Advances in Nanofabrication Technology for Nutraceuticals: New Insights and Future Trends
Abstract
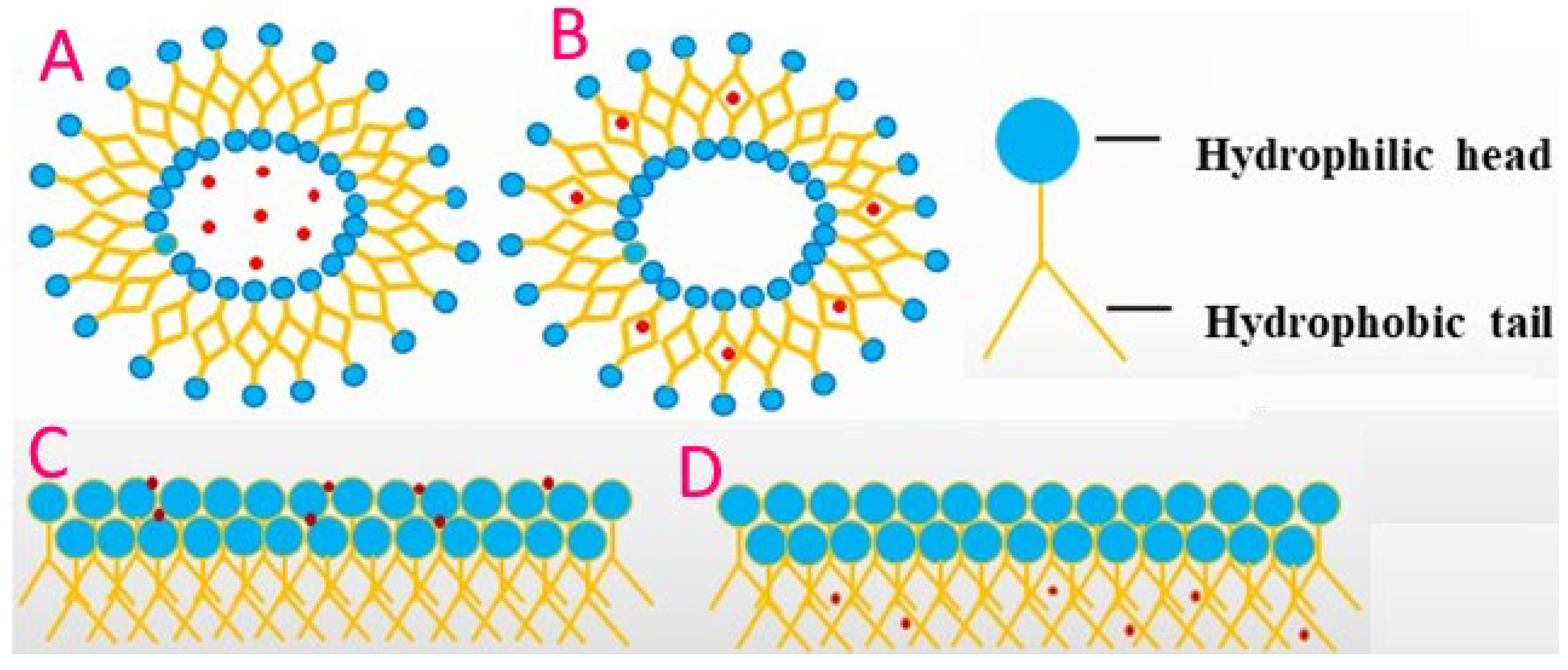
1. Introduction
| Bioactive Compounds | Health-Promoting Property | Occurrence | Molecular Weight (g/mol) | Structure | Reference |
|---|---|---|---|---|---|
| Quercetin | Promoting cardiovascular health properties and helping in blood flow. | Fruits and vegetables, especially in onions, grapes, lemon tea, citrus, etc., | 302.236 | 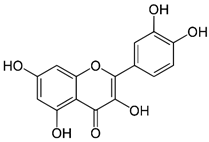 | [5] |
| Luteolin | Anticarcinogenic activity | Green pepper carrots, Broccoli, oregano | 286.24 | 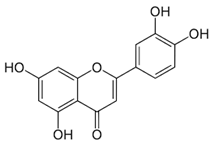 | [6] |
| Kaempferol | Antioxidant activity and Anticarcinogenic activity | Tomatoes, apples, grapes, green tea, broccoli, lettuce, peaches | 286.23 | 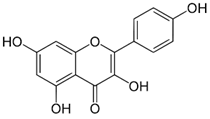 | [7] |
| Curcumin | Antibacterial activity, antioxidant activity, and anti-inflammatory activity | Turmeric | 368.38 | 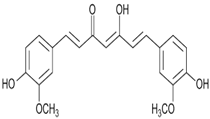 | [8] |
| Berberine | Treatment of breast cancer, colon cancer, pancreatic cancer, gastric cancer, liver cancer, oral cancer, etc., | Widely present in barks, leaves, twigs, rhizomes, roots, and stems of several medicinal plant species. | 336.3612 g/mol | 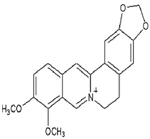 | [9] |
| Rutin& Quercetin | Protection against cancer and some other diseases. Lowers cholesterol, mainly used in skin aging. | invasive plant species, Carpobrotus edulis | 610.517 g/mol | 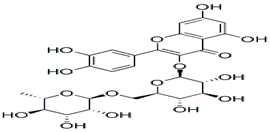 | [10] |
| Astaxanthin | Protection from UV skin damage, Reduction in inflammation, Supports Immune system. | algae, yeast, salmon, trout, krill, shrimp, and crayfish | 596.841 g/mol | 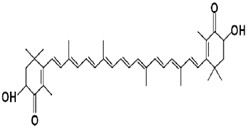 | [11] |
| Vitamin E | helps maintain healthy skin and eyes, and strengthen the body’s natural defense against illness and infection (the immune system). | Plant-based oils, nuts, seeds, fruits, and vegetables. | 430.71 g/mol | 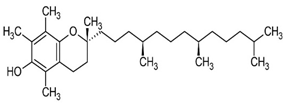 | [12] |
2. Nano Formulation of Bioactive Compounds
2.1. Nutraceuticals and Nanobiotechnology
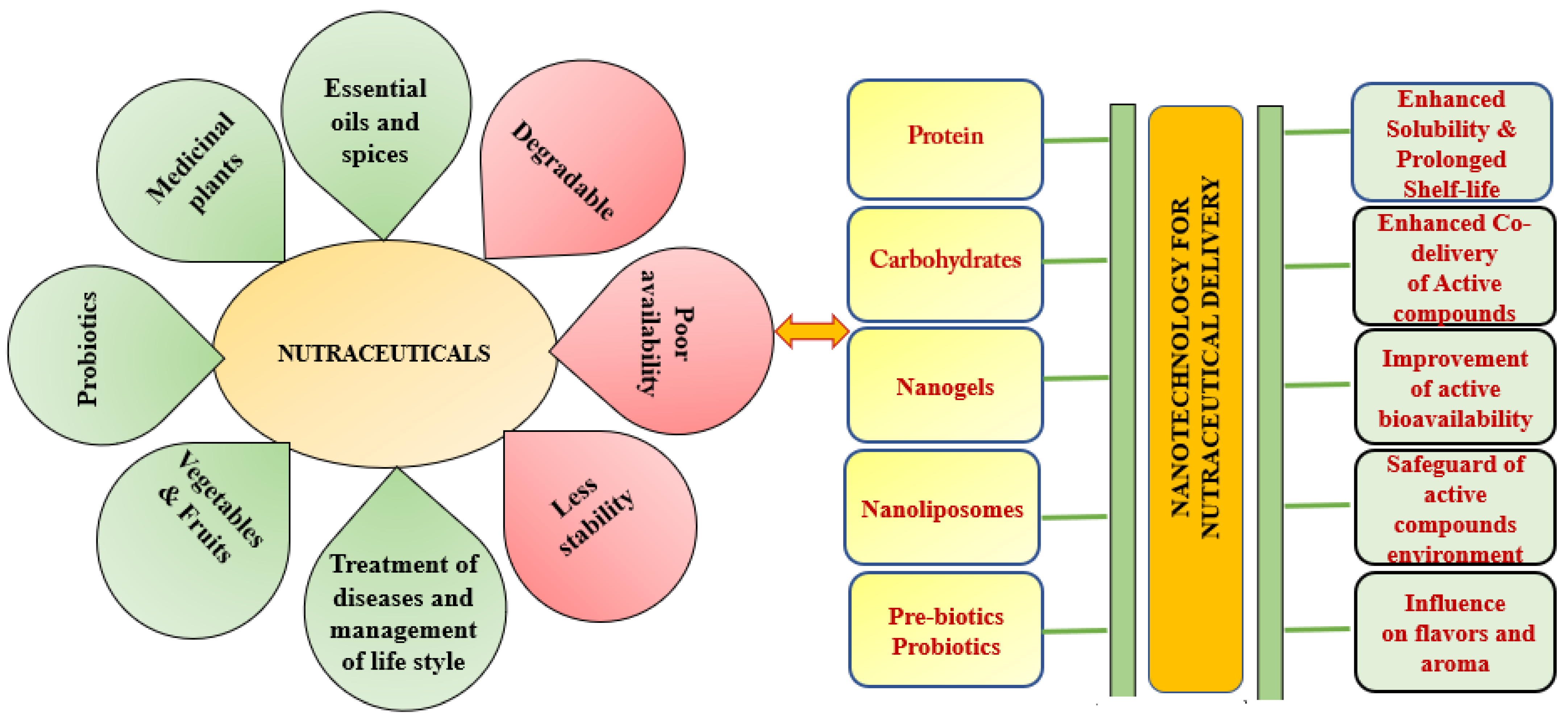
2.2. Definition and General Classification of Nutraceuticals
2.3. Dietary Supplements
2.4. Functional Foods
2.5. Medicinal Nutraceuticals and Pharmaceuticals
2.6. Role of Nutraceuticals in Human Health
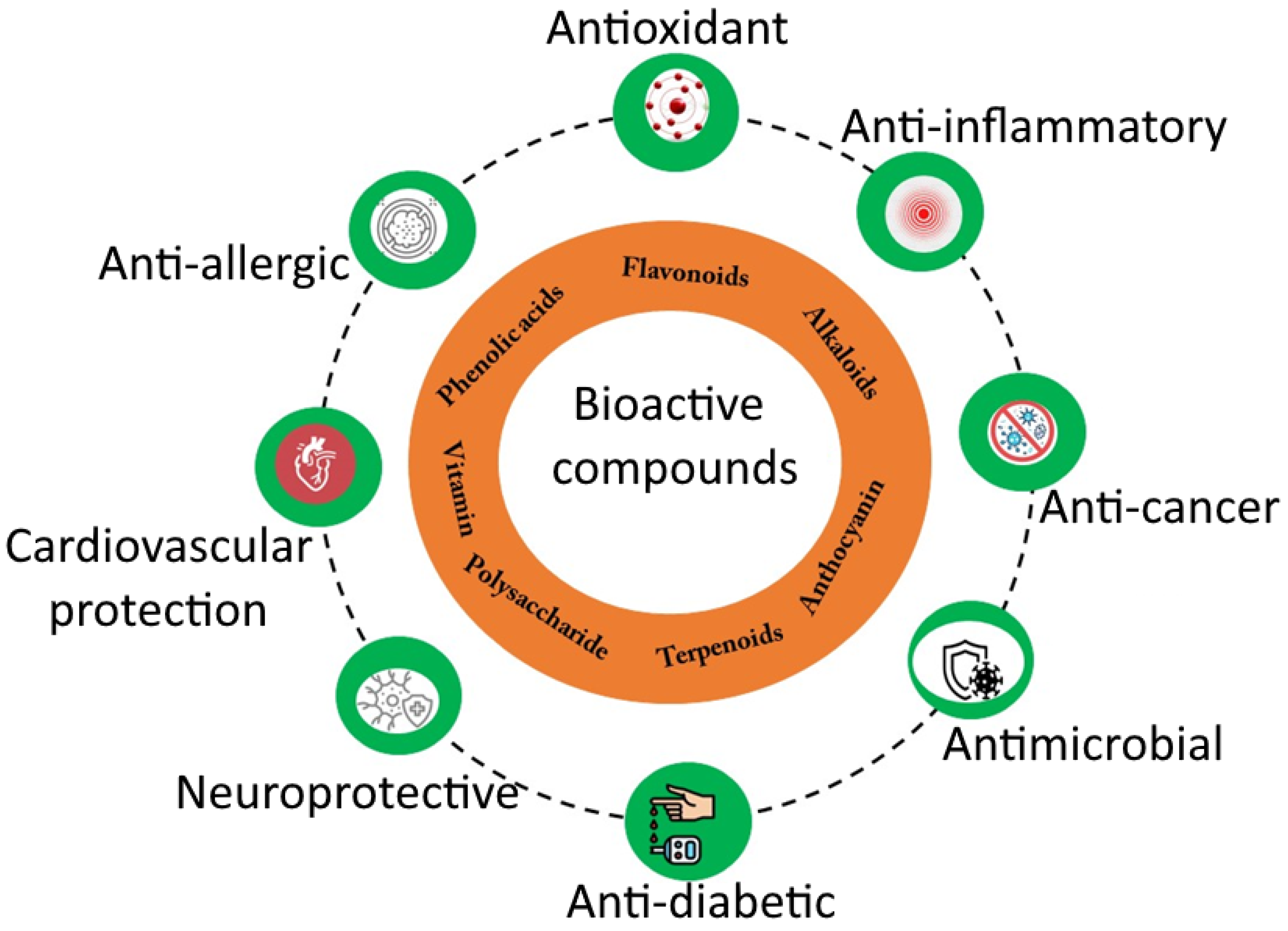
2.7. Favorable Nutraceuticals Properties on Human Health
3. Nanotechnology as a Nutraceutical Properties Enhancement Strategy
3.1. Nano Probiotics
3.2. Nano Prebiotics
3.3. Food Grade-Nanofabricated Delivery Systems
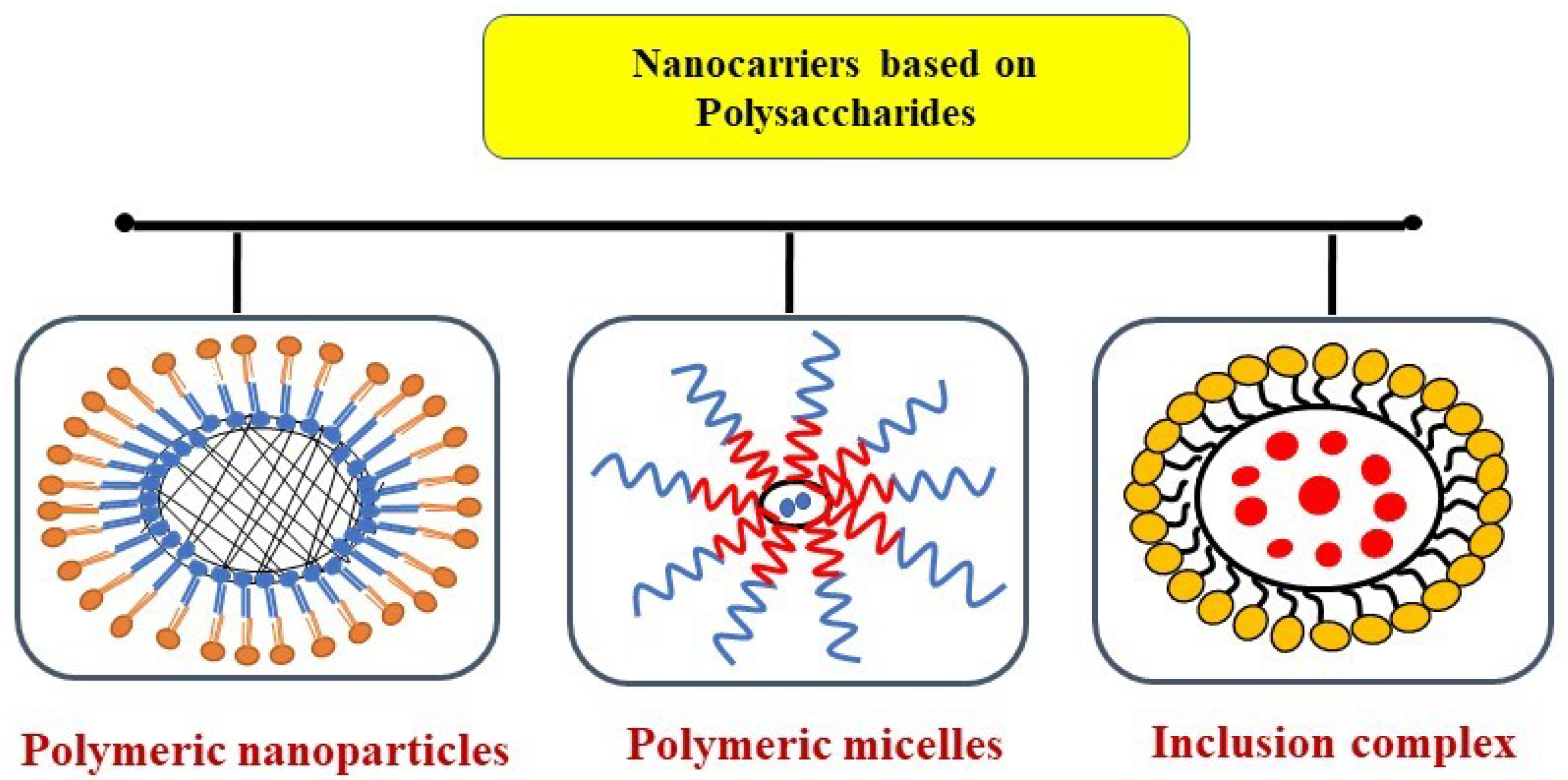
3.4. Carbohydrate/Polysaccharides Delivery Systems
3.5. Protein-Based Delivery Systems
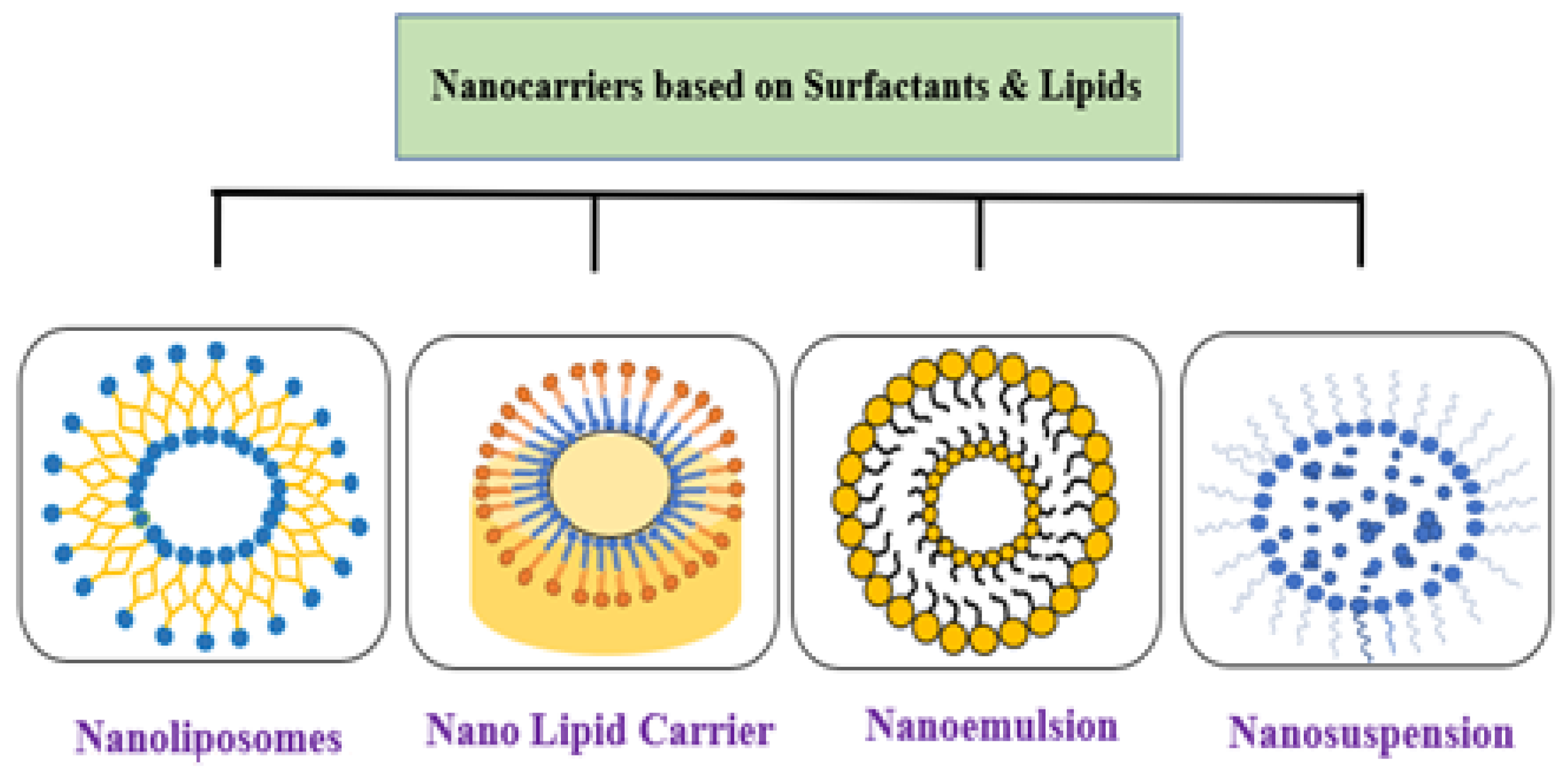
3.6. Lipid-Based Nano Delivery Systems
3.7. Nano-Emulsions
3.8. Nanoliposomes
3.9. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carrier (NLC)
3.10. Nanogels
4. Future Perspectives and Regulatory Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, Y.; Wang, Q.; Zhang, Y. Biopolymer-Based Nanotechnology Approaches to Deliver Bioactive Compounds for Food Applications: A Perspective on the Past, Present, and Future. J. Agric. Food Chem. 2020, 68, 12993–13000. [Google Scholar] [CrossRef] [PubMed]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 2019, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sarwal, A.; Rawat, N.; Singh, G.; Sinha, V.R.; Sharma, S.; Kumar, D. Nanonutraceuticals in Central Nervous System Disorders. In NanoNutraceuticals; CRC Press: Boca Raton, FL, USA, 2018; pp. 91–104. [Google Scholar] [CrossRef]
- Sasi, S. Nutraceuticals—A review. Int. J. Ind. Biotechnol. Biomater. 2017, 3, 25–29. [Google Scholar]
- Deepika; Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef] [PubMed]
- Sagi, S.S. Quercetin: A Potential Flavanol with Multiple Health Benefits. Arch. Food Sci. Nutr. Res. 2021, 2, 1002. [Google Scholar]
- Yang, L.; Gao, Y.; Bajpai, V.K.; El-Kammar, H.A.; Simal-Gandara, J.; Cao, H.; Cheng, K.W.; Wang, M.; Arroo, R.R.; Zou, L.; et al. Advance toward isolation, extraction, metabolism and health benefits of kaempferol, a major dietary flavonoid with future perspectives. Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef]
- Guest, P.C.; Sahebkar, A. Research in the Middle East into the Health Benefits of Curcumin. In Studies on Biomarkers and New Targets in Aging Research in Iran; Springer: Cham, Switzerland, 2021; pp. 1–13. [Google Scholar] [CrossRef]
- Xu, X.; Yi, H.; Wu, J.; Kuang, T.; Zhang, J.; Li, Q.; Du, H.; Xu, T.; Jiang, G.; Fan, G. Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence. Biomed. Pharmacother. 2021, 133, 110984. [Google Scholar] [CrossRef]
- Zhou, L.; Cai, L.; Ruan, H.; Zhang, L.; Wang, J.; Jiang, H.; Wu, Y.; Feng, S.; Chen, J. Electrospun chitosan oligosaccharide/polycaprolactone nanofibers loaded with wound-healing compounds of Rutin and Quercetin as antibacterial dressings. Int. J. Biol. Macromol. 2021, 183, 1145–1154. [Google Scholar] [CrossRef]
- Raza, S.H.A.; Naqvi, S.R.Z.; Abdelnour, S.A.; Schreurs, N.; Mohammedsaleh, Z.M.; Khan, I.; Shater, A.F.; El-Hack, M.E.A.; Khafaga, A.F.; Quan, G.; et al. Beneficial effects and health benefits of Astaxanthin molecules on animal production: A review. Res. Vet. Sci. 2021, 138, 69–78. [Google Scholar] [CrossRef]
- Zaaboul, F.; Liu, Y. Vitamin E in foodstuff: Nutritional, analytical, and food technology aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 964–998. [Google Scholar] [CrossRef]
- Mangla, B.; Kohli, K. Combination of natural agent with synthetic drug for the breast cancer therapy. Int. J. Drug Dev. Res. 2018, 10, 22–26. [Google Scholar]
- Majumdar, M.; Shivalkar, S.; Pal, A.; Verma, M.L.; Sahoo, A.K.; Roy, D.N. Nanotechnology for enhanced bioactivity of bioactive compounds. In Biotechnological Production of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 433–466. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.; Weiss, J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari-Movahed, M.; Mondal, A.; Farzaei, M.H.; Bishayee, A. Quercetin- and rutin-based nano-formulations for cancer treatment: A systematic review of improved efficacy and molecular mechanisms. Phytomedicine 2022, 97, 153909. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Inbaraj, B.S.; Dikkala, P.K.; Sridhar, K.; Mude, A.N.; Narsaiah, K. Preparation of Curcumin Hydrogel Beads for the Development of Functional Kulfi: A Tailoring Delivery System. Foods 2022, 11, 182. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Tan, H.; Zhang, R.; McClements, D.J. Vitamin E Encapsulation within Oil-in-Water Emulsions: Impact of Emulsifier Type on Physicochemical Stability and Bioaccessibility. J. Agric. Food Chem. 2019, 67, 1521–1529. [Google Scholar] [CrossRef]
- Wang, P.-P.; Luo, Z.-G.; Peng, X.-C. Encapsulation of Vitamin E and Soy Isoflavone Using Spiral Dextrin: Comparative Structural Characterization, Release Kinetics, and Antioxidant Capacity during Simulated Gastrointestinal Tract. J. Agric. Food Chem. 2018, 66, 10598–10607. [Google Scholar] [CrossRef]
- Fan, L.; Lu, Y.; Ouyang, X.-K.; Ling, J. Development and characterization of soybean protein isolate and fucoidan nanoparticles for curcumin encapsulation. Int. J. Biol. Macromol. 2021, 169, 194–205. [Google Scholar] [CrossRef]
- Bolat, Z.B.; Islek, Z.; Demir, B.N.; Yilmaz, E.N.; Sahin, F.; Ucisik, M.H. Curcumin- and Piperine-Loaded Emulsomes as Combinational Treatment Approach Enhance the Anticancer Activity of Curcumin on HCT116 Colorectal Cancer Model. Front. Bioeng. Biotechnol. 2020, 8, 50. [Google Scholar] [CrossRef]
- Oueslati, M.H.; Ben Tahar, L.; Harrath, A.H. Catalytic, antioxidant and anticancer activities of gold nanoparticles synthesized by kaempferol glucoside from Lotus leguminosae. Arab. J. Chem. 2020, 13, 3112–3122. [Google Scholar] [CrossRef]
- Loo, Y.S.; Madheswaran, T.; Rajendran, R.; Bose, R.J. Encapsulation of berberine into liquid crystalline nanoparticles to enhance its solubility and anticancer activity in MCF7 human breast cancer cells. J. Drug Deliv. Sci. Technol. 2020, 57, 101756. [Google Scholar] [CrossRef]
- Campos, E.; Proença, P.L.F.; Oliveira, J.L.; Pereira, A.; Ribeiro, L.N.D.M.; Fernandes, F.O.; Gonçalves, K.C.; Polanczyk, R.A.; Pasquoto-Stigliani, T.; Lima, R.; et al. Carvacrol and linalool co-loaded in β-cyclodextrin-grafted chitosan nanoparticles as sustainable biopesticide aiming pest control. Sci. Rep. 2018, 8, 7623. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Soto-Añual, M.; Leal-Calderon, F.; Bustamante, M. Food-grade Pickering emulsion as a novel astaxanthin encapsulation system for making powder-based products: Evaluation of astaxanthin stability during processing, storage, and its bioaccessibility. Food Res. Int. 2020, 134, 109244. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Prabu, S.L.; Suriyaprakash, T.; Dinesh, K.; Suresh, K.; Ragavendran, T. Nutraceuticals: A review. Elixir Pharm. 2012, 46, 8372–8377. [Google Scholar]
- Crawford, C.; Boyd, C.; Paat, C.F.; Meissner, K.; Lentino, C.; Teo, L.; Berry, K.; Deuster, P. Dietary Ingredients as an Alternative Approach for Mitigating Chronic Musculoskeletal Pain: Evidence-Based Recommendations for Practice and Research in the Military. Pain Med. 2019, 20, 1236–1247. [Google Scholar] [CrossRef]
- Zanella, M.; Ciappellano, S.G.; Venturini, M.; Tedesco, E.; Manodori, L.; Benetti, F. Nutraceuticals and nanotechnology. Diet. Ingred. Suppl. 2015, 26, 26–31. [Google Scholar]
- Subramani, T.; Ganapathyswamy, H. An overview of liposomal nano-encapsulation techniques and its applications in food and nutraceutical. J. Food Sci. Technol. 2020, 57, 3545–3555. [Google Scholar] [CrossRef]
- Singh, A.R.; Desu, P.K.; Nakkala, R.K.; Kondi, V.; Devi, S.; Alam, M.S.; Hamid, H.; Athawale, R.B.; Kesharwani, P. Nanotechnology-based approaches applied to nutraceuticals. Drug Deliv. Transl. Res. 2022, 12, 485–499. [Google Scholar] [CrossRef]
- Sahani, S.; Sharma, Y.C. Advancements in applications of nanotechnology in global food industry. Food Chem. 2021, 342, 128318. [Google Scholar] [CrossRef]
- Manocha, S.; Dhiman, S.; Grewal, A.S.; Guarve, K. Nanotechnology: An approach to overcome bioavailability challenges of nutraceuticals. J. Drug Deliv. Sci. Technol. 2022, 72, 103418. [Google Scholar] [CrossRef]
- Bronzwaer, S.; Kass, G.; Robinson, T.; Tarazona, J.; Verhagen, H.; Verloo, D.; Vrbos, D.; Hugas, M. Food Safety Regulatory Research Needs 2030. EFSA J. 2019, 17, e170622. [Google Scholar] [CrossRef] [PubMed]
- Whitman, M. Understanding the perceived need for complementary and alternative nutraceuticals: Lifestyle issues. Clin. J. Oncol Nurs. 2001, 5, 190–194. [Google Scholar]
- Ghani, U.; Naeem, M.; Rafeeq, H.; Imtiaz, U.; Amjad, A.; Ullah, S.; Rehman, A.; Qasim, F. A Novel Approach towards Nutraceuticals and Biomedical Applications. Sch. Int. J. Biochem. 2019, 2, 245–252. [Google Scholar] [CrossRef]
- Palmer, M.E.; Haller, C.; McKinney, P.E.; Klein-Schwartz, W.; Tschirgi, A.; Smolinske, S.C.; Woolf, A.; Sprague, B.M.; Ko, R.; Everson, G.; et al. Adverse events associated with dietary supplements: An observational study. Lancet 2003, 361, 101–106. [Google Scholar] [CrossRef]
- Dable-Tupas, G.; Otero, M.C.B.; Bernolo, L. Functional foods and health benefits. In Functional Foods and Nutraceuticals; Springer: Cham, Switzerland, 2020; pp. 1–11. [Google Scholar]
- Crowe, K.M.; Francis, C. Position of the Academy of Nutrition and Dietetics: Functional Foods. J. Acad. Nutr. Diet. 2013, 113, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Rawson, E.S.; Miles, M.P.; Larson-Meyer, D.E. Dietary supplements for health, adaptation, and recovery in athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 188–199. [Google Scholar] [CrossRef]
- Psota, T.L.; Gebauer, S.K.; Kris, E.P. Dietary omega-3 fatty acid intake and cardiovascular risk. Am. J. Cardiol. 2006, 98, 3–18. [Google Scholar] [CrossRef]
- Chandra, S.; Saklani, S.; Kumar, P.; Kim, B.; Coutinho, H.D.M. Nutraceuticals: Pharmacologically Active Potent Dietary Supplements. BioMed Res. Int. 2022, 2022, 2051017. [Google Scholar] [CrossRef] [PubMed]
- Abbadi, A.; Domergue, F. Biosynthesis of very long- chain polyunsaturated fatty acids in transgenic oilseeds: Constraints on their accumulation. Plant Cell 2004, 16, 2734–2748. [Google Scholar] [CrossRef]
- Wu, G.; Truksa, M.; Datla, N. Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat. Biotechnol. 2005, 23, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, R.; Reale, A.; Mazzeo, M.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A New Perspective for Functional Foods and Nutraceuticals. Nutrients 2021, 13, 1225. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, A. Functional Foods and Nutraceuticals as Dietary Intervention in Chronic Diseases; Novel Perspectives for Health Promotion and Disease Prevention. J. Diet. Suppl. 2018, 15, 977–1009. [Google Scholar] [CrossRef]
- Gruss, S.M.; Nhim, K.; Gregg, E.; Bell, M.; Luman, E.; Albright, A. Public Health Approaches to Type 2 Diabetes Prevention: The US National Diabetes Prevention Program and Beyond. Curr. Diabetes Rep. 2019, 19, 78. [Google Scholar] [CrossRef]
- De Felice, S. FIM rationale and proposed guidelines for the nutraceutical research & education act–NREA. In Proceedings of the FIM’s 10th Nutraceutical Conference, The Waldorf-Astoria, New York, NY, USA, 10–11 November 2002; Available online: https://fimdefelice.org/fim-rationale-and-proposed-guidelines-for-the-nutraceutical-research-education-act-nrea/ (accessed on 12 August 2022).
- Rajasekaran, A.; Sivagnanam, G.; Xavier, R. Nutraceuticals as therapeutic agents, a review research. Res. J. Pharm. Technol. 2008, 1, 328–340. [Google Scholar]
- Ozdal, T.; Tomas, M.; Toydemir, G.; Kamiloglu, S.; Capanoglu, E. Introduction to nutraceuticals, medicinal foods, and herbs. In Aromatic Herbs in Food; Academic Press: Cambridge, MA, USA, 2021; pp. 1–34. [Google Scholar] [CrossRef]
- Jagannathan, R.; Patel, S.A.; Ali, M.K.; Narayan, K.M.V. Global Updates on Cardiovascular Disease Mortality Trends and Attribution of Traditional Risk Factors. Curr. Diabetes Rep. 2019, 19, 44. [Google Scholar] [CrossRef]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Gonçalves, R.F.S.; Martins, J.T.; Duarte, C.M.M.; Vicente, A.A.; Pinheiro, A.C. Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation. Trends Food Sci. Technol. 2018, 78, 270–291. [Google Scholar] [CrossRef]
- Gopi, S.; Balakrishnan, P. Handbook of Nutraceuticals and Natural Products; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022. [Google Scholar]
- Shahgholian, N. Introduction to Nutraceuticals and Natural Products. In Handbook of Nutraceuticals and Natural Products: Biological, Medicinal, and Nutritional Properties and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; pp. 1–14. [Google Scholar]
- Durazzo, A.; Lucarini, M.; Santini, A. Nutraceuticals in Human Health. Foods 2020, 9, 370. [Google Scholar] [CrossRef]
- Natarajan, T.D.; Ramasamy, J.R.; Palanisamy, K. Nutraceutical potentials of synergic foods: A systematic review. J. Ethn. Foods 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Ghaffari, S.; Roshanravan, N. The role of nutraceuticals in prevention and treatment of hypertension: An updated review of the literature. Food Res. Int. 2020, 128, 108749. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, N.; Mohammadi, H. The Role of Nutraceuticals in Gestational Diabetes Mellitus. In Nutraceuticals for Prenatal, Maternal and Offspring’s Nutritional Health; CRC Press: Boca Raton, FL, USA, 2019; pp. 153–167. [Google Scholar] [CrossRef]
- Allahveran, A.; Farokhzad, A.; Asghari, M.; Sarkhosh, A. Foliar application of ascorbic and citric acids enhanced ‘Red Spur’apple fruit quality, bioactive compounds and antioxidant activity. Physiol. Mol. Biol. Plants 2018, 24, 433–440. [Google Scholar] [CrossRef]
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Estevinho, L.M. Special issue “Nutraceuticals in human health and disease”. Int. J. Mol. Sci. 2018, 19, 1213. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.; Jiang, Y.; Ho, C.-T.; Huang, Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Böhm, V.; Borge, G.I.A.; Cano, M.P.; Fikselová, M.; Gruskiene, R.; Lavelli, V.; Loizzo, M.R.; Mandić, A.I.; Brahm, P.M.; et al. Carotenoids: Considerations for Their Use in Functional Foods, Nutraceuticals, Nutricosmetics, Supplements, Botanicals, and Novel Foods in the Context of Sustainability, Circular Economy, and Climate Change. Annu. Rev. Food Sci. Technol. 2021, 12, 433–460. [Google Scholar] [CrossRef] [PubMed]
- Leonard, N.B. Stability testing of nutraceuticals and functional foods. In Handbook of Nutraceuticals and Functional Foods; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef]
- Jones, D.; Caballero, S.; Davidov-Pardo, G. Chapter Six—Bioavailability of nanotechnology-based bioactives and nutraceuticals. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2019; Volume 88, pp. 235–273. [Google Scholar]
- Punia, S.; Sandhu, K.S.; Kaur, M.; Siroha, A.K. Nanotechnology: A successful approach to improve nutraceutical bioavailability. In Nanobiotechnology in Bioformulations; Springer: Cham, Switzerland, 2019; pp. 119–133. [Google Scholar]
- Helal, N.A.; Eassa, H.A.; Amer, A.M.; Eltokhy, M.A.; Edafiogho, I.; Nounou, M.I. Nutraceuticals’ novel formulations: The good, the bad, the unknown and patents involved. Recent Pat. Drug Deliv. Formul. 2019, 13, 105–156. [Google Scholar] [CrossRef]
- Prasad, R.; Kumar, V.; Kumar, M.; Choudhary, D.K. (Eds.) Nanobiotechnology in Bioformulations; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Huang, Q.; Yu, H.; Ru, Q. Bioavailability and Delivery of Nutraceuticals Using Nanotechnology. J. Food Sci. 2010, 75, R50–R57. [Google Scholar] [CrossRef]
- Pandey, M.; Verma, R.K.; Saraf, S.A. Nutraceuticals: New era of medicine and health. Asian J. Pharm. Clin. Res. 2010, 3, 11–15. [Google Scholar]
- Rachmale, M.; Rajput, N.; Jadav, T.; Sahu, A.K.; Tekade, R.K.; Sengupta, P. Implication of metabolomics and transporter modulation based strategies to minimize multidrug resistance and enhance site-specific bioavailability: A needful consideration toward modern anticancer drug discovery. Drug Metab. Rev. 2022, 54, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, A.; Sakat, S.S.; Joshi, K.; Joshi, K.; Sharma, V.; Ranjan, R.; Bhattacharya, K.; Varshney, A. Cytokines driven anti-Inflammatory and anti-psoriasis like efficacies of nutraceutical sea buckthorn (hippophaerhamnoides) oil. Front. Pharm. 2019, 10, 1186. [Google Scholar] [CrossRef] [PubMed]
- Younis, S.A.; Kim, K.-H.; Shaheen, S.M.; Antoniadis, V.; Tsang, Y.F.; Rinklebe, J.; Deep, A.; Brown, R.J. Advancements of nanotechnologies in crop promotion and soil fertility: Benefits, life cycle assessment, and legislation policies. Renew. Sustain. Energy Rev. 2021, 152, 111686. [Google Scholar] [CrossRef]
- Bortolotti, M.; Mercatelli, D.; Polito, L. Momordica charantia, a Nutraceutical Approach for Inflammatory Related Diseases. Front. Pharmacol. 2019, 10, 486. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Methodologies for simulation of gastrointestinal digestion of different controlled delivery systems and further uptake of encapsulated bioactive compounds. Trends Food Sci. Technol. 2021, 114, 510–520. [Google Scholar] [CrossRef]
- DeRosa, G.; Limas, C.P.; Macías, P.C.; Estrella, A.; Maffioli, P. Dietary and nutraceutical approach to type 2 diabetes. Arch. Med Sci. 2014, 10, 336–344. [Google Scholar] [CrossRef]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, G. Nutraceutical Supplements in the Management and Prevention of Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef]
- Pashte, V.V.; Pashte, S.V.; Said, P.P. Nutraceutical properties of natural honey to fight health issues: A comprehensive review. J. Pharmacogn. Phytochem. 2020, 9, 234–242. [Google Scholar]
- Abbas, M.; Shah, I.; Nawaz, M.A.; Pervez, S.; Hussain, Y.; Niaz, K.; Khan, F. Honey Against Cancer. In Nutraceuticals and Cancer Signaling; Springer: Cham, Switzerland, 2021; pp. 401–418. [Google Scholar]
- Ajibola, A.; Chamunorwa, J.P.; Erlwanger, K.H. Nutraceutical values of natural honey and its contribution to human health and wealth. Nutr. Metab. 2012, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Ansary, J.; Gil, E.; Amici, A.; Bompadre, S.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Phenolic compounds from Mediterranean foods as nutraceutical tools for the prevention of cancer: The effect of honey polyphenols on colorectal cancer stem-like cells from spheroids. Food Chem. 2020, 325, 126881. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Three-arm, placebo-controlled, randomized clinical trial evaluating the metabolic effect of a combined nutraceutical containing a bergamot standardized flavonoid extract in dyslipidemic overweight subjects. Phytother. Res. 2019, 33, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Puc, J.F.; Cetzal-Ix, W.; Basu, S.K.; Enríquez-Nolasco, J.R.; Magaña-Magaña, M.A. Nutraceutical and medicinal properties of native stingless bees honey and their contribution to human health. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases, Academic Press: Cambridge, MA, USA, 2022; pp. 481–489.
- Poli, A.; Barbagallo, C.M.; Cicero, A.F.; Corsini, A.; Manzato, E.; Trimarco, B.; Bernini, F.; Visioli, F.; Bianchi, A.; Canzone, G.; et al. Nutraceuticals and functional foods for the control of plasma cholesterol levels. An intersociety position paper. Pharmacol. Res. 2018, 134, 51–60. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Stoian, A.P.; Vrablik, M.; Al Rasadi, K.; Banach, M.; Toth, P.P.; Rizzo, M. Nutraceuticals in the Management of Dyslipidemia: Which, When, and for Whom? Could Nutraceuticals Help Low-Risk Individuals with Non-optimal Lipid Levels? Curr. Atheroscler. Rep. 2021, 23, 57. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, H.; Hao, S.; Pan, D.; Wang, G.; Yu, W. Evaluation of phenolic composition and antioxidant properties of different varieties of Chinese citrus. Food Chem. 2021, 364, 130413. [Google Scholar] [CrossRef]
- Alcaide-Hidalgo, J.M.; Romero, M.; Duarte, J.; López-Huertas, E. Antihypertensive Effects of Virgin Olive Oil (Unfiltered) Low Molecular Weight Peptides with ACE Inhibitory Activity in Spontaneously Hypertensive Rats. Nutrients 2020, 12, 271. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Abhari, F.M. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Sahebkar, A.; Serban, M.-C.; Gluba-Brzózka, A.; Mikhailidis, D.P.; Cicero, A.F.; Rysz, J.; Banach, M. Lipid-modifying effects of nutraceuticals: An evidence-based approach. Nutrition 2016, 32, 1179–1192. [Google Scholar] [CrossRef]
- Bianconi, V.; Mannarino, M.R.; Sahebkar, A.; Cosentino, T.; Pirro, M. Cholesterol-lowering nutraceuticals affecting vascular function and cardiovascular disease risk. Curr. Cardiol. Rep. 2018, 20, 1–20. [Google Scholar] [CrossRef]
- Paolino, D.; Mancuso, A.; Cristiano, M.; Froiio, F.; Lammari, N.; Celia, C.; Fresta, M. Nanonutraceuticals: The New Frontier of Supplementary Food. Nanomaterials 2021, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Raghuvanshi, R.; Ceylan, F.D.; Bolling, B.W. Quercetin and Its Metabolites Inhibit Recombinant Human Angiotensin-Converting Enzyme 2 (ACE2) Activity. J. Agric. Food Chem. 2020, 68, 13982–13989. [Google Scholar] [CrossRef] [PubMed]
- Cimaglia, P.; Sega, F.V.D.; Vitali, F.; Lodolini, V.; Bernucci, D.; Passarini, G.; Fortini, F.; Marracino, L.; Aquila, G.; Rizzo, P.; et al. Effectiveness of a Novel Nutraceutical Compound Containing Red Yeast Rice, Polymethoxyflavones and Antioxidants in the Modulation of Cholesterol Levels in Subjects with Hypercholesterolemia and Low-Moderate Cardiovascular Risk: The NIRVANA Study. Front. Physiol. 2019, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Laganà, G.; Leuzzi, U.; Smeriglio, A.; Trombetta, D.; Bellocco, E. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L., variety Bronte) hulls. Food Chem. 2016, 196, 493–502. [Google Scholar] [CrossRef]
- Mandalari, G.; Barreca, D.; Gervasi, T.; Roussell, M.A.; Klein, B.; Feeney, M.J.; Carughi, A. Pistachio Nuts (Pistacia vera L.): Production, Nutrients, Bioactives and Novel Health Effects. Plants 2021, 11, 18. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of NutraceuticalsAnd bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Tenore, G.C.; Caruso, D.; D’Avino, M.; Buonomo, G.; Caruso, G.; Ciampaglia, R.; Schiano, E.; Maisto, M.; Annunziata, G.; Novellino, E. A Pilot Screening of Agro-Food Waste Products as Sources of Nutraceutical Formulations to Improve Simulated Postprandial Glycaemia and Insulinaemia in Healthy Subjects. Nutrients 2020, 12, 1292. [Google Scholar] [CrossRef]
- Varzakas, T.; Zakynthinos, G.; Verpoort, F. Plant Food Residues as a Source of Nutraceuticals and Functional Foods. Foods 2016, 5, 88. [Google Scholar] [CrossRef]
- Enrico, C. Nanotechnology-Based Drug Delivery of Natural Compounds and Phytochemicals for the Treatment of Cancer and Other Diseases. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 62, pp. 91–123. [Google Scholar] [CrossRef]
- Khorasani, S.; Danaei, M.; Mozafari, M. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. Technol. 2018, 79, 106–115. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Martins, N. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Tahir, A.; Ahmad, R.S.; Imran, M.; Ahmad, M.H.; Khan, M.K.; Muhammad, N.; Nisa, M.U.; Nadeem, M.T.; Yasmin, A.; Tahir, H.S.; et al. Recent approaches for utilization of food components as nano-encapsulation: A review. Int. J. Food Prop. 2021, 24, 1074–1096. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and controlled delivery for bioactive compounds: Outlining challenges for new “smart-foods” for health. Foods 2018, 7, 72. [Google Scholar] [CrossRef]
- Persano, F.; Gigli, G.; Leporatti, S. Lipid-polymer hybrid nanoparticles in cancer therapy: Current overview and future directions. Nano Express 2021, 2, 012006. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Villalba-Rodríguez, A.M.; Romero-Castillo, K.D.; Zavala-Yoe, R.; Bilal, M.; Ramirez-Mendoza, R.A.; Parra-Saldivar, R.; Iqbal, H.M. Poly-3-hydroxybutyrate-based constructs with novel characteristics for drug delivery and tissue engineering applications—A review. Polym. Eng. Sci. 2020, 60, 1760–1772. [Google Scholar] [CrossRef]
- Sonawane, P.G.; Doshi, Y.S.; Shah, M.U.; Bajaj, M.; Kevadia, V. Probiotics: The Nano Soldiers for Periodontium. J. PEARLDENT 2017, 8, 1. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, F.; Wang, X.; Feng, T.; Xia, S.; Zhang, X. Chitosan decoration improves the rapid and long-term antibacterial activities of cinnamaldehyde-loaded liposomes. Int. J. Biol. Macromol. 2021, 168, 59–66. [Google Scholar] [CrossRef]
- Hills, S.; Walker, M.; Barry, A.E. Sport as a vehicle for health promotion: A shared value example of corporate social responsibility. Sport Manag. Rev. 2019, 22, 126–141. [Google Scholar] [CrossRef]
- Khangwal, I.; Yadav, M.; Mandeep; Shukla, P. Probiotics and Prebiotics: Techniques Used and Its Relevance. In Microbial Enzymes and Biotechniques; Springer: Singapore, 2020; pp. 193–206. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Galiwango, E.; Tamiello-Rosa, C.; Abdullah, H.; Esposito, G.; Hunashal, Y.; Obaid, R.S.; Hamed, F. Characterization, bioactivities, and rheological properties of exopolysaccharide produced by novel probiotic Lactobacillus plantarum C70 isolated from camel milk. Int. J. Biol. Macromol. 2020, 144, 938–946. [Google Scholar] [CrossRef]
- Ghanavati, R.; Asadollahi, P.; Shapourabadi, M.B.; Razavi, S.; Talebi, M.; Rohani, M. Inhibitory effects of Lactobacilli cocktail on HT-29 colon carcinoma cells growth and modulation of the Notch and Wnt/β-catenin signaling pathways. Microb. Pathog. 2020, 139, 103829. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Parmar, I.; Neir, S.V. Biotransformation of Cranberry Proanthocyanidins to Probiotic Metabolites by Lactobacillus rhamnosus Enhances Their Anticancer Activity in HepG2 Cells In Vitro. Oxidative Med. Cell. Longev. 2019, 2019, 4750795. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Su, M.; Duan, Y.; Huang, Y. Eurotium cristatum, a potential probiotic fungus from Fuzhuan brick tea, alleviated obesity in mice by modulating gut microbiota. Food Funct. 2019, 10, 5032–5045. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Baruah, R.; Goyal, A. Prebiotic Chondroitin Sulfate Disaccharide Isolated from Chicken Keel Bone Exhibiting Anticancer Potential Against Human Colon Cancer Cells. Nutr. Cancer 2019, 71, 825–839. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, M.; Yang, F.; Liu, J. Antioxidant activity of high purity blueberry anthocyanins and the effects on human intestinal microbiota. LWT 2020, 117, 108621. [Google Scholar] [CrossRef]
- Ohara, T.; Mori, T. Antiproliferative Effects of Short-chain Fatty Acids on Human Colorectal Cancer Cells via Gene Expression Inhibition. Anticancer Res. 2019, 39, 4659–4666. [Google Scholar] [CrossRef]
- Li, E.; Yang, S.; Zou, Y.; Cheng, W.; Li, B.; Hu, T.; Li, Q.; Wang, W.; Liao, S.; Pang, D. Purification, Characterization, Prebiotic Preparations and Antioxidant Activity of Oligosaccharides from Mulberries. Molecules 2019, 24, 2329. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Pandey, K.; Naik, S.; Vakil, B. Probiotics, prebiotics and synbiotics—a review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, Nano-guard for Pesticides: A New Window for Safe Application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef]
- Xiao, J.; Cao, Y.; Huang, Q. Edible Nanoencapsulation Vehicles for Oral Delivery of Phytochemicals: A Perspective Paper. J. Agric. Food Chem. 2017, 65, 6727–6735. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Martín, A.; McClements, D.J. Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci. Technol. 2014, 39, 18–39. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.; Li-Chan, E.C. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Hoseyni, S.Z.; Jafari, S.M.; Tabarestani, H.S.; Ghorbani, M.; Assadpour, E.; Sabaghi, M. Release of catechin from Azivash gum-polyvinyl alcohol electrospun nanofibers in simulated food and digestion media. Food Hydrocoll. 2021, 112, 106366. [Google Scholar] [CrossRef]
- Kurd, F.; Fathi, M.; Shekarchizadeh, H. Nanoencapsulation of hesperetin using basil seed mucilage nanofibers: Characterization and release modeling. Food Biosci. 2019, 32, 100475. [Google Scholar] [CrossRef]
- Remanan, M.K.; Zhu, F. Encapsulation of rutin using quinoa and maize starch nanoparticles. Food Chem. 2021, 353, 128534. [Google Scholar] [CrossRef]
- Rajabi, H.; Jafari, S.M.; Rajabzadeh, G.; Sarfarazi, M.; Sedaghati, S. Chitosan-gum Arabic complex nanocarriers for encapsulation of saffron bioactive components. Colloids Surf. A Physicochem. Eng. Asp. 2020, 578, 123644. [Google Scholar] [CrossRef]
- Fathi, M.; Donsi, F.; McClements, D.J. Protein-Based Delivery Systems for the Nanoencapsulation of Food Ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17, 920–936. [Google Scholar] [CrossRef]
- Ahmad, U.; Ali, A.; Khan, M.M.; Siddiqui, M.A.; Akhtar, J.; Ahmad, F.J. Nanotechnology-Based Strategies for Nutraceuticals: A Review of Current Research Development. Nanosci. Technol. Int. J. 2019, 10, 133–155. [Google Scholar] [CrossRef]
- Zhang, R.; Han, Y.; Xie, W.; Liu, F.; Chen, S. Advances in Protein-Based Nanocarriers of Bioactive Compounds: From Microscopic Molecular Principles to Macroscopical Structural and Functional Attributes. J. Agric. Food Chem. 2022, 70, 6354–6367. [Google Scholar] [CrossRef]
- Mozafari, M.R.; Flanagan, J.; Matia-Merino, L.; Awati, A.; Omri, A.; Suntres, Z.E.; Singh, H. Recent trends in the lipid-based nanoencapsulation of antioxidants and their role in foods. J. Sci. Food Agric. 2006, 86, 2038–2045. [Google Scholar] [CrossRef]
- Akhavan, S.; Assadpour, E.; Katouzian, I.; Jafari, S.M. Lipid Nano Scale Cargos for The Protection and Delivery of Food Bioactive Ingredients and Nutraceuticals. Trends Food Sci. Technol. 2018, 74, 132–146. [Google Scholar] [CrossRef]
- Livney, Y.D. Nanostructured delivery systems in food: Latest developments and potential future directions. Curr. Opin. Food Sci. 2015, 3, 125–135. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. An overview of specialized equipment for nanoencapsulation of food ingredients. In Nanoencapsulation of Food Ingredients by Specialized Equipment; Jafari, S., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–30. [Google Scholar] [CrossRef]
- Rashidi, L. Different nano-delivery systems for delivery of nutraceuticals. Food Biosci. 2021, 43, 101258. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Control. Release 2019, 298, 38–67. [Google Scholar] [CrossRef]
- Xia, S.; Tan, C.; Zhang, Y.; Abbas, S.; Feng, B.; Zhang, X.; Qin, F. Modulating effect of lipid bilayer–carotenoid interactions on the property of liposome encapsulation. Colloids Surf. B Biointerfaces 2015, 128, 172–180. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Lopes, N.A.; Pinilla, C.M.B.; Brandelli, A. Antimicrobial activity of lysozyme-nisin co-encapsulated in liposomes coated with polysaccharides. Food Hydrocoll. 2019, 93, 1–9. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, C.; Ma, L.; Zou, L.; Liu, W.; Li, R.; Cao, Y.; Liu, Y.; Ruan, R.; Li, J. The Formation of Chitosan-Coated Rhamnolipid Liposomes Containing Curcumin: Stability and In Vitro Digestion. Molecules 2021, 26, 560. [Google Scholar] [CrossRef]
- Dalmoro, A.; Bochicchio, S.; Lamberti, G.; Bertoncin, P.; Janssens, B.; Barba, A.A. Micronutrients encapsulation in enhanced nanoliposomal carriers by a novel preparative technology. RSC Adv. 2019, 9, 19800–19812. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Meng, Q.; Zhou, J.; Chen, B.; Xi, J.; Long, P.; Zhang, L.; Hou, R. Nanoemulsion delivery system of tea polyphenols enhanced the bioavailability of catechins in rats. Food Chem. 2019, 242, 527–532. [Google Scholar] [CrossRef]
- Katouzian, I.; Esfanjani, A.F.; Jafari, S.M.; Akhavan, S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci. Technol. 2017, 68, 14–25. [Google Scholar] [CrossRef]
- Fathi, M.; Mozafari, M.R.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid nanoparticles as carriers for bioactive delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Wang, J.; Jin, Z.; Qiu, C. A review of nanostructured delivery systems for the encapsulation, protection, and delivery of silymarin: An emerging nutraceutical. Food Res. Int. 2022, 156, 111314. [Google Scholar] [CrossRef]
- Peres, L.B.; dos Anjos, R.S.; Tappertzhofen, L.C.; Feuser, P.E.; de Araújo, P.H.; Landfester, K.; Muñoz-Espí, R. pH-responsive physically and chemically cross-linked glutamic-acid-based hydrogels and nanogels. Eur. Polym. J. 2018, 101, 341–349. [Google Scholar] [CrossRef]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K.; Iyer, A.K. pH Responsive 5-Fluorouracil Loaded Biocompatible Nanogels for Topical Chemotherapy of Aggressive Melanoma. Colloids Surf. B Biointerfaces 2019, 174, 232–245. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Zhang, N.; Yang, C.; Xu, H.; Wang, Z.; Li, B.; Ding, J.; Chen, X. X-ray-responsive polypeptide nanogel for concurrent chemoradiotherapy. J. Control. Release 2021, 332, 1–9. [Google Scholar] [CrossRef]
- Mullen, E.; Morris, M. Green Nanofabrication Opportunities in the Semiconductor Industry: A Life Cycle Perspective. Nanomaterials 2021, 11, 1085. [Google Scholar] [CrossRef]
- Bazana, M.T.; Codevilla, C.F.; de Menezes, C.R. Nanoencapsulation of bioactive compounds: Challenges and perspectives. Curr. Opin. Food Sci. 2019, 26, 47–56. [Google Scholar] [CrossRef]

| Nano formulated Bioactive Compounds | Solubility | Stability | Nanofabricated Method/Source | Bioavailability/Release Kinetics | Main Findings | References |
|---|---|---|---|---|---|---|
| Rutin and Quercetin | Water-insoluble | Sensitive to light, oxidation, pH | PLGA | Release of quercetin ~64% after 3 days of injection, in vivo | Study of anticancer activity | [16] |
| Curcumin | Poor water solubility | Sensitive to oxygen, light | Curcumin Hydrogel beads (CHBs) | Cucumin release was 67% after 2 h, and 67% after 4 h | Mask bitterness, enhance solubility, and increase the bioavailability | [17] |
| Vitamin E | Water- insoluble | Sensitive to oxygen, light, pH | Oil –in water emulsion | Bioaccessibility of developed emulsions was in the range of 65–85% | Increased storage stability (Vitamin E fortified emulsions) | [18] |
| Vitamin E | Water -insoluble | Sensitive to oxygen, light, pH | Spiral dextrin inclusion complexes | 95% of Vit E and 98% of soy isoflavone released after 80 min | Study of release kinetics of bioactive compound and Antioxidant capacity during the simulated gastrointestinal tract | [19] |
| Curcumin | Water-insoluble | Sensitive to light, heat, iron ion | Spi-Fuc polymer-core-shell nanoparticles | Release rates of curcumin were 96.25% and 82.69% after 4 h | Stability studies, delivering lipid soluble active ingredients | [20] |
| Curcumin and piperine | Water-insoluble | Sensitive to light, heat, iron ion | Nanoemulsion | Release of curcumin 40%, release of piperine 7.5% after 72 h | The activity of curcumin on HCT 116 Colorectal Cancer Model | [21] |
| Kaempferol glucoside | Water insoluble | Sensitive to oxidation, light, pH | Gold nanoparticles | - | Catalytic, antioxidant, and anticancer activities of gold nanoparticles | [22] |
| Berberine | Low water solubility | Sensitive to heat and pH | Liquid crystalline nanoparticle | 80% of berberine released after 24 h | Anticancer activity in MCF7 human breast cancer cells | [23] |
| Carvacrol and linalool | Water insoluble | Sensitive to oxidation, light, pH | β-cyclodextrin-grafted chitosan | Carvacrol released—49% after 600 min, linalool released—71% after 460 min | sustainable biopesticide aiming pest control | [24] |
| Astaxanthin | Low water solubility | Sensitive to oxygen, light, heat, pH | Lupin protein-based Pickering emulsion | Astaxanthin powder exhibited 80% bioaccessibility | Usage as a food ingredientLupin protein-based particles | [25] |
| Type | Micro-Organisms | Activity | Study | Reference |
|---|---|---|---|---|
| Probiotic | Lactobacilli plantarum C70 | Anticancer effect | Lactobacilli plantarum C70 by releasing the exopolysaccharide, caused 73.1% and 88.1% cytotoxic properties against breast and colon cancers, respectively. | [117] |
| Probiotic | Lactobacilli cocktail | Anticancer effect | HT-29, a human colorectal carcinoma cell line, was controlled by Lactobacilli cocktail via the modulation of the Notch and Wnt/β-catenin signaling pathways | [118] |
| Probiotic | L. rhamnosus | Anticancer effect | The bioconversion of cranberry proanthocyanidins to Lactobacillus rhamnosus could result in the IC50 values of 20.1 and 47.8 µg/mL | [119] |
| Probiotic | Eurotium cristatum | Anti-obesity effect | The administration of Eurotium cristatum showed anti-obesity activity in mice fed a high-fat diet (HFD) through the modulation of gut microbiota | [120] |
| Type | Active Compounds | Activity | Study | Reference |
|---|---|---|---|---|
| Prebiotic | Chondroitin Sulfate Disaccharide | Anticancer effect | The growth of HT-29, human colon cancer cell line, was controlled by Chondroitin sulfate (CS)-Keel disaccharide (CSD) generated by chondroitin AC lyase, estimated at 80% antiproliferative activity. | [121] |
| Prebiotic | Blueberry anthocyanins | Antioxidant effect | The density and composition of intestinal microbiota in human models were increased by consumption of high purity blueberry anthocyanins through the increase in the modulatory and prebiotic activities. | [122] |
| Prebiotic | Short-chain fatty acids | Antiproliferative effects | The administration of short-chain fatty acids (SCFAs) prevented the expression of genes involved in the human colorectal cancer cell. | [123] |
| Prebiotic | Oligosaccharides | Antioxidant effect | The water-soluble oligosaccharide of EMOS-1a showed a 1420% proliferation level | [124] |
| Nutraceuticals | Encapsulating Materials | Encapsulation Techniques | Target | Reference |
|---|---|---|---|---|
| Catechin | Azivash (Corchorus olitorius L.) gum-polyvinyl alcohol | Electrospinning process | Simulated gastric fluid and simulated intestinal fluid, EE%. | [131] |
| Hesperetin (HSP) | Nanofibers: Basil seed mucilage/polyvinylalcohol | Electrospinning | Characterization of nanofibers. Release models, EE% | [132] |
| Rutin | Quinoa and maize starch NPs | Ultrasonication | Characterization, EE%, simulated in vitro digestion. | [133] |
| Saffron Bioactive components | Nanoparticles: chitosan (CS) and gum arabic (GA) | Ionic gelation (IG) | Characterization NPs, EE%, Release of Saffron in acidic and natural media | [134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puttasiddaiah, R.; Lakshminarayana, R.; Somashekar, N.L.; Gupta, V.K.; Inbaraj, B.S.; Usmani, Z.; Raghavendra, V.B.; Sridhar, K.; Sharma, M. Advances in Nanofabrication Technology for Nutraceuticals: New Insights and Future Trends. Bioengineering 2022, 9, 478. https://doi.org/10.3390/bioengineering9090478
Puttasiddaiah R, Lakshminarayana R, Somashekar NL, Gupta VK, Inbaraj BS, Usmani Z, Raghavendra VB, Sridhar K, Sharma M. Advances in Nanofabrication Technology for Nutraceuticals: New Insights and Future Trends. Bioengineering. 2022; 9(9):478. https://doi.org/10.3390/bioengineering9090478
Chicago/Turabian StylePuttasiddaiah, Rachitha, Rohitha Lakshminarayana, Nandini Lalithadripura Somashekar, Vijai Kumar Gupta, Baskaran Stephen Inbaraj, Zeba Usmani, Vinay Basavegowda Raghavendra, Kandi Sridhar, and Minaxi Sharma. 2022. "Advances in Nanofabrication Technology for Nutraceuticals: New Insights and Future Trends" Bioengineering 9, no. 9: 478. https://doi.org/10.3390/bioengineering9090478
APA StylePuttasiddaiah, R., Lakshminarayana, R., Somashekar, N. L., Gupta, V. K., Inbaraj, B. S., Usmani, Z., Raghavendra, V. B., Sridhar, K., & Sharma, M. (2022). Advances in Nanofabrication Technology for Nutraceuticals: New Insights and Future Trends. Bioengineering, 9(9), 478. https://doi.org/10.3390/bioengineering9090478











