral flower of extant angiosperms: The ‘war of the whorls’ is heating up. J. Exp. Bot. 2019, 70, 2615–2622. [Google Scholar] [CrossRef]
- Sokoloff, D.; Remizowa, M.V.; Bateman, R.M.; Rudall, P.J. Was the ancestral angiosperm flower whorled throughout? Am. J. Bot. 2018, 105, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledford, H. Deatabe blooms over anatomy of the world’s first flower. Nature 2018, 554, 153–154. [Google Scholar] [CrossRef] [Green Version]
- APG. APG IV: An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Endress, P.K. The morphological relationship between carpels and ovules in angiosperms: Pitfalls of morphological interpretation. Bot. J. Linn. Soc. 2019, 189, 201–227. [Google Scholar] [CrossRef] [Green Version]
- Florin, R. Evolution in cordaites and conifers. Acta Horti Berginal 1951, 15, 285–388. [Google Scholar]
- Schweitzer, H.-J. Der weibliches Zapfen von Pseudovoltzia liebeana und seine Bedeutung fuer die Phylogenie der Koniferen. Paläontographica B 1963, 113, 1–29. [Google Scholar]
- Wang, Z. A bizarre Palissya ovulate organ from Upper Triassic strata of the Zixing coal field, Hunan Province, China. Chin. Sci. Bull. 2012, 57, 1169–1177. [Google Scholar] [CrossRef] [Green Version]
- Pattemore, G.A.; Rigby, J.F.; Playford, G. Palissya: A global review and reassessment of Eastern Gondwanan material. Rev. Palaeobot. Palynol. 2014, 210, 50–61. [Google Scholar] [CrossRef]
- Delevoryas, T.; Hope, R.C. More evidence for conifer diversity in the upper Triassic of North Carolina. Am. J. Bot. 1981, 68, 1003–1007. [Google Scholar] [CrossRef]
- Arndt, S. Morphologie und Systematik ausgewählter Mesozoischer Koniferen. Paläontographica B 2002, 262, 1–23. [Google Scholar]
- Kerp, H.; Bomfleur, B. Photography of plant fossils-new techniques, old tricks. Rev. Palaeobot. Palynol. 2011, 166, 117–151. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.A.; Wang, X. Pre-Carpels from the Middle Triassic of Spain. Plants 2022, 11, 2833. https://doi.org/10.3390/plants11212833
Santos AA, Wang X. Pre-Carpels from the Middle Triassic of Spain. Plants. 2022; 11(21):2833. https://doi.org/10.3390/plants11212833
Chicago/Turabian StyleSantos, Artai A., and Xin Wang. 2022. "Pre-Carpels from the Middle Triassic of Spain" Plants 11, no. 21: 2833. https://doi.org/10.3390/plants11212833




