Expression Profiling of Pdx1, Ngn3, and MafA in the Liver and Pancreas of Recovering Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Induction of Diabetes and Treatment with GE
2.3. Tissue Sample Collection and RNA Extraction
2.4. Measurement of Blood Glucose and Insulin Concentrations after Treatment with GE or Normal Saline
2.5. Conversion of RNA to cDNA
2.6. Real-Time PCR
2.7. Statistical Analysis
3. Results
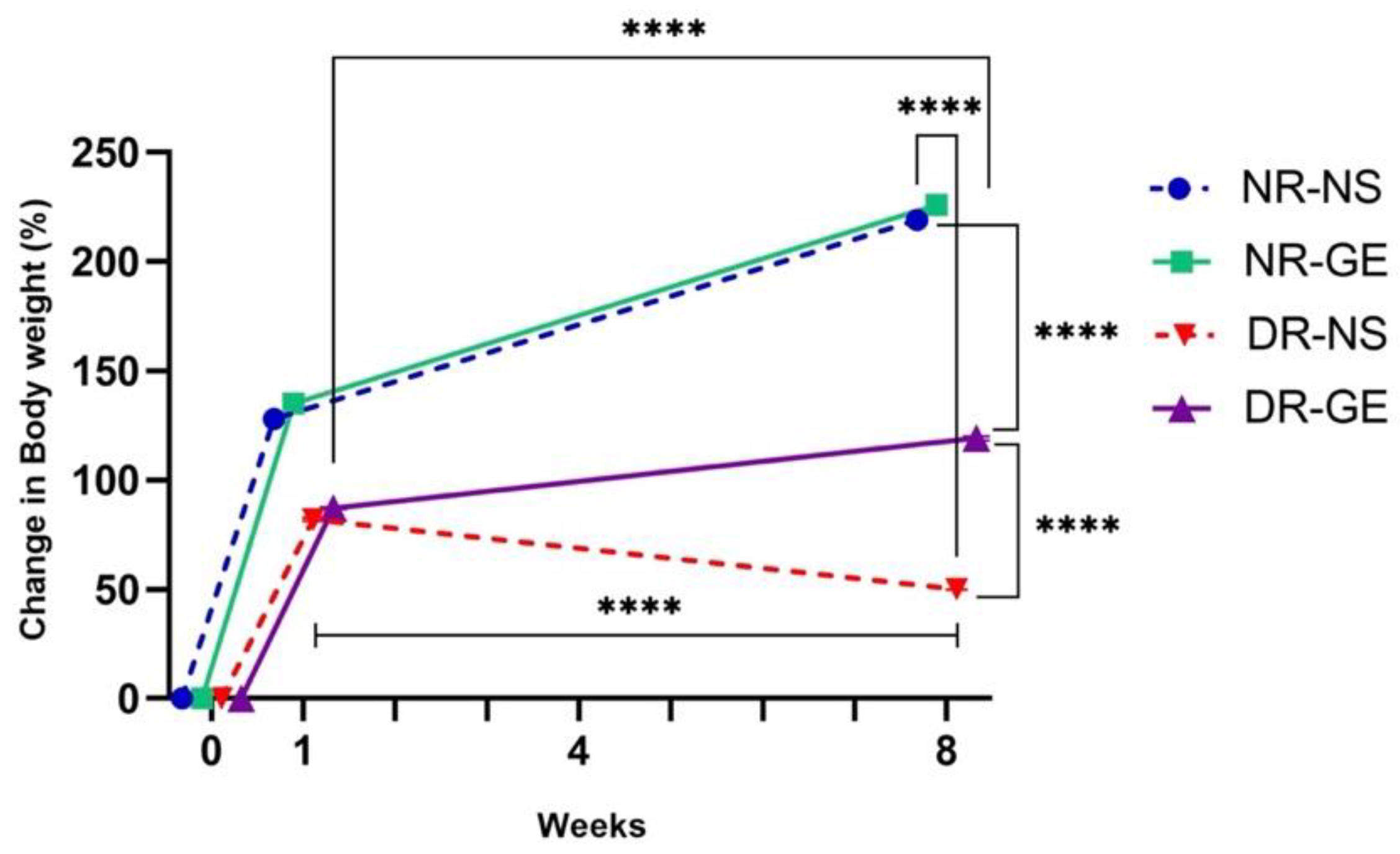
3.1. FBG and Serum Insulin Concentrations
3.2. Real Time-PCR Profiling
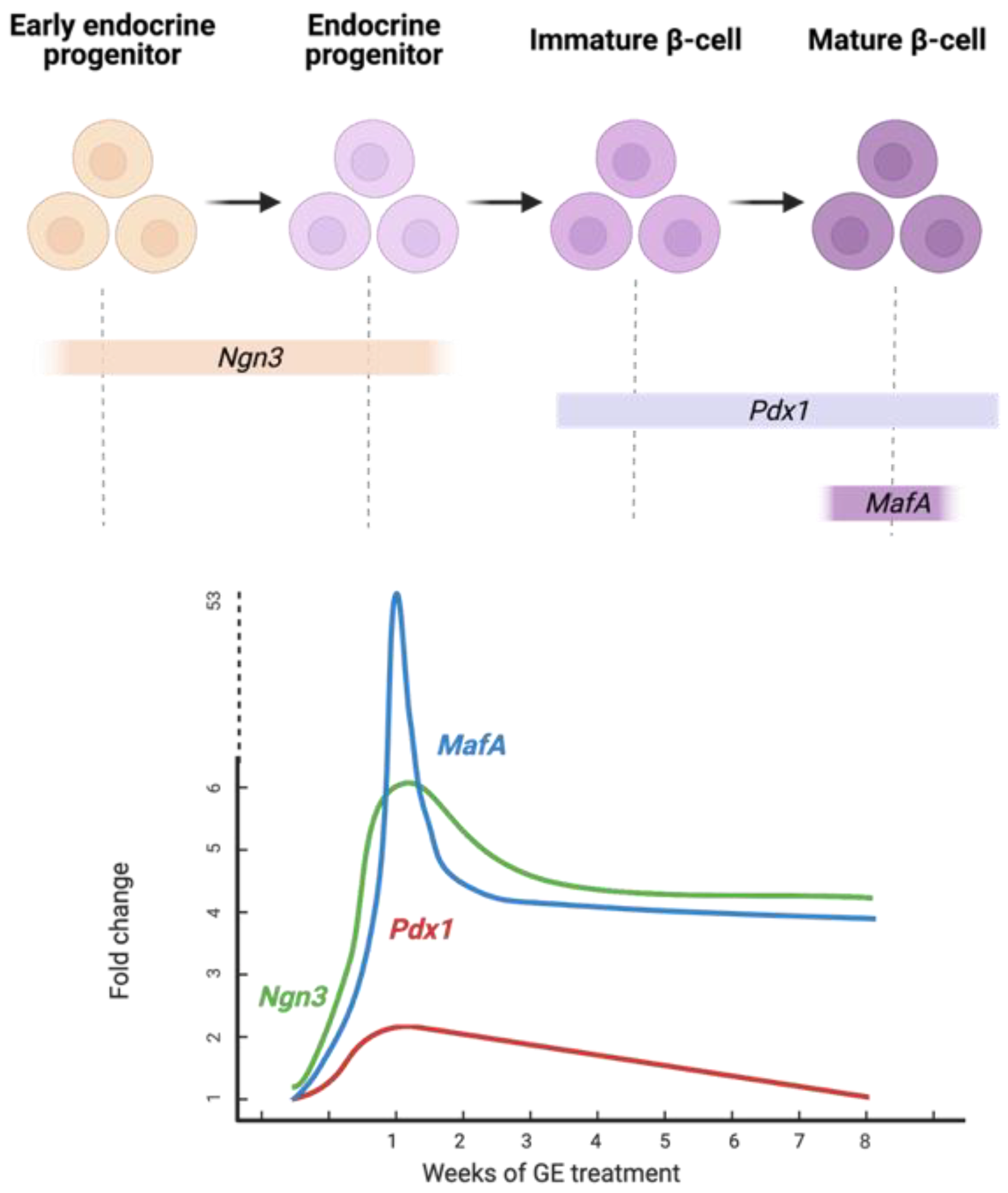
3.2.1. Pancreas Gene Expression Profiling
3.2.2. Liver Gene Expression Profiling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Diabetes Care. 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Alkandari, A.; Alarouj, M.; Elkum, N.; Sharma, P.; Devarajan, S.; Abu-Farha, M.; Al-Mulla, F.; Tuomilehto, J.; Bennakhi, A. Adult Diabetes and Prediabetes Prevalence in Kuwait: Data from the Cross-Sectional Kuwait Diabetes Epidemiology Program. J. Clin. Med. 2020, 9, 3420. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.; Al-Qattan, K.K.; Bordia, T.; Ali, M. Including Garlic in the Diet May Help Lower Blood Glucose, Cholesterol, and Triglycerides. J. Nutr. 2006, 136 (Suppl. S3), 800S–802S. [Google Scholar] [CrossRef]
- Baeyens, L.; Lemper, M.; Staels, W.; De Groef, S.; De Leu, N.; Heremans, Y.; German, M.S.; Heimberg, H. (Re) Generating Human Beta Cells: Status, Pitfalls, and Perspectives. Physiol. Rev. 2018, 98, 1143–1167. [Google Scholar] [CrossRef]
- Memon, B.; Abdelalim, E.M. Stem Cell Therapy for Diabetes: Beta Cells versus Pancreatic Progenitors. Cells 2020, 9, 283. [Google Scholar] [CrossRef]
- Zhou, Q.; Melton, D.A. Pancreas Regeneration. Nature. 2018, 557, 351–358. [Google Scholar] [CrossRef]
- Gittes, G.K. Developmental Biology of the Pancreas: A Comprehensive Review. Dev. Biol. 2009, 326, 4–35. [Google Scholar] [CrossRef]
- Habener, J.F.; Kemp, D.M.; Thomas, M.K. Minireview: Transcriptional Regulation in Pancreatic Development. Endocrinology 2005, 146, 1025–1034. [Google Scholar] [CrossRef]
- Matsuoka, T.A.; Artner, I.; Henderson, E.; Means, A.; Sander, M.; Stein, R. The MafA Transcription Factor Appears to Be Responsible for Tissue-Specific Expression of Insulin. Proc. Natl. Acad. Sci. USA 2004, 101, 2930–2933. [Google Scholar] [CrossRef]
- Olbrot, M.; Rud, J.; Moss, L.G.; Sharma, A. Identification of β-Cell-Specific Insulin Gene Transcription Factor RIPE3b1 as Mammalian MafA. Proc. Natl. Acad. Sci. USA 2002, 99, 6737–6742. [Google Scholar] [CrossRef] [Green Version]
- Pan, F.C.; Wright, C. Pancreas Organogenesis: From Bud to Plexus to Gland. Dev. Dyn. 2011, 240, 530–565. [Google Scholar] [CrossRef] [PubMed]
- Melloul, D.; Marshak, S.; Cerasi, E. Regulation of Insulin Gene Transcription. Diabetologia 2002, 45, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Q.; Zhou, Z.; Ikeda, Y. PDX1, Neurogenin-3, and MAFA: Critical Transcription Regulators for Beta Cell Development and Regeneration. Stem Cell Res. Ther. 2017, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Brown, J.; Kanarek, A.; Rajagopal, J.; Melton, D.A. In Vivo Reprogramming of Adult Pancreatic Exocrine Cells to β-Cells. Nature 2008, 455, 627–632. [Google Scholar] [CrossRef]
- Lima, M.J.; Muir, K.R.; Docherty, H.M.; McGowan, N.W.; Forbes, S.; Heremans, Y.; Heimberg, H.; Casey, J.; Docherty, K. Generation of Functional Beta-Like Cells from Human Exocrine Pancreas. PLoS ONE 2016, 11, e0156204. [Google Scholar] [CrossRef]
- Matsuoka, T.A.; Kawashima, S.; Miyatsuka, T.; Sasaki, S.; Shimo, N.; Katakami, N.; Kawamori, D.; Takebe, S.; Herrera, P.L.; Kaneto, H.; et al. Mafa Enables Pdx1 to Effectively Convert Pancreatic Islet Progenitors and Committed Islet α-Cells Into β-Cells In Vivo. Diabetes 2017, 66, 1293–1300. [Google Scholar] [CrossRef]
- Saleh, M.; Gittes, G.K.; Prasadan, K. Alpha-to-Beta Cell Trans-differentiation for Treatment of Diabetes. Biochem. Soc. Trans. 2021, 49, 2539–2548. [Google Scholar] [CrossRef]
- Cim, A.; Sawyer, G.J.; Zhang, X.; Su, H.; Collins, L.; Jones, P.; Antoniou, M.; Reynes, J.P.; Lipps, H.J.; Fabre, J.W. In Vivo Studies on Non-Viral Transdifferentiation of Liver Cells towards Pancreatic β Cells. J. Endocrinol. 2012, 214, 277–288. [Google Scholar] [CrossRef]
- Chen, Y.J.; Finkbeiner, S.R.; Weinblatt, D.; Emmett, M.J.; Tameire, F.; Yousefi, M.; Yang, C.; Maehr, R.; Zhou, Q.; Shemer, R.; et al. De Novo Formation of Insulin-Producing “Neo-β Cell Islets” From Intestinal Crypts. Cell Rep. 2014, 6, 1046–1058. [Google Scholar] [CrossRef]
- Szkudelski, T. The Mechanism of Alloxan and Streptozotocin Action in B Cells of the Rat Pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar]
- Jensen, J. Gene Regulatory Factors in Pancreatic Development. Dev. Dyn. 2004, 229, 176–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yan, J.; Anderson, D.A.; Xu, Y.; Kanal, M.C.; Cao, Z.; Wright, C.V.; Gu, G. Neurog3 Gene Dosage Regulates Allocation of Endocrine and Exocrine Cell Fates in the Developing Mouse Pancreas. Dev. Biol. 2010, 339, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Cerf, M.E. Transcription Factors Regulating β-Cell Function. Eur. J. Endocrinol. 2006, 155, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.; Al-Qattan, K.K.; Js, D.; Ali, M. Anti-Diabetic and Anti-Oxidant Potential of Aged Garlic Extract (AGE) in Streptozotocin-Induced Diabetic Rats. BMC Complement. Altern. Med. 2016, 16, 17. [Google Scholar] [CrossRef]
- Wickramasinghe, A.S.D.; Kalansuriya, P.; Attanayake, A.P. Herbal Medicines Targeting the Improved β-Cell Functions and β-Cell Regeneration for the Management of Diabetes Mellitus. Evid. Based Complement. Alternat. Med. 2021, 2021, 2920530. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C. Targeting β-Cell Dedifferentiation and Transdifferentiation: Opportunities and Challenges. Endocr. Connect. 2021, 10, R213–R228. [Google Scholar] [CrossRef]
- Ferber, S.; Halkin, A.; Cohen, H.; Ber, I.; Einav, Y.; Goldberg, I.; Barshack, I.; Seijffers, R.; Kopolovic, J.; Kaiser, N.; et al. Pancreatic and Duodenal Homeobox Gene 1 Induces Expression of Insulin Genes in Liver and Ameliorates Streptozotocin-Induced Hyperglycemia. Nat. Med. 2000, 6, 568–572. [Google Scholar] [CrossRef]
- Shternhall-Ron, K.; Quintana, F.J.; Perl, S.; Meivar-Levy, I.; Barshack, I.; Cohen, I.R.; Ferber, S. Ectopic PDX-1 Expression in Liver Ameliorates Type 1 Diabetes. J. Autoimmun. 2007, 28, 134–142. [Google Scholar] [CrossRef]
- Lu, J.; Xia, Q.; Zhou, Q. How to Make Insulin-Producing Pancreatic β Cells for Diabetes Treatment. Sci. China Life Sci. 2017, 60, 239–248. [Google Scholar] [CrossRef]
- Gradwohl, G.; Dierich, A.; LeMeur, M.; Guillemot, F. Neurogenin3 Is Required for the Development of the Four Endocrine Cell Lineages of the Pancreas. Proc. Natl. Acad. Sci. USA 2000, 97, 1607–1611. [Google Scholar] [CrossRef]
- Bechard, M.E.; Bankaitis, E.D.; Hipkens, S.B.; Ustione, A.; Piston, D.W.; Yang, Y.P.; Magnuson, M.A.; Wright, C.V. Precommitment Low-Level Neurog3 Expression Defines a Long-Lived Mitotic Endocrine-Biased Progenitor Pool That Drives Production of Endocrine-Committed Cells. Genes Dev. 2016, 30, 1852–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrali, S.S.; Sampley, M.L.; Vanderford, N.L.; Özcan, S. Glucose Regulation of Insulin Gene Expression in Pancreatic β-Cells. Biochem. J. 2008, 415, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nasteska, D.; Fine, N.; Ashford, F.B.; Cuozzo, F.; Viloria, K.; Smith, G.; Dahir, A.; Dawson, P.; Lai, Y.C.; Bastidas-Ponce, A.; et al. PDX1LOW MAFALOW β-cells contribute to islet function and insulin release. Nat. Commun. 2021, 12, 674–693. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Adsani, A.M.; Al-Otaibi, A.N.; Barhoush, S.A.; Al-Qattan, K.K.; Al-Bustan, S.A. Expression Profiling of Pdx1, Ngn3, and MafA in the Liver and Pancreas of Recovering Streptozotocin-Induced Diabetic Rats. Genes 2022, 13, 1625. https://doi.org/10.3390/genes13091625
Al-Adsani AM, Al-Otaibi AN, Barhoush SA, Al-Qattan KK, Al-Bustan SA. Expression Profiling of Pdx1, Ngn3, and MafA in the Liver and Pancreas of Recovering Streptozotocin-Induced Diabetic Rats. Genes. 2022; 13(9):1625. https://doi.org/10.3390/genes13091625
Chicago/Turabian StyleAl-Adsani, Amani M., Anoud N. Al-Otaibi, Sahar A. Barhoush, Khaled K. Al-Qattan, and Suzanne A. Al-Bustan. 2022. "Expression Profiling of Pdx1, Ngn3, and MafA in the Liver and Pancreas of Recovering Streptozotocin-Induced Diabetic Rats" Genes 13, no. 9: 1625. https://doi.org/10.3390/genes13091625
APA StyleAl-Adsani, A. M., Al-Otaibi, A. N., Barhoush, S. A., Al-Qattan, K. K., & Al-Bustan, S. A. (2022). Expression Profiling of Pdx1, Ngn3, and MafA in the Liver and Pancreas of Recovering Streptozotocin-Induced Diabetic Rats. Genes, 13(9), 1625. https://doi.org/10.3390/genes13091625






