Evaluation of the Effectiveness of Iontophoresis with Perskindol Gel in Patients with Osteoarthritis of the Knee Joints
Abstract
:1. Introduction
1.1. Assumptions and Goals of the Study
1.2. Methodology
1.3. Clinical Evaluation
1.4. Statistical Analysis
2. Results
2.1. The VAS Pain Assessment Scale
2.2. The Laitinen Questionnaire
2.3. The Lequesne Index
2.4. The Lysholm Knee Index
- The Physical Component Summary (PCS). There were no statistically significant differences between the applied forms of treatment.
- The Mental Component Summary (MCS). Statistically, the MCS score before treatment did not significantly differ between the three groups. After the galvanic treatment, the MCS score decreased on average by −0.13 (median = −0.13). After “−” iontophoresis, the score increased by 3.78 (median = 3.78), whereas after “+” iontophoresis, it increased by an average of 5.10 (median = 5.10). A statistically significant and better effect of MCS growth was obtained after “+” iontophoresis compared to anodic galvanic treatment (Kruskal–Wallis post-hoc test, p = 0.007) (Figure 1, Table 1).
3. Discussion
4. Conclusions
- In the group of patients who underwent iontophoresis with Perskindol gel introduced from the positive pole, statistically significant improvements in the degree of pain intensity, functional capacity, and performance of the knee joints, in addition to an improvement in the quality of life, was demonstrated in relation to the patients who underwent the anodic galvanic treatment.
- In the group of patients receiving iontophoresis with Perskindol gel introduced from the positive pole, a significant improvement was achieved in the degree of pain intensity, as assessed according to the Laitinen questionnaire, in relation to the patients treated with iontophoresis with the use of this preparation applied from the negative pole.
- In the studied group of patients with OA of the knee joints, the most favorable therapeutic effect was obtained using iontophoresis with the use of Perskindol gel introduced from the positive pole.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yegane, Y.; Mottaghi, A.; Moghimi, J. Correlation of quantified MRI, physical exam and knee radiography in patients with osteoarthritis. Tehran Univ. Med. Sci. 2011, 9, 185–190. [Google Scholar]
- Loew, L.; Brosseau, L.; Wells, G.A.; Tugwell, P.; Kenny, G.P.; Reid, R.; Maetzel, A.; Huijbregts, M.; McCullough, C.; De Angelis, G.; et al. Ottawa panel evidence-based clinical practice guidelines for aerobic walking programs in the management of osteoarthrosis. Arch. Phys. Med. Rehabil. 2012, 93, 1269–1285. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.W. Treatment of knee osteoarthritis. Am. Fam. Phys. 2018, 98, 603–606. [Google Scholar]
- Carlesso, L.C.; Segal, N.A.; Frey-Law, L.; Zhang, Y.; Na, L.; Nevitt, M.; Lewis, C.E.; Neogi, T. Pain Susceptibility Phenotypes in Those Free of Knee Pain with or at Risk of Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis Rheumatol. 2019, 71, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Osteoarthritis of the knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [Green Version]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef]
- Mańkowska, A.; Kasprzak, W. Medycyna fizykalna w praktyce klinicznej. In Physical Medicine in Clinical Practice; PZWL: Warszawa, Poland, 2020. [Google Scholar]
- Akinbo, S.R.; Aiyejusunle, C.B.; Akinyemi, O.A.K. Comparison of the therapeutic efficacy of phonophoresis and iontophoresis using dexamethasone sodium phosphate in the management of patients with knee osteoarthritis. Niger. Postgrad. Med. J. 2007, 14, 190–194. [Google Scholar]
- Bello, A.; Kuwornu, S. 5% Ibuprofen Iontophoresis Compared with Transcutaneous Electrical Nerve Stimulation in the Management of Knee Osteoarthritis: A Feasibility Study. OJTR 2014, 2, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Rezasoltani, A.; Roustaei, M.; Hasanzadeh, K. A survey on the effects of iontophoresis of Piroxicam Gel on pain and knee muscles strenght patients with knee osteoarthritis. Sci. J. Rehabil. Med. 2012, 1, 27–33. [Google Scholar]
- Turhanoğlu, A.D.; Güler, H.; İnanoğlu, D.; İnanoğlu, K.; Turhanoğlu, S. Tramadol iontophoresis added to treatment of knee osteoarthritis. Turk. J. Rheumatol. 2010, 25, 174–178. [Google Scholar] [CrossRef]
- Dyszkiewicz, A. Środki farmakologiczne do jonoforezy—cz. I. [Pharmacological agents for iontophoresis—part 1]. Rehabil. Prakt. 2006, 1, 40–42. [Google Scholar]
- Onigbinde, A.T.; Owolabi, A.R.; Lasisi, K.; Sarah, O.I.; Ibikunle, A.F. Symptoms-modifying effects of electromotive administration of glucosamine sulphate among patients with knee osteoarthritis. Hong Kong Physiother. J. 2018, 38, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyszkiewicz, A. Środki farmakologiczne do jonoforezy—cz.II [Pharmacological agents for iontophoresis—part 2]. Rehabil. Prakt. 2006, 2, 37–40. [Google Scholar]
- Malanga, G.A.; Yan, N.; Stark, J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad. Med. 2015, 127, 57–65. [Google Scholar] [CrossRef]
- Liu, B.; Fan, L.; Balakrishna, S.; Sui, A.; Morris, J.B.; Jordt, S.-E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 2013, 154, 2169–2177. [Google Scholar] [CrossRef] [Green Version]
- Farco, J.A.; Grundmann, O. Menthol-pharmacology of an important naturally medicinal “cool”. Mini Rev. Med. Chem. 2013, 13, 124–131. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, R.; Sun, L.; Huang, C.; Wang, C.; Zhang, D.-M.; Zhang, T.-T.; Du, G.-H. Anti-inflammatory activity of methyl salicylate glycosides isolated from Gaultheria yunnanensis(Franch.) Rehder. Molecules 2011, 16, 3875–3884. [Google Scholar] [CrossRef] [Green Version]
- Mason, L.; Moore, R.A.; Edwards, J.E.; McQuay, H.J.; Derry, S.; Wiffen, P.J. Systematic review of efficacy of topical rubefacients containing salicylates for the treatment of acute and chronic pain. BMJ 2004, 328, 995. [Google Scholar] [CrossRef] [Green Version]
- Barkin, R.L. The pharmacology of topical analgesics. Postgrad. Med. 2013, 125, 7–18. [Google Scholar] [CrossRef]
- Wu, K.K. Aspirin and salicylate: An old remedy with a new twist. Circulation 2000, 102, 2022–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Judzentiene, A.; Kupcinskiene, E. Chemical Composition on Essential Oils from Needles of Pinus sylvestris L. grown in Northern Lithuania. J. Essent. Oil Res. 2008, 20, 26–29. [Google Scholar] [CrossRef]
- Vigo, E.; Cepeda, A.; Gualillo, O.; Perez-Fernandez, R. In-vitro anti-inflammatory activity of Pinus sylvestris and Plantago lanceolate extracts: Effect on inducible NOS, COX-1, COX-2 and their products in J774A.1 murine macrophages. J. Pharm. Pharm. 2005, 57, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Bruschetta, G.; Di Paola, R.; Ahmad, A.; Campolo, M.; Cuzzocrea, S.; Esposito, E.; Navarra, M. The anti-inflammatory and antioxidant effects of bergamot juice extract (BJe) in an experimental model of inflammatory bowel disease. Clin. Nutr. 2014, 34, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, T.; Mizoguchi, H.; Kuwahata, H.; Katsuyama, S.; Komatsu, T.; Morrone, L.A.; Corasaniti, M.T.; Bagetta, G.; Sakurada, S. Intraplantar injection of bergamot essential oil induces peripheral antinociception mediated by opioid mechanism. Pharmacol. Biochem. Behav. 2010, 97, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-Y.; Liao, M.-H.; Wang, Y.-K.; Huang, Y.-S.; Wen, H.-C. Effect of Lavender Essential Oil on LPS-Stimulated Inflammation. Am. J. Chin. Med. 2012, 40, 845–859. [Google Scholar] [CrossRef] [PubMed]
- DA Silva, G.L.; Luft, C.; Lunardelli, A.; Amaral, R.H.; Melo, D.A.D.S.; Donadio, M.V.; Nunes, F.B.; DE Azambuja, M.S.; Santana, J.C.; Moraes, C.M.; et al. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. An. Da Acad. Bras. De Ciênc. 2015, 87, 1397–1408. [Google Scholar] [CrossRef] [Green Version]
- Takaki, I.; Bersani-Amado, L.E.; Vendruscolo, A.; Sartoretto, S.M.; Diniz, S.P.; Bersani-Amado, C.A.; Cuman, R.K.N. Anti-inflammatory and antinociceptive effects of Rosmarinus officinalis L. essential oil in experimental animal models. J. Med. Foot 2008, 11, 741–746. [Google Scholar]
- Galeotti, N.; Mannelli, L.D.C.; Mazzanti, G.; Bartolini, A.; Ghelardini, C. Menthol: A natural analgesic compound. Neurosci. Lett. 2001, 322, 145–148. [Google Scholar] [CrossRef]
- Liu, W.-R.; Qiao, W.-L.; Liu, Z.-Z.; Wang, X.-H.; Jiang, R.; Li, S.-Y.; Shi, R.-B.; She, G.-M. Gaultheria: Phytochemical and Pharmacological Characteristics. Molecules 2013, 18, 12071–12108. [Google Scholar] [CrossRef]
- Nikolić, M.; Marković, T.; Mojović, M.; Pejin, B.; Savić, A.; Perić, T.; Marković, D.; Stević, T.; Soković, M. Chemical composition and biological activity of Gaultheria procumbens L. essential oil. Ind. Crops Prod. 2013, 49, 561–567. [Google Scholar] [CrossRef]
- Haeseler, G.; Maue, D.; Grosskreutz, J.; Bufler, J.; Nentwig, B.; Piepenbrock, S.; Dengler, R.; Leuwer, M. Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol. Eur. J. Anaesthesiol. 2002, 19, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Craighead, D.H.; Alexander, L.M. Topical menthol increases cutaneous blood flow. Microvasc. Res. 2016, 107, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-J.; Yang, Y.-J.; Li, Y.-S.; Zhang, W.K.; Tang, H.-B. α-Pinene, linalool, and 1-octanol contribute to the topical anti-inflammatory and analgesic activities of frankincense by inhibiting COX-2. J. Ethnopharmacol. 2016, 179, 22–26. [Google Scholar] [CrossRef]
- Ribnicky, D.M.; Poulev, A.; Raskin, I. The Determination of Salicylates in Gaultheria procumbens for Use as a Natural Aspirin Alternative. J. Nutraceuticals Funct. Med. Foods 2003, 4, 39–52. [Google Scholar] [CrossRef]
- Kellgren Lawrence, J. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Kohn, M.; Sassoon, A.; Fernando, N. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef] [Green Version]
- Schiphof, D.; Boers, M.; Bierma-Zeinstra, S. Differences in Descriptions of Kellgren and Lawrence Grades of Knee Osteoarthritis. Ann. Rheum. Dis. 2008, 67, 1034–1036. [Google Scholar] [CrossRef]
- Sangha, O. Epidemiology of Rheumatic Diseases. Rheumatology 2000, 39, 3–12. [Google Scholar] [CrossRef] [Green Version]
- sevni Kurniawati, W.; Ilyas, M.; Muis, M.; Faridin, F.; Asriyani, S. Correlation between Osteoarthritis Knee Damage Based on Ultrasound with Kellgren-Lawrence Classification. Mutiara Med. J. Kedokt. Dan Kesehat. 2021, 21, 50–58. [Google Scholar] [CrossRef]
- Aiyejusunle, C.B.; Kola-Korolo, T.A.; Ajiboye, O.A. Comparison of the effects of TENS and sodium salicylate iontophoresis in the management of osteoarthritis of the knee. Niger. Q. J. Hosp. Med. 2007, 17, 30–34. [Google Scholar]
- Onigbinde, A.T.; Talabi, A.E.; Okulaja, I.A.; Dominic, O. Comparative efficacy of cycle-ergometry exercise and glucosamine sulphate iontophoresis in pain management of subjects with sub-acute knee osteoarthritis. Med. Sport 2011, 7, 1517–1521. [Google Scholar]
- Briggs, K.K.; Lysholm, J.; Tegner, Y.; Rodkey, W.G.; Kocher, M.S.; Steadman, J.R. The reliability, validity and responsiveness of the Lysholm score and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am. J. Sports Med. 2009, 37, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Lysholm, J.; Gillquist, J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am. J. Sports Med. 1982, 10, 150–154. [Google Scholar] [CrossRef]
- Ware, J.E.; Gandek, B. Overview of the SF-36 Health survey and the International Quality of Life Assessment (IQOLA) Project. J. Clin. Epidemiol. 1998, 51, 903–912. [Google Scholar] [CrossRef]
- Lobo, S.; Yan, G. Evaluation iontophoretic delivery of a cationic ketoprofen prodrug for treating nociceptive symptoms in monosodium iodoacetate induced osteoarthritic rat model. Int. J. Pharm. 2019. [Google Scholar] [CrossRef]
- Kuryliszyn-Moskal, A.; Kaniewska, K.; Dzięcioł-Anikiej, Z.; Klimiuk, P.A. Evaluation of foot static disturbances in patients with rheumatic diseases. Reumatologia 2017, 55, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Dzięcioł, Z.; Dzięcioł, J. Application of plantography examination to the assessment of foot deformity in patients with rheumatoid arthritis. Arch. Med. Sci. 2015, 11, 1015–1020. [Google Scholar]
- Kuryliszyn-Moskal, A.; Hryniewicz, A.; Bagiński, N.; Moskal-Jasińska, D.; Dzięcioł-Anikiej, Z.; Dzięcioł, J. Foot static disturbances and clinical features in overweight patients with rheumatoid arthritis. Arch. Med. Sci. 2020. [Google Scholar] [CrossRef]
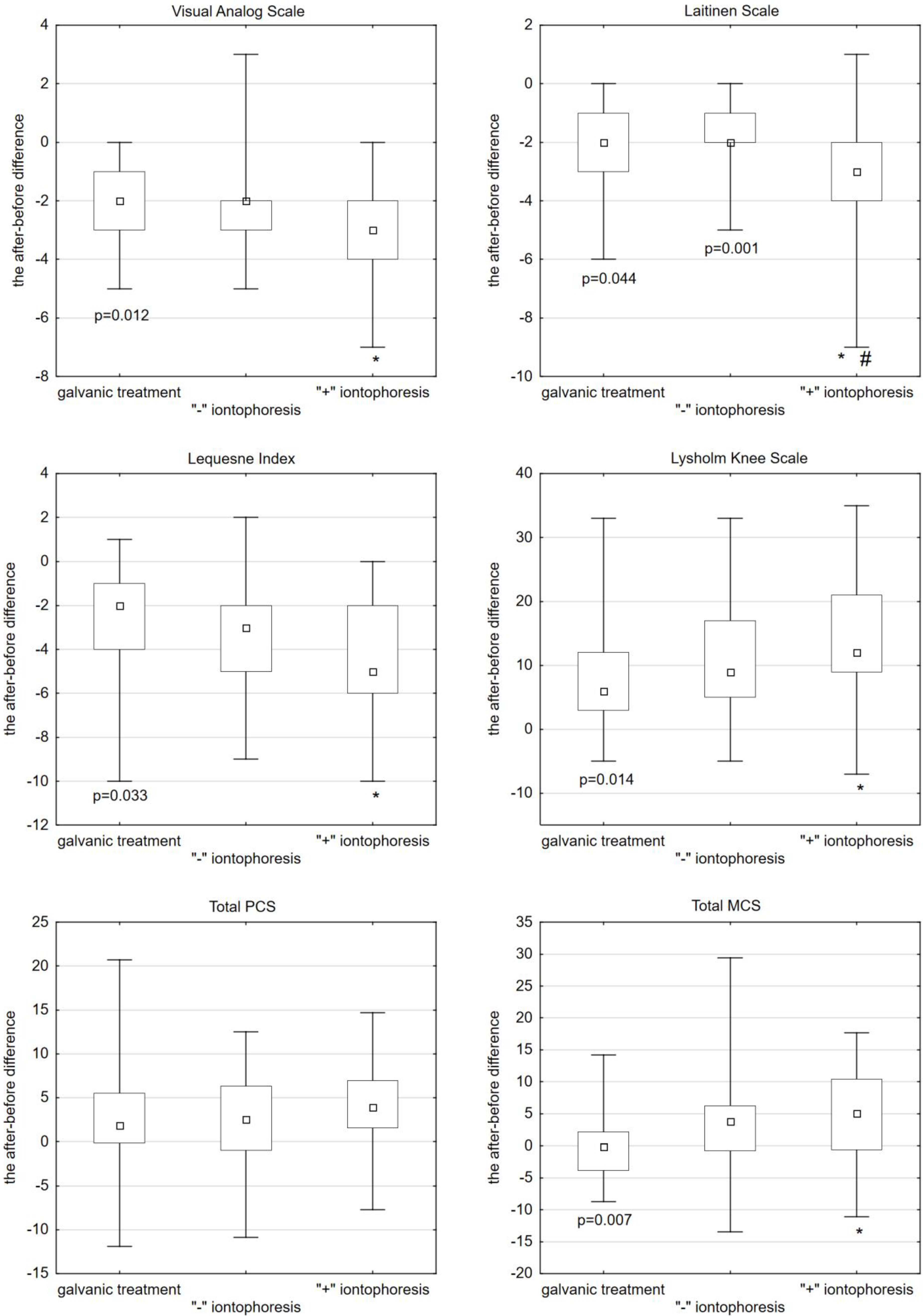
| Scale | (1) Galvanic Treatment | (2) ‘’−” Iontophoresis | (3) ‘’+” Iontophoresis | 1 vs. 2 1 vs. 3 2 vs. 3 |
|---|---|---|---|---|
| VAS | −2.00 (−3.00; −1.00) | −2.00 (−3.00; −2.00) | −3.00 (−4.00; −2.00) | n/a p = 0.012 n/a |
| Laitinen Questionnaire | −2.00 (−3.00; −1.00) | −2.00 (−2.00; −1.00) | −3.00 (−4.00; −2.00) | n/a p = 0.044 p = 0.001 |
| Lequesne Index | −2.00 (−4.00; −1.00) | −3.00 (−5.00; −2.00) | −5.00 (−6.00; −2.00) | n/a p = 0.033 n/a |
| Lysholm Knee Index | 6.00 (3.00; 12.00) | 9.00 (5.00; 17.00) | 12.00 (9.00; 21.00) | n/a p = 0.014 n/a |
| MCS Index | −0.13 (−3.84; 2.14) | 3.78 (−0.73; 6.21) | 5.10 (−0.60; 10.44) | n/a p = 0.007 n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dakowicz, A.; Dzięcioł-Anikiej, Z.; Hryniewicz, A.; Judycka, M.; Ciołkiewicz, M.; Moskal-Jasińska, D.; Kuryliszyn-Moskal, A. Evaluation of the Effectiveness of Iontophoresis with Perskindol Gel in Patients with Osteoarthritis of the Knee Joints. Int. J. Environ. Res. Public Health 2022, 19, 8489. https://doi.org/10.3390/ijerph19148489
Dakowicz A, Dzięcioł-Anikiej Z, Hryniewicz A, Judycka M, Ciołkiewicz M, Moskal-Jasińska D, Kuryliszyn-Moskal A. Evaluation of the Effectiveness of Iontophoresis with Perskindol Gel in Patients with Osteoarthritis of the Knee Joints. International Journal of Environmental Research and Public Health. 2022; 19(14):8489. https://doi.org/10.3390/ijerph19148489
Chicago/Turabian StyleDakowicz, Agnieszka, Zofia Dzięcioł-Anikiej, Anna Hryniewicz, Małgorzata Judycka, Mariusz Ciołkiewicz, Diana Moskal-Jasińska, and Anna Kuryliszyn-Moskal. 2022. "Evaluation of the Effectiveness of Iontophoresis with Perskindol Gel in Patients with Osteoarthritis of the Knee Joints" International Journal of Environmental Research and Public Health 19, no. 14: 8489. https://doi.org/10.3390/ijerph19148489
APA StyleDakowicz, A., Dzięcioł-Anikiej, Z., Hryniewicz, A., Judycka, M., Ciołkiewicz, M., Moskal-Jasińska, D., & Kuryliszyn-Moskal, A. (2022). Evaluation of the Effectiveness of Iontophoresis with Perskindol Gel in Patients with Osteoarthritis of the Knee Joints. International Journal of Environmental Research and Public Health, 19(14), 8489. https://doi.org/10.3390/ijerph19148489






