Development and Characterization of Unmodified and Modified Natural Rubber Composites Filled with Modified Clay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Rubber–Clay Composites
2.3. Characterization of Rubber-Clay Composites
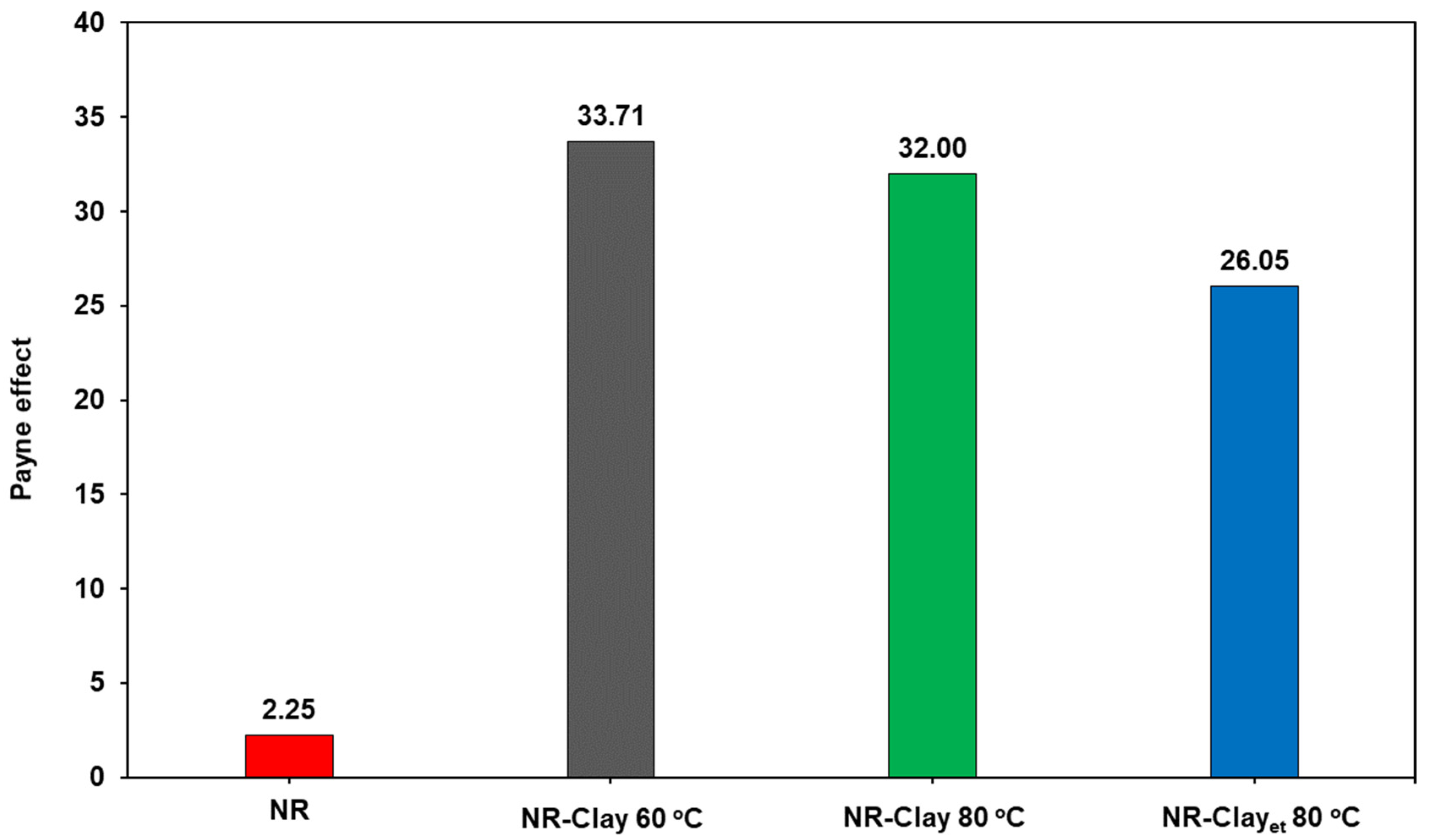
2.3.1. Dynamic Mechanical Rheology and Payne Effect
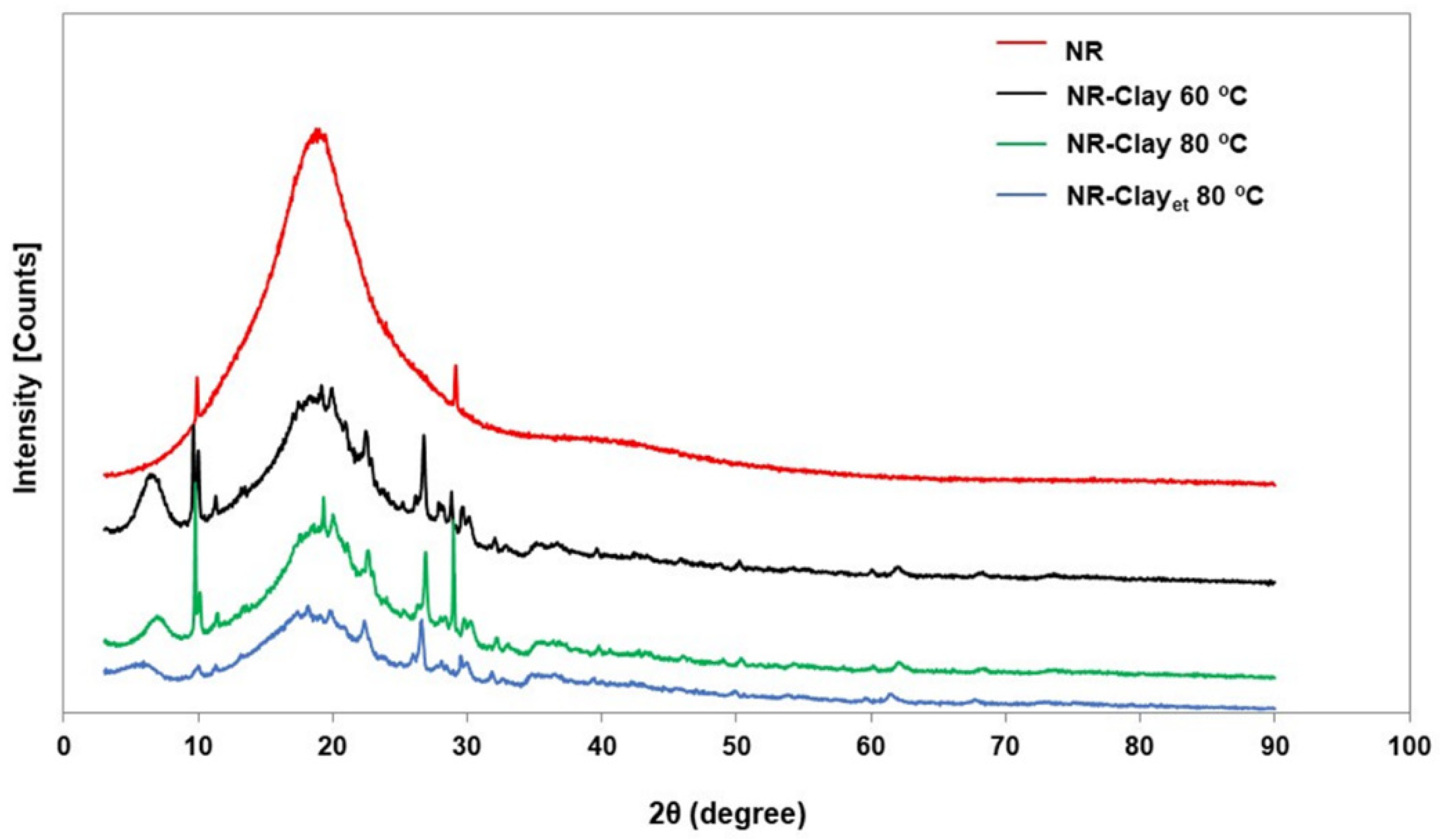
2.3.2. X-ray Diffraction
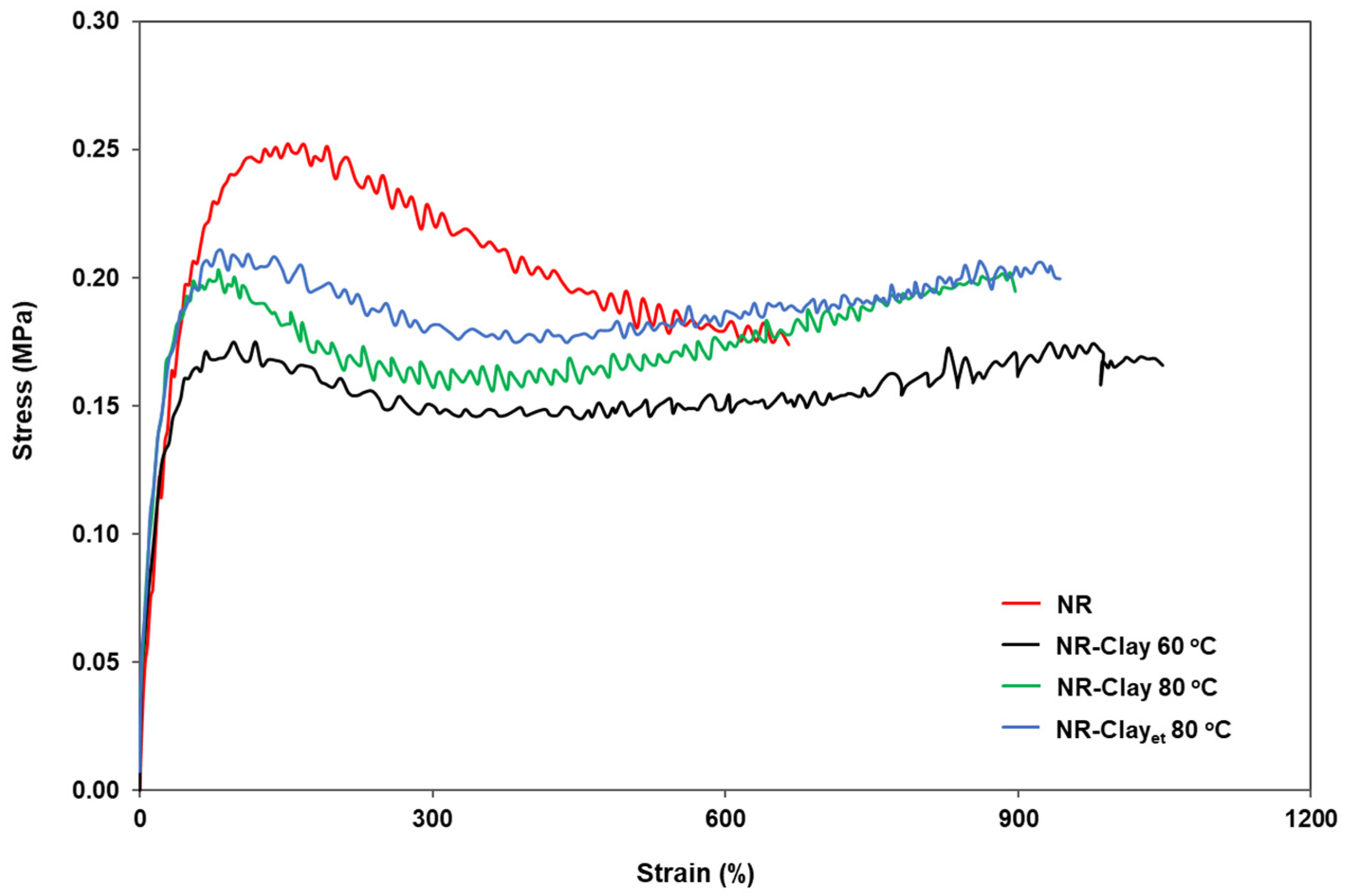
2.3.3. Mechanical Properties
3. Results and Discussion
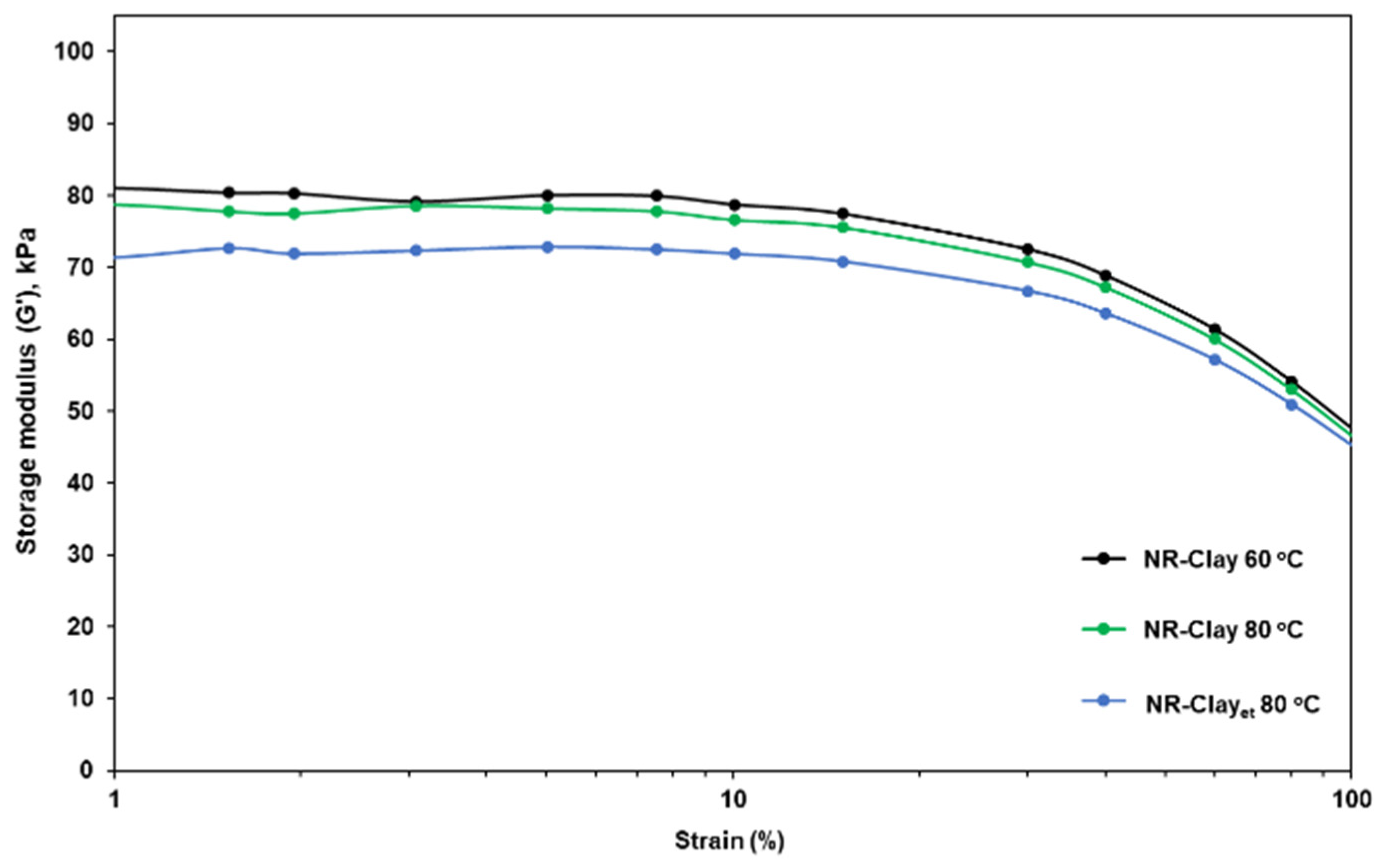
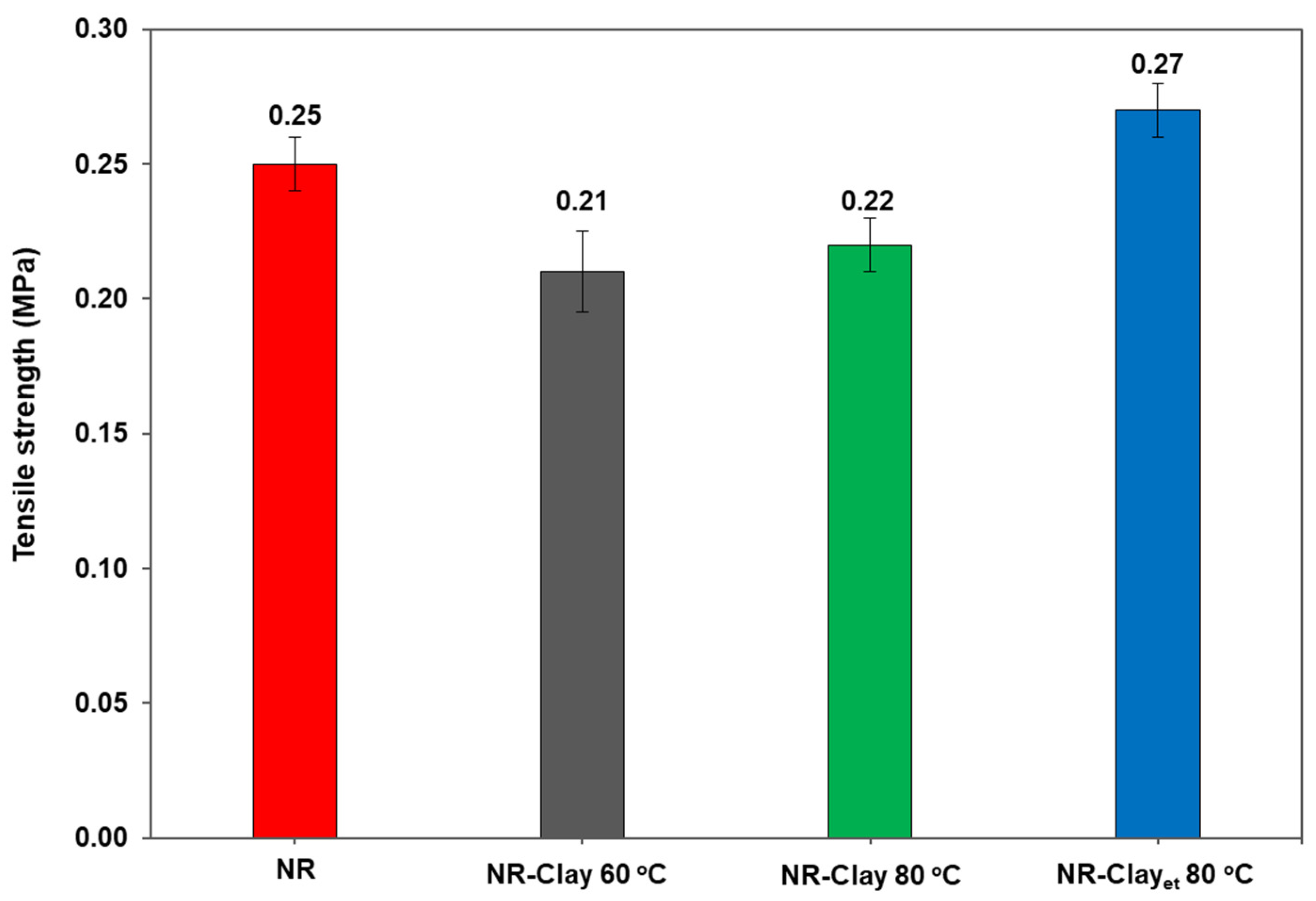
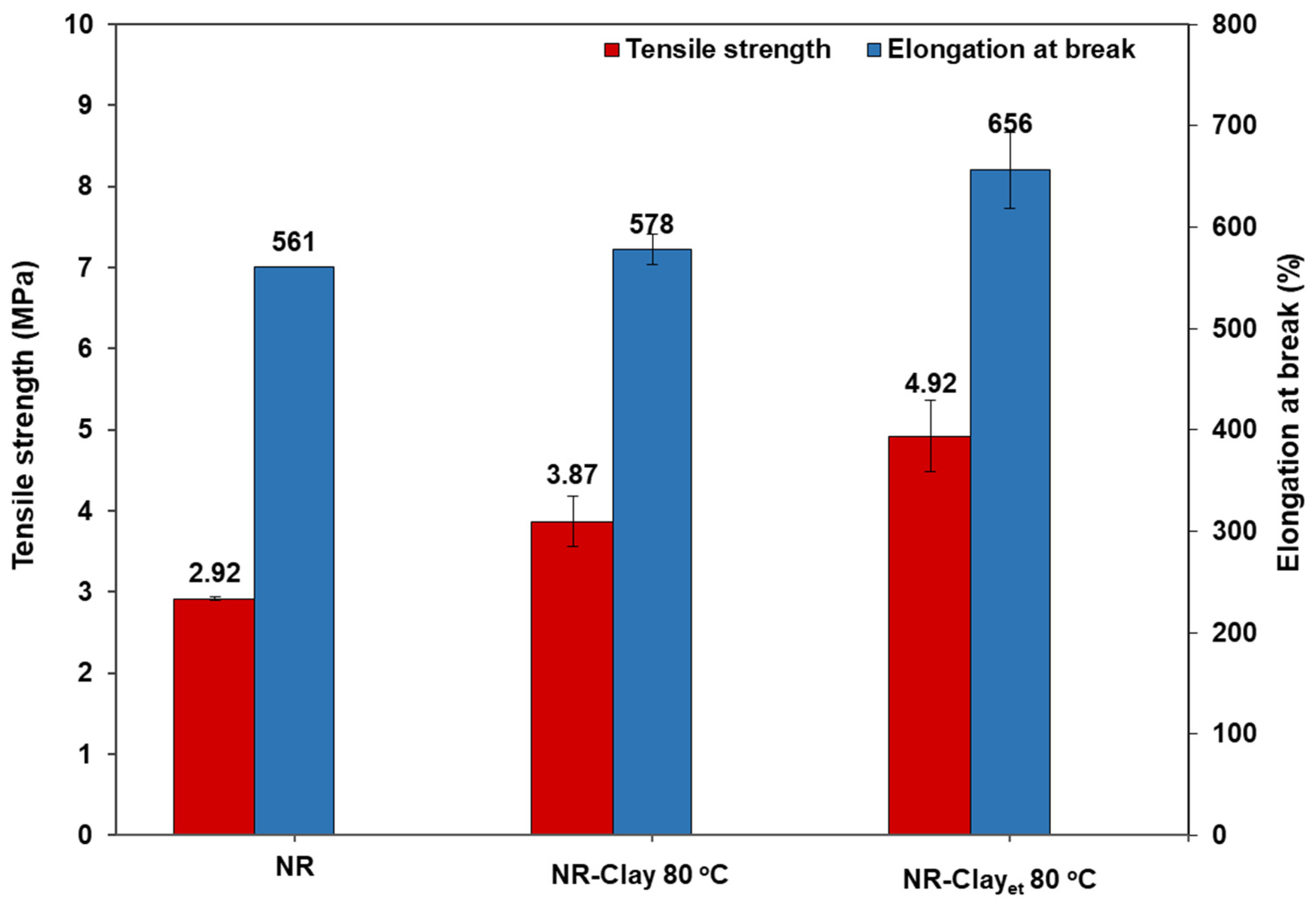
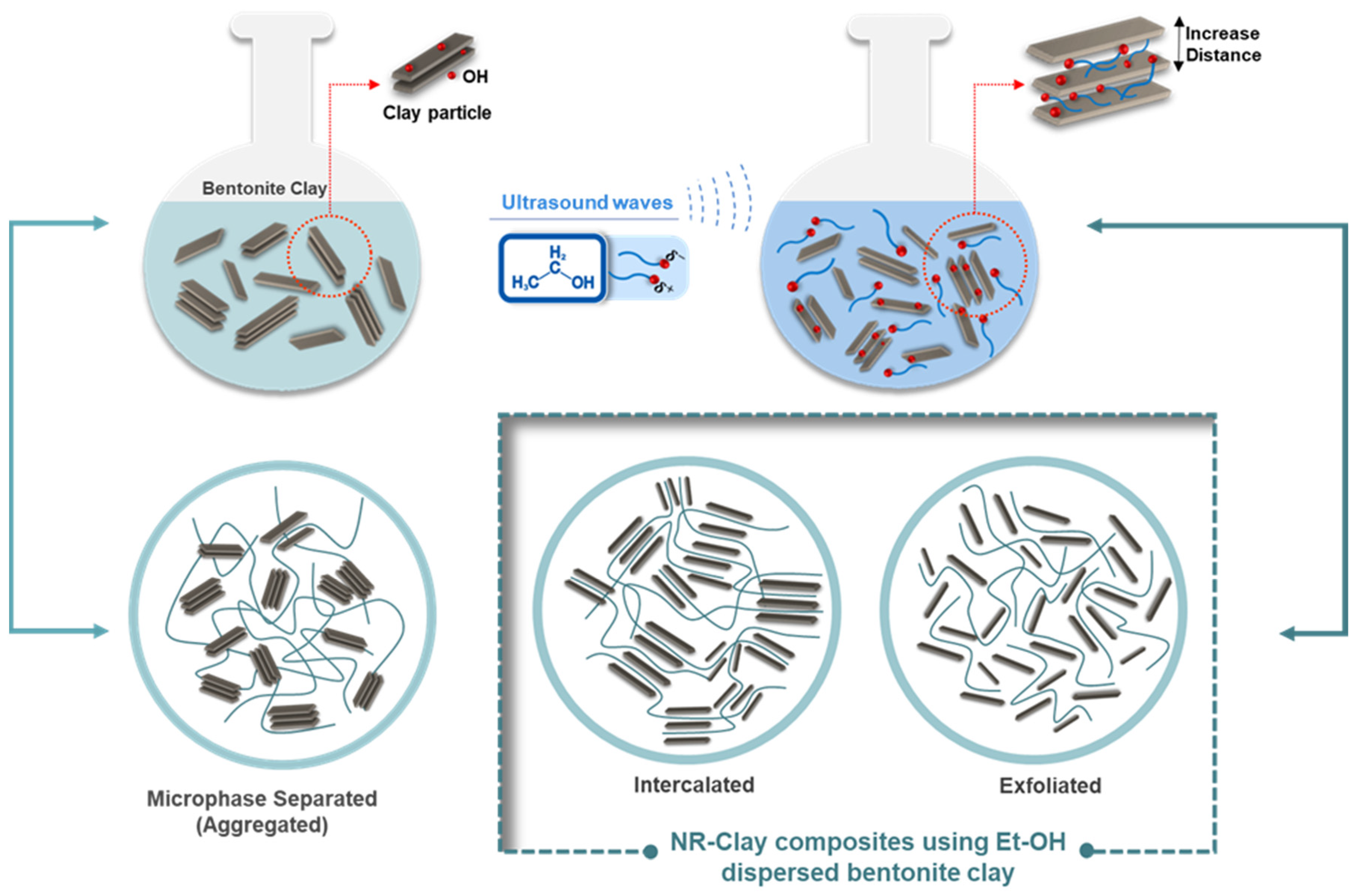
3.1. Effect of Et-OH on Bentonite Clay Dispersion in NR and NR-Clay Composites
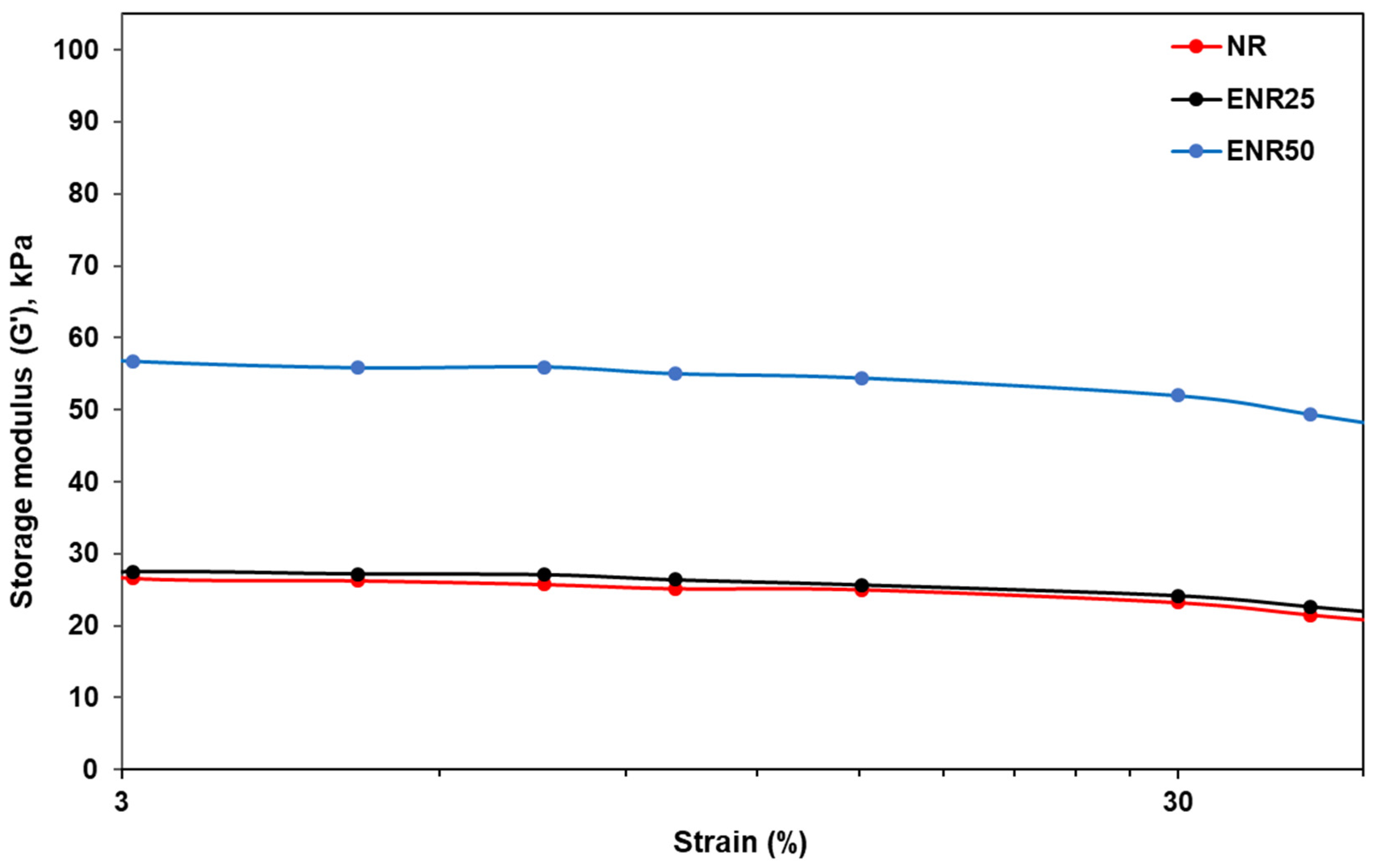
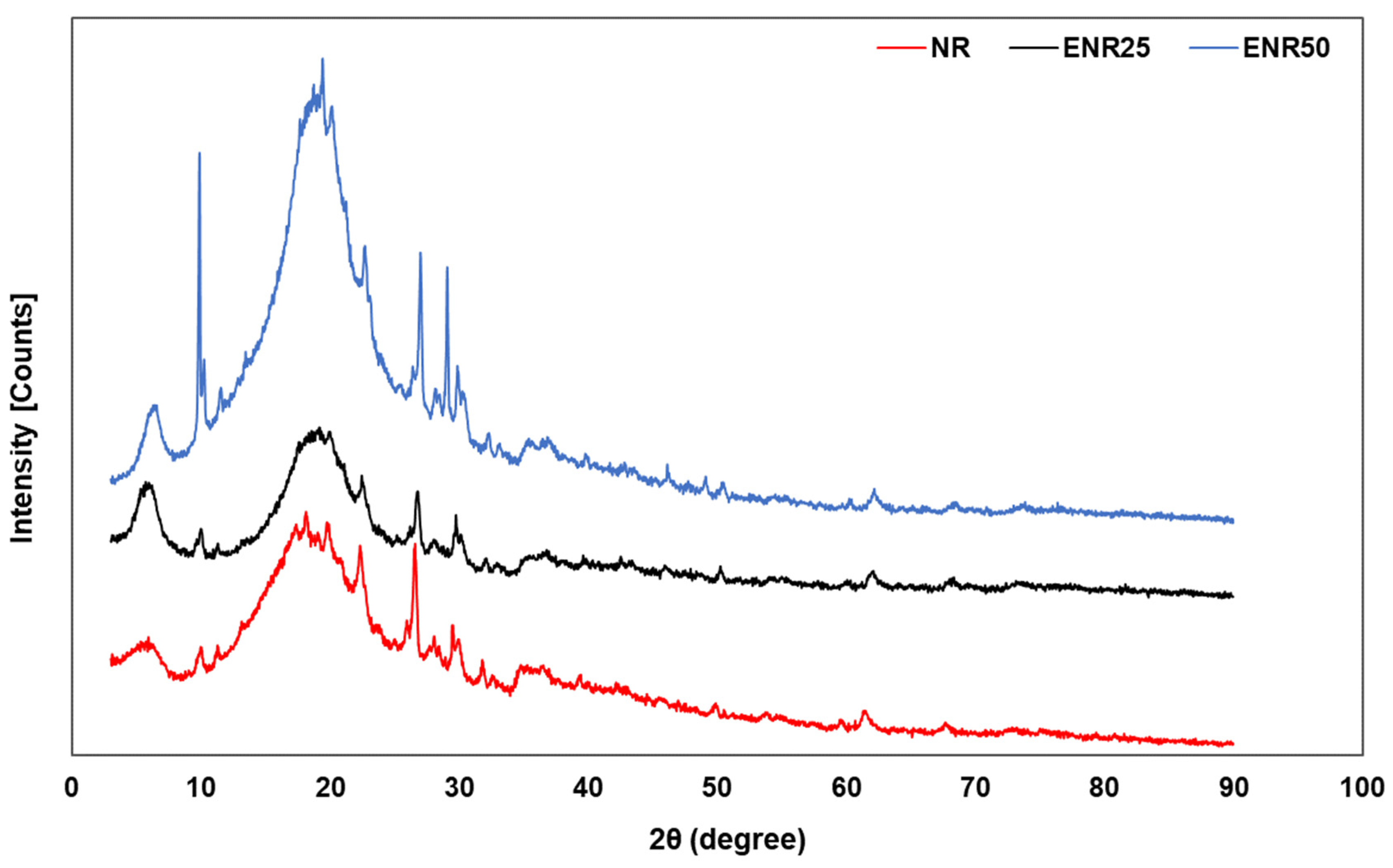
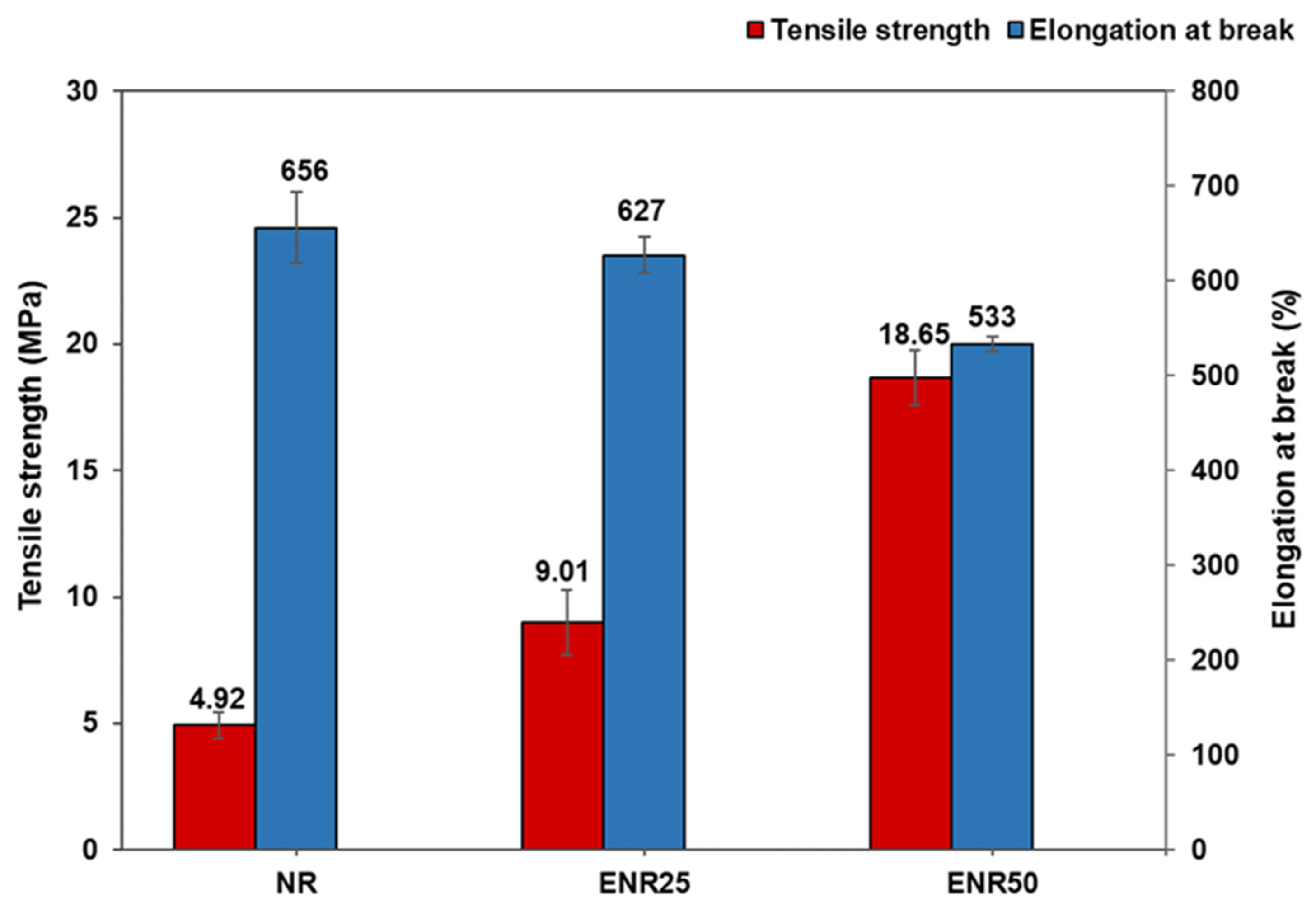
3.2. Effect of Rubber Types in NR-Clay Composites on the Properties of NR/Clay Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mente, P.; Motaung, T.E.; Hlangothi, S.P. Natural rubber and reclaimed rubber composites—A systematic review. Polym Sci. 2016, 2, 1. [Google Scholar] [CrossRef]
- Ku, H.; Wang, H.; Pattarachaiyakoop, N.; Trada, N. A review on the tensile properties of natural fiber reinforced polymer composites. Comp. Part B 2011, 42, 856–873. [Google Scholar]
- Ngo, T.-D. Introduction to Composite Materials; Intechopen: London, UK, 2020. [Google Scholar] [CrossRef]
- Ho, C.C. Fundamentals and Recent Applications of Natural Rubber Latex in Dipping Technology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 317–361. [Google Scholar] [CrossRef]
- Low, D.Y.S.; Supramaniam, J.; Soottitantawat, A.; Charinpanitkul, T.; Tanthapanichakoon, W.; Tan, K.W.; Tang, S.Y. Recent developments in nanocellulose-reinforced rubber matrix composites: A review. Polymers 2021, 13, 550. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Rajasekar, R.; Heinrich, G.; Das, A.; Das, C.K. Development of SBR-Nanoclay composites with epoxidized natural rubber as compatibilizer. Res. Lett. Nanotechnol. 2009, 2009, 405153. [Google Scholar] [CrossRef]
- Riza, M.; Mulyati, S.; Mulana, F.; Rihayat, T. The ultrasound effect of cationic surfactant on the preparation of natural bentonite Nisam, North Aceh. IOP Conf. Ser. J. Phys. 2017, 953, 012013. [Google Scholar]
- Singla, P.; Mehta, R.; Upadhyay, S.N. Clay Modification by the Use of Organic Cations. Green Sustain. Chem. 2012, 2, 21–25. [Google Scholar] [CrossRef]
- Miedzianowska, J.; Masłowski, M.; Rybiński, P.; Strzelec, K. Modified Nanoclays/Straw Fillers as Functional Additives of Natural Rubber Biocomposites. Polymers 2021, 13, 799. [Google Scholar] [CrossRef]
- Khar’kova, E.M.; Mendeleev, D.I.; Levin, I.S.; Sorokin, S.E.; Gerasin, V.A. Influence of small amounts of water and ethanol on Na+-montmorillonite solid-state modification by inorganic and organic intercalants. Appl. Clay Sci. 2020, 195, 105734. [Google Scholar] [CrossRef]
- El-Sabbagh, S.H.; Mahmoud, D.S.; Ahmed, N.M.; Ward, A.A.; Sabaa, M.W. Composites of styrene butadiene rubber/modified clay: Mechanical, dielectric and morphological properties. Pigm. Resin Technol. 2017, 46, 161–171. [Google Scholar]
- Wang, Y.; Zhang, L.; Tang, C.; Yu, D. Preparation and Characterization of Rubber-Clay Nanocomposites. J. Appl. Polym. Sci. 2000, 78, 1879–1883. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/Layered Silicate Nanocomposites: A Review from Preparation to Processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Khattab, R.M.; Saad, E.M.; Gado, R.A. Effect of Surfactant Types and Their Concentration on the Structural Characteristics of Nanoclay. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 616–623. [Google Scholar] [CrossRef]
- Linhares, F.N.; Gabriel, C.F.S.; de Sousa, A.M.F.; Nunes, R.C.R. Mechanical and rheological properties of nitrile rubber/fluoromica composites. Appl. Clay Sci. 2018, 162, 165–174. [Google Scholar] [CrossRef]
- Sapkota, J. Influence of Clay Modification on Curing Kinetics of Natural Rubber Nanocomposites. Master of Science Thesis, Material Research, Tampere University, Tampere, Finland, 8 June 2011. [Google Scholar] [CrossRef]
- Bokobza, L. Natural rubber nanocomposites: A review. Nanomaterials 2018, 9, 12. [Google Scholar]
- Kumar, A.; Lingfa, P. Sodium bentonite and kaolin clays: Comparative study on their FT-IR, XRF, and XRD. Mater. Today Proc. 2020, 22, 737–742. [Google Scholar] [CrossRef]
- Sharif, J.; Zin Wan Yunus, W.M.; Mohd Dahlan, K.Z.H.; Ahmad, M.H. Preparation and properties of radiation crosslinked natural rubber/clay nanocomposites. Polym. Test. 2005, 24, 211–217. [Google Scholar] [CrossRef]
- Saeed, A.; Hanif, M.A.; Nawaz, H.; Qadri, R.W.K. The production of biodiesel from plum waste oil using nano-structured catalyst loaded into supports. Sci. Rep. 2021, 11, 24120. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, L.; Wu, Y. Relationship between dynamic fatigue crack propagation properties and viscoelasticity of natural rubber/silicone rubber composites. RSC Adv. 2019, 9, 29813–29820. [Google Scholar] [CrossRef]
- Arroyo, M.; Lopez-Manchado, M.A.; Valentin, J.L.; Carretero, J. Morphology/behaviour relationship of nanocomposites based on natural rubber/epoxidized natural rubber blends. Compos. Sci. Technol. 2007, 67, 1330–1339. [Google Scholar] [CrossRef]
- George, S.C.; Rajan, R.; Aprem, A.S.; Thomas, S.; Kim, S.S. The fabrication and properties of natural rubber-clay nanocomposites. Polym. Test. 2017, 51, 165–173. [Google Scholar] [CrossRef]
- Yokkhun, P.; Thongnuanchan, B.; Nakason, C. Influence of epoxide levels in epoxidized natural rubber (ENR) molecules on cure characteristics, dynamic properties and mechanical properties of ENR montmorillonite clay nanocomposites. Adv. Mater. Res. 2014, 844, 247–250. [Google Scholar] [CrossRef]
- Tan, J.-H.; Wang, X.-P.; Liu, Y.-W.; Luo, Y.-F.; Jia, D.-M.; Liu, Y.-J.; Xiong, Y.-F.; Wang, W.-T. Effects of epoxidized natural rubber as a compatibilizer on latex compounded natural rubber-clay nanocomposites. J. Polym. Eng. 2017, 37, 43–51. [Google Scholar] [CrossRef]

| Ingredients | Function | Designation | |||
|---|---|---|---|---|---|
| NR | NR-Clay | ENR25-Clay | ENR50-Clay | ||
| Content (phr 1) | |||||
| 2 NR | Matrix | 100 | 100 | - | - |
| 3 ENR25 | Matrix | - | - | 100 | - |
| 4 ENR50 | Matrix | - | - | - | 100 |
| Bentonite clay (dispersion in Et-OH) | Dispersion agent | 0 | 20 | 20 | 20 |
| Zinc oxide | Activator | 5 | 5 | 5 | 5 |
| Stearic acid | Activator | 1 | 1 | 1 | 1 |
| 5 MBTS | Accelerator | 1 | 1 | 1 | 1 |
| Sulfur | Crosslinking agent | 2.5 | 2.5 | 2.5 | 2.5 |
| NR-Clay Composite | Two Theta (Degree) | d-Spacing (nm) |
|---|---|---|
| NR | - | - |
| NR-Clay 60 °C | 11.28 | 7.84 |
| 19.94 | 3.85 | |
| 22.86 | 3.89 | |
| 27.86 | 3.20 | |
| 28.81 | 3.10 | |
| NR-Clay 80 °C | 10.12 | 8.74 |
| 19.29 | 4.20 | |
| 22.60 | 3.93 | |
| 26.88 | 3.31 | |
| 28.33 | 3.15 | |
| NR-Clayet 80 °C | 10.03 | 8.82 |
| 18.15 | 4.99 | |
| 22.67 | 3.92 | |
| 26.52 | 3.36 | |
| 28.06 | 3.18 |
| Properties | NR | NR-Clay 80 °C | NR-Clayet 80 °C |
|---|---|---|---|
| 100% modulus (MPa) | 0.46 ± 0.01 | 0.47 ± 0.02 | 0.45 ± 0.08 |
| 300% modulus (MPa) | 0.97 ± 0.03 | 1.00 ± 0.02 | 1.12 ± 0.03 |
| Tensile strength (MPa) | 2.92 ± 0.02 | 3.87 ± 0.31 | 4.92 ± 0.44 |
| Rubber-Clay Composite | Two Theta (Degree) | d-Spacing (nm) |
|---|---|---|
| NR | 19.98 | 3.87 |
| ENR25 | 19.91 | 4.46 |
| ENR50 | 19.20 | 4.92 |
| Properties | NR | ENR25 | ENR50 |
|---|---|---|---|
| 100% modulus (MPa) | 0.45 ± 0.08 | 0.90 ± 0.34 | 1.41 ± 0.03 |
| 300% modulus (MPa) | 1.12 ± 0.03 | 1.56 ± 0.02 | 4.05 ± 0.01 |
| Reinforcing index (MPa) | 2.49 ± 0.37 | 1.73 ± 0.06 | 4.05 ± 0.33 |
| Tensile strength (MPa) | 4.92 ± 0.54 | 9.01 ± 1.28 | 18.65 ± 1.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keereerak, A.; Sukkhata, N.; Lehman, N.; Nakaramontri, Y.; Sengloyluan, K.; Johns, J.; Kalkornsurapranee, E. Development and Characterization of Unmodified and Modified Natural Rubber Composites Filled with Modified Clay. Polymers 2022, 14, 3515. https://doi.org/10.3390/polym14173515
Keereerak A, Sukkhata N, Lehman N, Nakaramontri Y, Sengloyluan K, Johns J, Kalkornsurapranee E. Development and Characterization of Unmodified and Modified Natural Rubber Composites Filled with Modified Clay. Polymers. 2022; 14(17):3515. https://doi.org/10.3390/polym14173515
Chicago/Turabian StyleKeereerak, Adisak, Nusara Sukkhata, Nussana Lehman, Yeampon Nakaramontri, Karnda Sengloyluan, Jobish Johns, and Ekwipoo Kalkornsurapranee. 2022. "Development and Characterization of Unmodified and Modified Natural Rubber Composites Filled with Modified Clay" Polymers 14, no. 17: 3515. https://doi.org/10.3390/polym14173515








