Indoxyl Sulfate Alters the Humoral Response of the ChAdOx1 COVID-19 Vaccine in Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Ethics
2.2. Theory and Calculation
2.2.1. Study Design
2.2.2. Measurement of Indoxyl Sulfate and Antibody Titers after ChAdOx1 Vaccine Injection
2.2.3. Statistics
3. Results
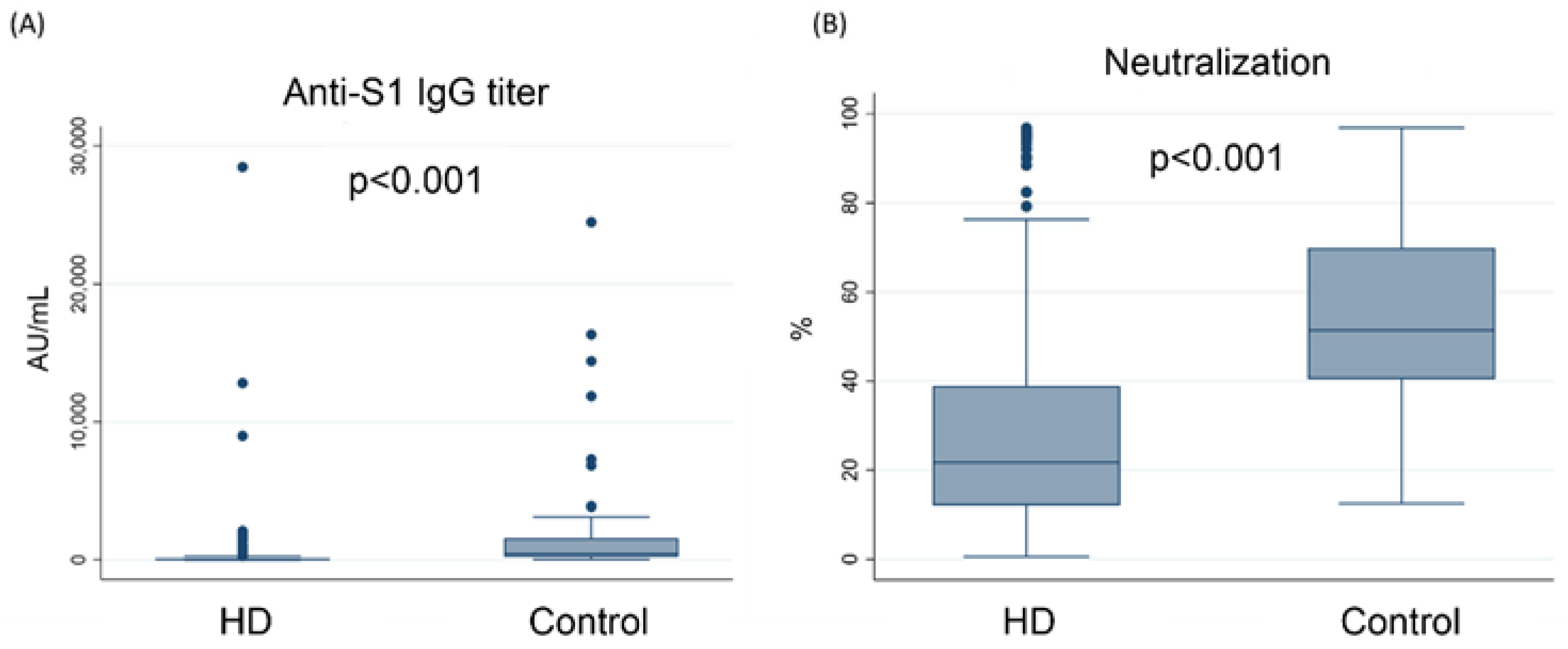
3.1. The Concentration of Anti-S1 IgG Was Lower in Hemodialysis Patients Than in the Control Group
3.2. The Humoral Response against SARS-CoV-2 Was Lower in Hemodialysis Patients
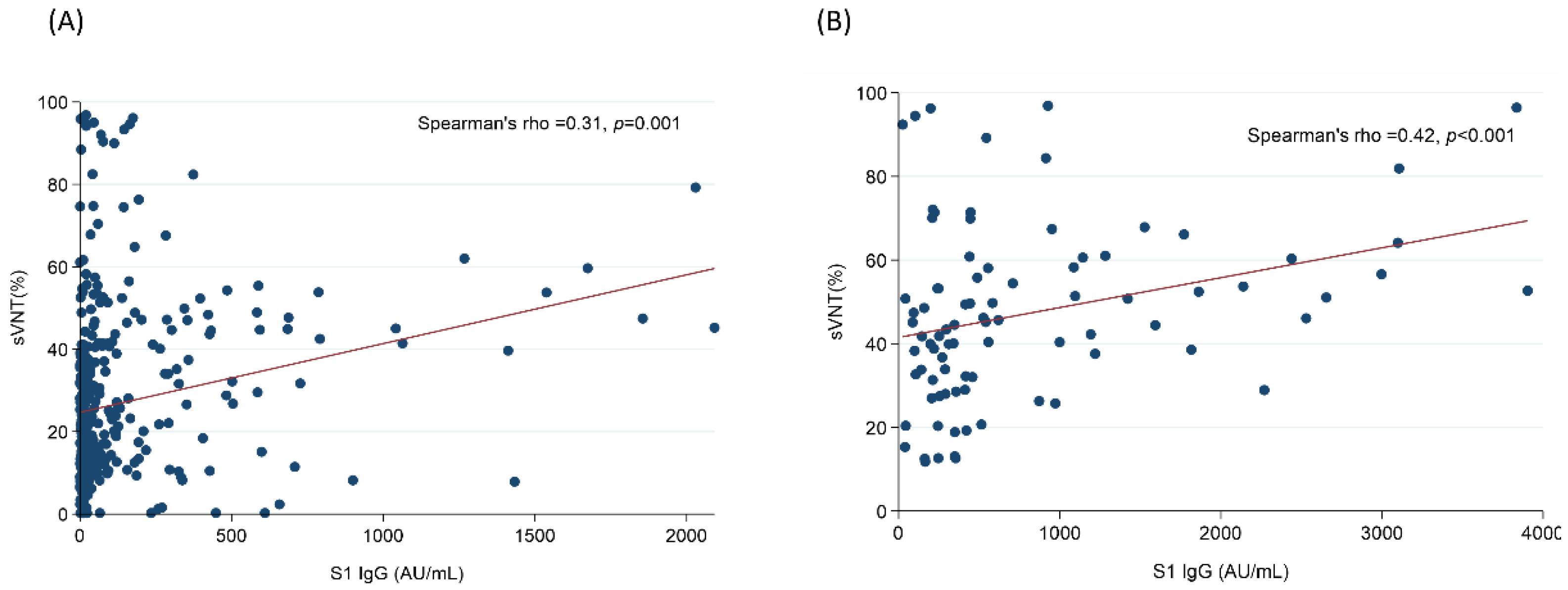
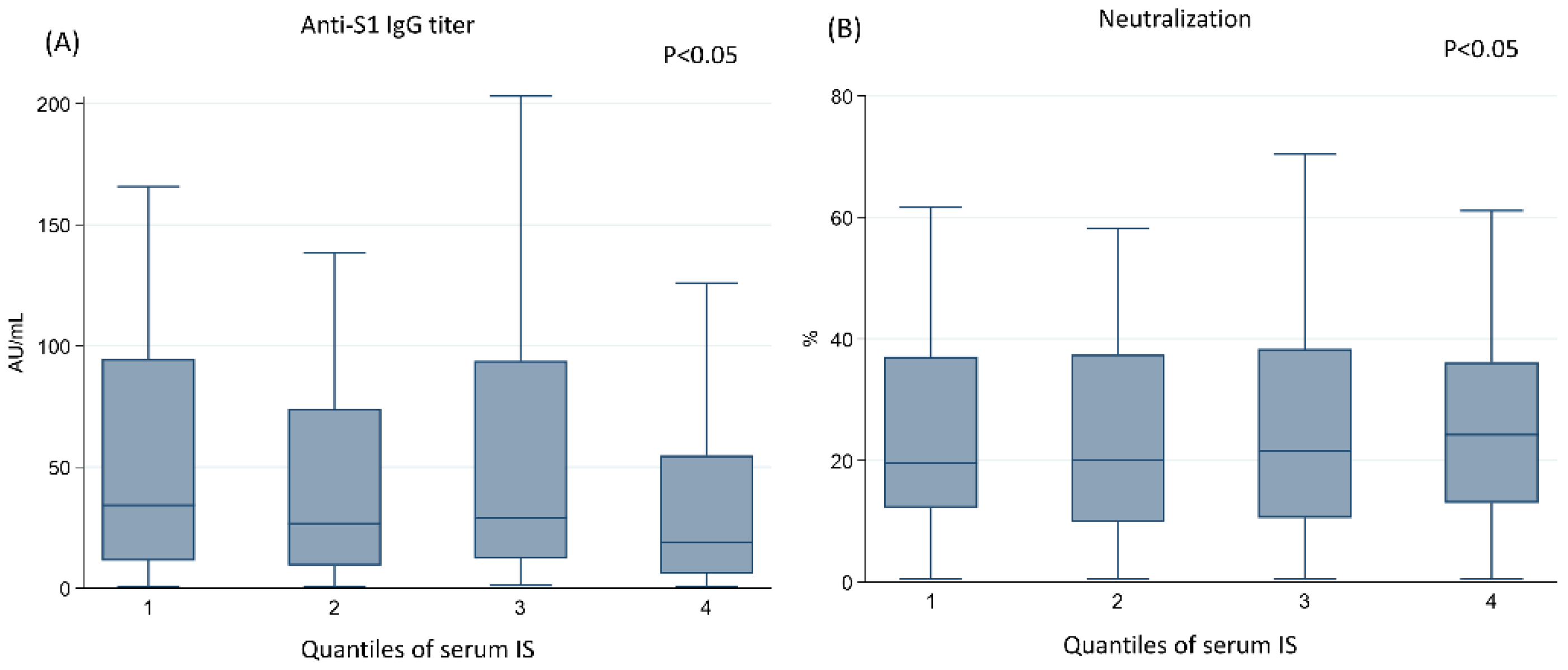
3.3. The Higher Concentration of IS Was Associated with the Lower Humoral Response after Vaccination with ChAdOx1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE-2 | angiotensin-converting enzyme 2 |
| CKD | chronic kidney disease |
| CX3CL1 | CX3C chemokine receptor 1 |
| COVID-19 | the coronavirus 2019 disease |
| ELISA | enzyme-linked immunosorbent assay |
| ESRD | end stage renal disease |
| IgG | immunoglobulin G |
| INF-γ | interferon gamma |
| IS | indoxyl sulfate |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| RBD | receptor binding domain |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| sVNT | surrogate virus neutralization test |
References
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.J.; Ran, L.; Xu, L.; Danesh, A.; Bermejo-Martin, J.F.; Cameron, C.M.; Muller, M.P.; Gold, W.L.; Richardson, S.E.; Poutanen, S.M.; et al. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J. Virol. 2007, 81, 8692–8706. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.S.; de Sá, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Mamun, A.; Dominic, A.; Le, N.-T. SARS-CoV-2 Mediated Endothelial Dysfunction: The Potential Role of Chronic Oxidative Stress. Front. Physiol. 2021, 11, 605908. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Clemens, S.A.C.; Folegatti, P.M.; Emary, K.R.W.; Weckx, L.Y.; Ratcliff, J.; Bibi, S.; De Almeida Mendes, A.V.; Milan, E.P.; Pittella, A.; Schwarzbold, A.V.; et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 lineages circulating in Brazil. Nat. Commun. 2021, 12, 5861. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 28, 202–221. [Google Scholar] [CrossRef]
- Hitchings, M.D.T.; Ranzani, O.T. Effectiveness of ChAdOx1 vaccine in older adults during SARS-CoV-2 Gamma variant circulation in São Paulo. Nat. Commun. 2021, 12, 6220. [Google Scholar] [CrossRef]
- Tang, Y.W.; Schmitz, J.E.; Persing, D.H.; Stratton, C.W. Laboratory Diagnosis of COVID-19: Current Issues and Challenges. J. Clin. Microbiol. 2020, 58, e00512-20. [Google Scholar] [CrossRef] [PubMed]
- Petherick, A. Developing antibody tests for SARS-CoV-2. Lancet 2020, 395, 1101–1102. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Reimerink, J. Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT). Emerg. Microbes Infect. 2020, 9, 2394–2403. [Google Scholar] [CrossRef]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273. JAMA 2021, 326, 1533–1535. [Google Scholar] [CrossRef]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Morillas Ramos, G.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Chia, W.N.; Zhu, F.; Ong, S.W.X.; Young, B.E.; Fong, S.-W.; Le Bert, N.; Tan, C.W.; Tiu, C.; Zhang, J.; Tan, S.Y.; et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: A longitudinal study. Lancet Microbe 2021, 2, e240–e249. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Kim, H.Y.; Yoo, T.-H.; Hwang, Y.; Lee, G.H.; Kim, B.; Jang, J.; Yu, H.T.; Kim, M.C.; Cho, J.-Y.; Lee, C.J.; et al. Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD). Sci. Rep. 2017, 7, 3057. [Google Scholar] [CrossRef]
- Adesso, S.; Popolo, A.; Bianco, G.; Sorrentino, R.; Pinto, A.; Autore, G.; Marzocco, S. The Uremic Toxin Indoxyl Sulphate Enhances Macrophage Response to LPS. PLoS ONE 2013, 8, e76778. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.L.; Zhao, J.F.; Huang, P.H.; Guo, B.C.; Tarng, D.C.; Lee, T.S. Indoxyl sulfate impairs valsartan-induced neovascularization. Redox Biol. 2020, 30, 101433. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Lu, K.C.; Kuo, K.L. The Efficacy of COVID-19 Vaccines in Chronic Kidney Disease and Kidney Transplantation Patients: A Narrative Review. Vaccines 2021, 9, 885. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Montez-Rath, M.E.; Han, J.; Garcia, P.; Cadden, L.; Hunsader, P.; Kerschmann, R.; Beyer, P.; Dittrich, M.; Block, G.A.; et al. Antibody Response to COVID-19 Vaccination in Patients Receiving Dialysis. J. Am. Soc. Nephrol. 2021, 32, 2435. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-C.; Su, S.-Y. Epidemic trend of COVID-19 in Taiwan, May to June 2021. J. Formos. Med. Assoc. 2021, 121, 580–581. [Google Scholar] [CrossRef]
- Daugirdas, J.T. Simplified equations for monitoring Kt/V, PCRn, eKt/V, and ePCRn. Adv. Ren. Replace. Ther. 1995, 2, 295–304. [Google Scholar] [CrossRef]
- Brill, L.; Rechtman, A.; Zveik, O.; Haham, N.; Oiknine-Djian, E.; Wolf, D.G.; Levin, N.; Raposo, C.; Vaknin-Dembinsky, A. Humoral and T-Cell Response to SARS-CoV-2 Vaccination in Patients With Multiple Sclerosis Treated With Ocrelizumab. JAMA Neurol. 2021, 78, 1510–1514. [Google Scholar] [CrossRef]
- Payne, R.P.; Longet, S.; Austin, J.A.; Skelly, D.T.; Dejnirattisai, W.; Adele, S.; Meardon, N.; Faustini, S.; Al-Taei, S.; Moore, S.C.; et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021, 184, 5699–5714.e5611. [Google Scholar] [CrossRef]
- Wickens, O.; Chinnadurai, R.; Mannan, F.; Svendsen, F.; Baig, M.Y.; Chukwu, C.; Ali, I.; Summersgill, C.; Evans, D.; Antoine, B.V.; et al. Investigating the utility of COVID-19 antibody testing in end-stage renal disease patients receiving haemodialysis: A cohort study in the United Kingdom. BMC Nephrol. 2021, 22, 154. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Bachelet, T.; Bourdenx, J.-P.; Martinez, C.; Mucha, S.; Martin-Dupont, P.; Perier, V.; Pommereau, A. Humoral response after SARS-CoV-2 mRNA vaccines in dialysis patients: Integrating anti-SARS-CoV-2 Spike-Protein-RBD antibody monitoring to manage dialysis centers in pandemic times. PLoS ONE 2021, 16, e0257646. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Arevalo, H.; Choi, M.; Stefanski, A.L.; Halleck, F.; Weber, U.; Szelinski, F.; Jahrsdörfer, B.; Schrezenmeier, H.; Ludwig, C.; Sattler, A.; et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci. Immunol. 2021, 6, eabj1031. [Google Scholar] [CrossRef]
- Goupil, R.; Benlarbi, M.; Beaubien-Souligny, W.; Nadeau-Fredette, A.C.; Chatterjee, D.; Goyette, G.; Gunaratnam, L.; Lamarche, C.; Tom, A.; Finzi, A.; et al. Short-term antibody response after 1 dose of BNT162b2 vaccine in patients receiving hemodialysis. Can. Med. Assoc. J. 2021, 193, E793–E800. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Lee, T.H.; Tian, Y.C.; Lee, C.C.; Fan, P.C.; Chang, C.H. Immunogenicity Rates After SARS-CoV-2 Vaccination in People With End-stage Kidney Disease: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2131749. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.C.; Kuo, K.L.; Huang, H.L.; Lin, C.C.; Tsai, T.H.; Wang, C.H.; Chen, J.W.; Lin, S.J.; Huang, P.H.; Tarng, D.C. Indoxyl sulfate suppresses endothelial progenitor cell-mediated neovascularization. Kidney Int. 2016, 89, 574–585. [Google Scholar] [CrossRef]
- Xiang, F.; Cao, X.; Shen, B.; Chen, X.; Guo, M.; Ding, X.; Zou, J. Transcriptome Profiling Reveals Indoxyl Sulfate Should Be Culpable of Impaired T Cell Function in Chronic Kidney Disease. Front. Med. 2020, 7, 178. [Google Scholar] [CrossRef]
- Nakano, T.; Katsuki, S.; Chen, M.; Decano, J.L.; Halu, A.; Lee, L.H.; Pestana, D.V.S.; Kum, A.S.T.; Kuromoto, R.K.; Golden, W.S.; et al. Uremic Toxin Indoxyl Sulfate Promotes Proinflammatory Macrophage Activation Via the Interplay of OATP2B1 and Dll4-Notch Signaling. Circulation 2019, 139, 78–96. [Google Scholar] [CrossRef]
- Shyu, J.F.; Liu, W.C.; Zheng, C.M.; Fang, T.C.; Hou, Y.C.; Chang, C.T.; Liao, T.Y.; Chen, Y.C.; Lu, K.C. Toxic Effects of Indoxyl Sulfate on Osteoclastogenesis and Osteoblastogenesis. Int. J. Mol. Sci. 2021, 22, 11265. [Google Scholar] [CrossRef]
- Liu, W.-C.; Tomino, Y.; Lu, K.-C. Impacts of Indoxyl Sulfate and p-Cresol Sulfate on Chronic Kidney Disease and Mitigating Effects of AST-120. Toxins 2018, 10, 367. [Google Scholar] [CrossRef]
- Belikov, A.V.; Schraven, B.; Simeoni, L. T cells and reactive oxygen species. J. Biomed. Sci. 2015, 22, 85. [Google Scholar] [CrossRef]
- Hartzell, S.; Bin, S.; Cantarelli, C.; Haverly, M.; Manrique, J.; Angeletti, A.; Manna, G.L.; Murphy, B.; Zhang, W.; Levitsky, J.; et al. Kidney Failure Associates With T Cell Exhaustion and Imbalanced Follicular Helper T Cells. Front. Immunol. 2020, 11, 583702. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.-H.; Yu, M.-C.; Wei, M.-J.; Kuo, K.-L. The Therapeutic Strategies for Uremic Toxins Control in Chronic Kidney Disease. Toxins 2021, 13, 573. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Shyu, J.-F.; Lin, Y.-F.; Chiu, H.-W.; Lim, P.S.; Lu, C.-L.; Zheng, C.-M.; Hou, Y.-C.; Chen, P.-H.; Lu, K.-C. Resveratrol Rescue Indoxyl Sulfate-Induced Deterioration of Osteoblastogenesis via the Aryl Hydrocarbon Receptor /MAPK Pathway. Int. J. Mol. Sci. 2020, 21, 7483. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, M.; Zaffina, S.; Fernandez Salinas, A.; Bocci, C.; Palomba, P.; Conti, M.G.; Terreri, S.; Frisullo, G.; Giorda, E.; Scarsella, M.; et al. Evolution of Human Memory B Cells From Childhood to Old Age. Front. Immunol. 2021, 12, 690534. [Google Scholar] [CrossRef]
- Ju, C.H.; Blum, L.K.; Kongpachith, S.; Lingampalli, N.; Mao, R.; Brodin, P.; Dekker, C.L.; Davis, M.M.; Robinson, W.H. Plasmablast antibody repertoires in elderly influenza vaccine responders exhibit restricted diversity but increased breadth of binding across influenza strains. Clin. Immunol. 2018, 193, 70–79. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B. Effects of aging on B cell function. Curr. Opin. Immunol. 2009, 21, 425–430. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Mendez, N.V.; Landin, A.M.; Ryan, J.G.; Blomberg, B.B. Young and elderly patients with type 2 diabetes have optimal B cell responses to the seasonal influenza vaccine. Vaccine 2013, 31, 3603–3610. [Google Scholar] [CrossRef][Green Version]
- Cheung, A.K.; Chang, T.I.; Cushman, W.C.; Furth, S.L.; Hou, F.F.; Ix, J.H.; Knoll, G.A.; Muntner, P.; Pecoits-Filho, R.; Sarnak, M.J.; et al. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021, 99, S1–S87. [Google Scholar] [CrossRef]
- Van Praet, J.; Reynders, M.; De Bacquer, D.; Viaene, L.; Schoutteten, M.; Caluwé, R.; Doubel, P.; Heylen, L.; De Bel, A.; Steensels, D.; et al. Predictors and Dynamics of the Humoral and Cellular Immune Response to SARS-CoV-2 mRNA Vaccines in Hemodialysis Patients: A Multicenter Observational Study. J. Am. Soc. Nephrol. 2021, 32, 3208–3220. [Google Scholar] [CrossRef]
- Bertrand, D.; Hamzaoui, M.; Lemée, V.; Lamulle, J.; Hanoy, M.; Laurent, C.; Lebourg, L.; Etienne, I.; Lemoine, M.; Le Roy, F.; et al. Antibody and T Cell Response to SARS-CoV-2 Messenger RNA BNT162b2 Vaccine in Kidney Transplant Recipients and Hemodialysis Patients. J. Am. Soc. Nephrol. 2021, 32, 2147. [Google Scholar] [CrossRef]
- Speer, C.; Göth, D.; Benning, L.; Buylaert, M.; Schaier, M.; Grenz, J.; Nusshag, C.; Kälble, F.; Kreysing, M.; Reichel, P.; et al. Early Humoral Responses of Hemodialysis Patients after COVID-19 Vaccination with BNT162b2. Clin. J. Am. Soc. Nephrol. 2021, 16, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, K.A.; Pindel, M.; Pietruczuk, K.; Kuźmiuk-Glembin, I.; Storoniak, H.; Dębska-Ślizień, A.; Witkowski, J.M. The influence of a single hemodialysis procedure on human T lymphocytes. Sci. Rep. 2019, 9, 5041. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J.T.; Depner, T.A.; Inrig, J.; Mehrotra, R.; Rocco, M.V.; Suri, R.S.; Weiner, D.E.; Greer, N.; Ishani, A.; MacDonald, R.; et al. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 Update. Am. J. Kidney Dis. 2015, 66, 884–930. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-N.; Wu, I.W.; Huang, Y.-F.; Peng, S.-Y.; Huang, Y.-C.; Ning, H.-C. Measuring serum total and free indoxyl sulfate and p-cresyl sulfate in chronic kidney disease using UPLC-MS/MS. J. Food Drug Anal. 2019, 27, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.Y.; Yen, Y.F.; Chen, S.Y.; Lee, T.I.; Huang, K.H.; Chan, T.C.; Tung, T.H.; Hsu, L.Y.; Chiu, T.Y.; Hsueh, P.R.; et al. Learning from the past: Taiwan’s responses to COVID-19 versus SARS. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021, 110, 469–478. [Google Scholar] [CrossRef]
- Liang, L.L.; Kao, C.T.; Ho, H.J.; Wu, C.Y. COVID-19 case doubling time associated with non-pharmaceutical interventions and vaccination: A global experience. J. Glob. Health 2021, 11, 05021. [Google Scholar] [CrossRef]

| Characteristics | Indoxyl Sulfate Concentrations | p Value | |||
|---|---|---|---|---|---|
| Quartile 1 (<95.03) | Quartile 2 (95.03–147.255) | Quartile 3 (147.255–211.59) | Quartile 4 (≥211.59) | ||
| Patient number | 91 | 88 | 90 | 89 | |
| Age group, years | 0.15 | ||||
| <65 | 45 (49.5) | 56 (63.6) | 58 (64.4) | 52 (58.4) | |
| ≥65 | 46 (50.5) | 32 (36.4) | 32 (35.6) | 37 (41.6) | |
| Mean (sd) | 67 (11.1) | 65.2 (12) | 67.4 (15) | 67.4 (11.7) | 0.59 |
| Sex, n (%) | 0.20 | ||||
| Male | 57 (62.6) | 42 (47.7) | 47 (52.2) | 52 (58.4) | |
| Female | 34 (37.4) | 46 (52.3) | 43 (47.8) | 37 (41.6) | |
| Diabetes mellitus, n (%) | 43 (47.3) | 48 (54.5) | 51 (56.7) | 42 (47.2) | 0.46 |
| WBC count, per 1000/μL | |||||
| Mean (sd) | 6 (1.6) | 6.2 (1.8) | 6.6 (2.2) | 6.3 (2) | 0.24 |
| Median (IQR) | 6 (5–7) | 5.9 (4.9–7.3) | 6.5 (5.1–7.8) | 5.8 (5–7.6) | 0.39 |
| ≥8.2 | 11 (12.1) | 10 (11.4) | 18 (20) | 15 (16.9) | 0.32 |
| Lymphocyte, % | |||||
| Mean (sd) | 22.6 (8.1) | 22.6 (6.6) | 21.9 (8.2) | 22.1 (8) | 0.64 |
| Median (IQR) | 21.7 (17.6–26.2) | 22.4 (18–27.2) | 21.8 (16.7–25.8) | 21.8 (17.3–26.4) | 0.77 |
| <20 | 44 (48.4) | 43 (48.9) | 58 (64.4) | 56 (62.9) | 0.04 |
| Kt/V | |||||
| Mean (sd) | 1.6 (0.3) | 1.6 (0.3) | 1.5 (0.3) | 1.6 (0.3) | 0.91 |
| Median (IQR) | 1.5 (1.4–1.8) | 1.6 (1.4–1.8) | 1.5 (1.3–1.7) | 1.6 (1.4–1.8) | 0.21 |
| ≥1.2 | 84 (92.3) | 82 (93.2) | 79 (87.8) | 84 (94.4) | 0.39 |
| Dialysis vintage, years | |||||
| Mean (sd) | 4.9 (5.6) | 5.4 (5.8) | 3.7 (3.8) | 5.4 (6.9) | 0.92 |
| Median (IQR) | 2.9 (1.4–6.4) | 3.5 (2.2–6.7) | 2.8 (1.5–4.4) | 3.3 (1.4–5.9) | 0.11 |
| ≥5 years | 44 (48.4) | 53 (60.2) | 36 (40) | 46 (51.7) | 0.06 |
| Indoxyl sulfate | |||||
| Mean (sd) | 74 (18.6) | 119.3 (14.6) | 176 (17.2) | 381.2 (443.3) | <0.001 |
| Median (IQR) | 78.8 (65.1–87.7) | 119.9 (106.6–131.2) | 172.8 (162.5–190.6) | 273.6 (249.1–357.4) | <0.01 |
| PCS | |||||
| Mean (sd) | 20.7 (20.1) | 24.1 (25.6) | 32.3 (72.3) | 81.4 (454.1) | 0.08 |
| Median (IQR) | 12.4 (4.6–31.7) | 9.6 (4.7–34.1) | 10.5 (4.9–31.5) | 9.5 (4.4–29.9) | 0.96 |
| Anti-S1 IgG, AU/mL | |||||
| Mean (sd) | 146.7 (254.7) | 134.9 (308.8) | 101 (195.1) | 176.6 (382.4) | 0.54 |
| Median (IQR) | 37.8 (14–126.4) | 29.8 (12.2–115.2) | 29.2 (12.3–102.7) | 27.6 (12–132.1) | 0.74 |
| sVNT (% neutralization) | |||||
| Mean (sd) | 28.2 (22.2) | 27.6 (20.5) | 27.5 (21) | 27.4 (20) | 0.79 |
| Median (IQR) | 20.5 (12.5–38.4) | 23.8 (11.3–40.7) | 21.9 (10.7–39.3) | 21.8 (12.7–35.8) | 0.98 |
| Crude Model | Adjusted Model a | |||
|---|---|---|---|---|
| Regression Coefficients (95% Confidence Intervals) | p Value | Regression Coefficients (95% Confidence Intervals) | p Value | |
| Indoxyl sulfate | ||||
| Quartile 1 | reference | reference | ||
| Quartile 2 | −110.5 (−200.1 to −20.8) | 0.016 | −119.1 (−207.8 to −30.4) | 0.009 |
| Quartile 3 | −189.4 (−301.9 to −77.0) | 0.001 | −225.1 (−340.2 to −110.1) | <0.001 |
| Quartile 4 | −352.4 (−511.9 to −192.9) | <0.001 | −448.2 (−622.0 to −274.5) | <0.001 |
| p for trend | <0.001 | <0.001 | ||
| Age ≥ 65 vs. age < 65 | 155.9 (107.4 to 204.4) | <0.001 | 61.5 (5.3 to 117.7) | 0.032 |
| Male vs. female sex | 105.3 (63.4 to 147.3) | <0.001 | −34.8 (−90.9 to 21.3) | 0.22 |
| Diabetes mellitus | 108.2 (66.2 to 150.2) | <0.001 | −45.1 (−101.4 to 11.2) | 0.12 |
| Dialysis vintage ≥ 5 vs. <5 years | 102.6 (46.2 to 159.0) | <0.001 | −37.1 (−97.7 to 23.5) | 0.23 |
| % Lymphocyte < 20% vs. ≥20% | 92.9 (51.4 to 134.4) | <0.001 | −66.0 (−124.1 to −7.8) | 0.026 |
| Crude Model | Adjusted Model a | |||
|---|---|---|---|---|
| Regression Coefficients (95% Confidence Intervals) | p Value | Regression Coefficients (95% Confidence Intervals) | p Value | |
| Indoxyl sulfate | ||||
| Quartile 1 | reference | reference | ||
| Quartile 2 | 1.7 (−6.8 to 10.3) | 0.69 | 2.2 (−6.2 to 10.7) | 0.61 |
| Quartile 3 | −8.8 (−19.4 to 1.9) | 0.11 | −5.8 (−16.6 to 5.1) | 0.29 |
| Quartile 4 | −27.2 (−42.4 to −12.0) | <0.001 | −21.2 (−37.8 to −4.5) | 0.013 |
| p for trend | <0.001 | <0.001 | ||
| Age ≥ 65 vs. age < 65 | 30.1 (24.6 to 35.6) | <0.001 | 8.8 (3.5 to 14.1) | 0.001 |
| Male vs. female | 25.4 (20.8 to 30.1) | <0.001 | 0.9 (−4.4 to 6.2) | 0.74 |
| Diabetic | 27.7 (23.2 to 32.1) | <0.001 | 3.4 (−1.9 to 8.7) | 0.21 |
| Dialysis vintage ≥ 5 vs. <5 years | 27.3 (20.8 to 33.9) | <0.001 | 3.2 (−2.5 to 8.9) | 0.27 |
| % Lymphocyte < 20% vs. ≥20% | 24.0 (19.4 to 28.7) | <0.001 | −3.2 (−8.7 to 2.3) | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.-C.; Wu, C.-L.; Lu, K.-C.; Kuo, K.-L. Indoxyl Sulfate Alters the Humoral Response of the ChAdOx1 COVID-19 Vaccine in Hemodialysis Patients. Vaccines 2022, 10, 1378. https://doi.org/10.3390/vaccines10091378
Hou Y-C, Wu C-L, Lu K-C, Kuo K-L. Indoxyl Sulfate Alters the Humoral Response of the ChAdOx1 COVID-19 Vaccine in Hemodialysis Patients. Vaccines. 2022; 10(9):1378. https://doi.org/10.3390/vaccines10091378
Chicago/Turabian StyleHou, Yi-Chou, Chia-Lin Wu, Kuo-Cheng Lu, and Ko-Lin Kuo. 2022. "Indoxyl Sulfate Alters the Humoral Response of the ChAdOx1 COVID-19 Vaccine in Hemodialysis Patients" Vaccines 10, no. 9: 1378. https://doi.org/10.3390/vaccines10091378
APA StyleHou, Y.-C., Wu, C.-L., Lu, K.-C., & Kuo, K.-L. (2022). Indoxyl Sulfate Alters the Humoral Response of the ChAdOx1 COVID-19 Vaccine in Hemodialysis Patients. Vaccines, 10(9), 1378. https://doi.org/10.3390/vaccines10091378






