A Novel Direct Butyrate Generator Reduces Belly Pain in a Randomized, Double-Blind, Placebo-Controlled Clinical Study
Abstract
1. Introduction
2. Materials and Methods
- Digestion-associated Quality of Life Questionnaire (DQLQ);
- PROMIS™ Belly Pain 5A*;
- PROMIS™ GI Gas and Bloating 13A*;
- PROMIS™ Constipation 9A*;
- PROMIS™ Diarrhea 6A*
- PROMIS™ Reflux 13A*;
- PROMIS™ Nausea and Vomiting 4A*.
- PROMIS™ Belly Pain 5A, which evaluated abdominal pain;
- PROMIS™ Reflux 13A, which assessed symptoms related to reflux;
- PROMIS™ Nausea and Vomiting 4A, which measured the severity of nausea and vomiting;
- PROMIS™ GI Gas and Bloating 13A, which evaluated gas and bloating;
- PROMIS™ Constipation 9A, which assessed constipation;
- PROMIS™ Diarrhea 6A, which measured the severity of diarrhea.
- GI-related Quality of Life (QOL) as assessed by the Digestion-associated QOL Questionnaire (DQLQ).
3. Results
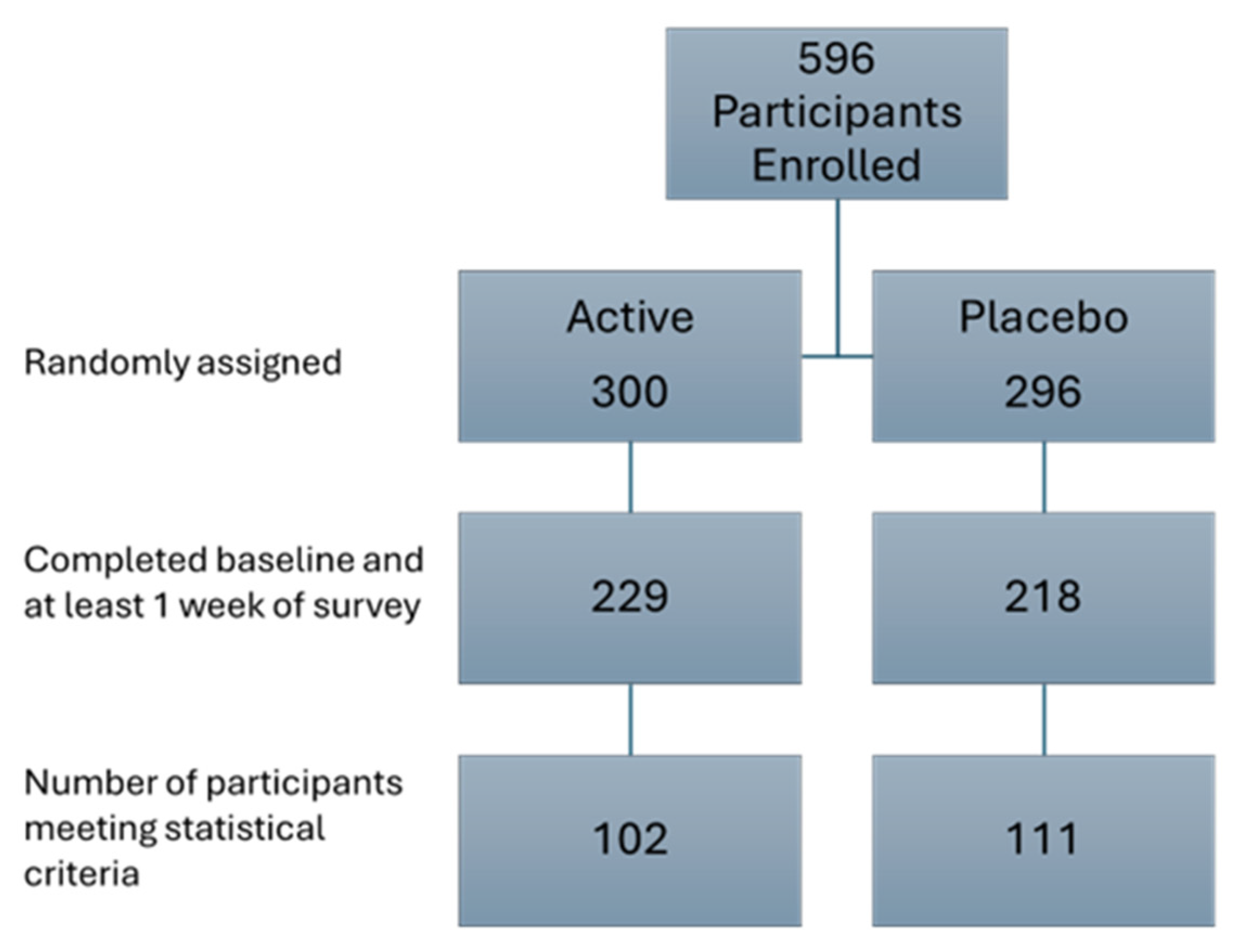
3.1. Study Demographics
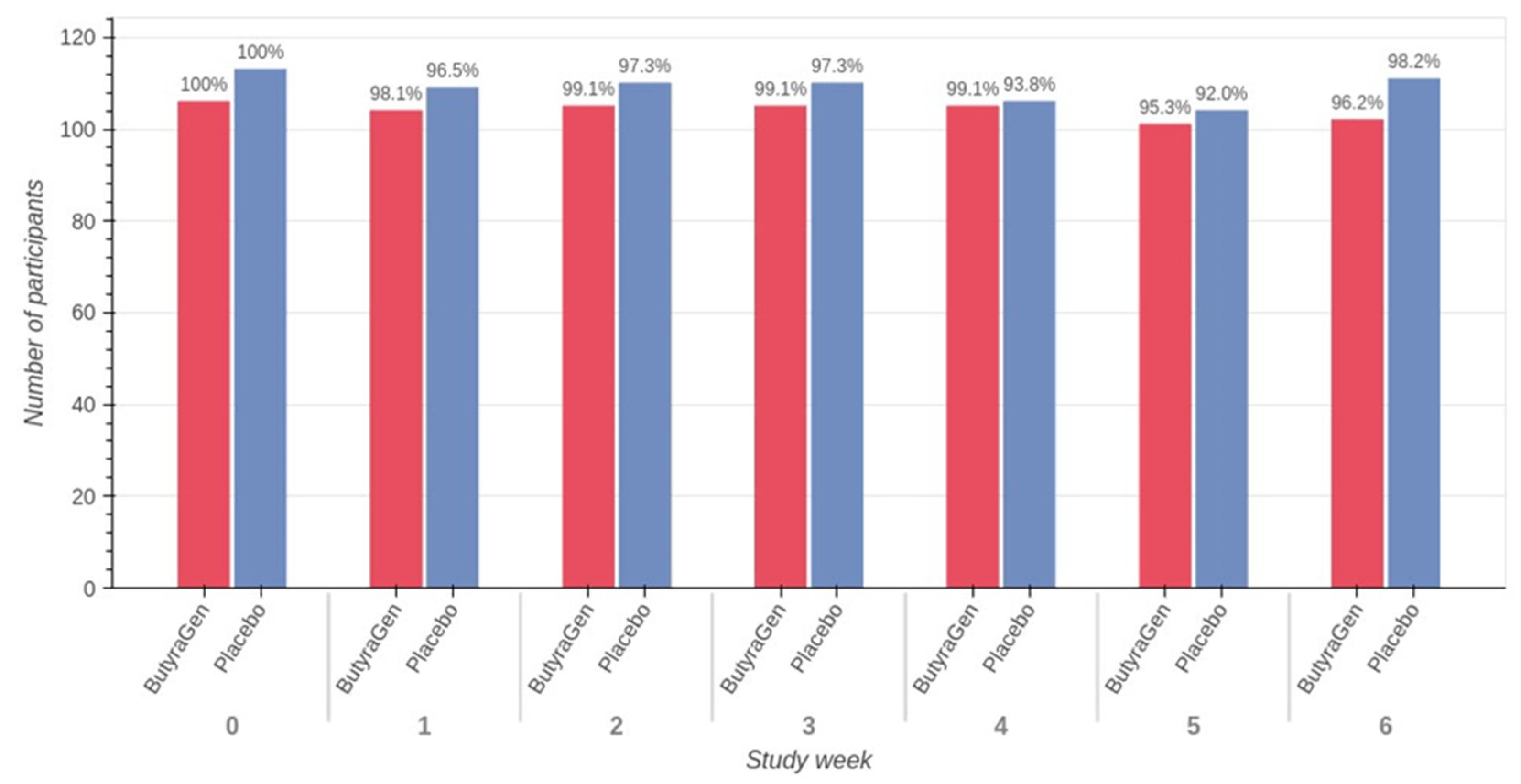
3.2. Study Compliance
3.3. Tolerability
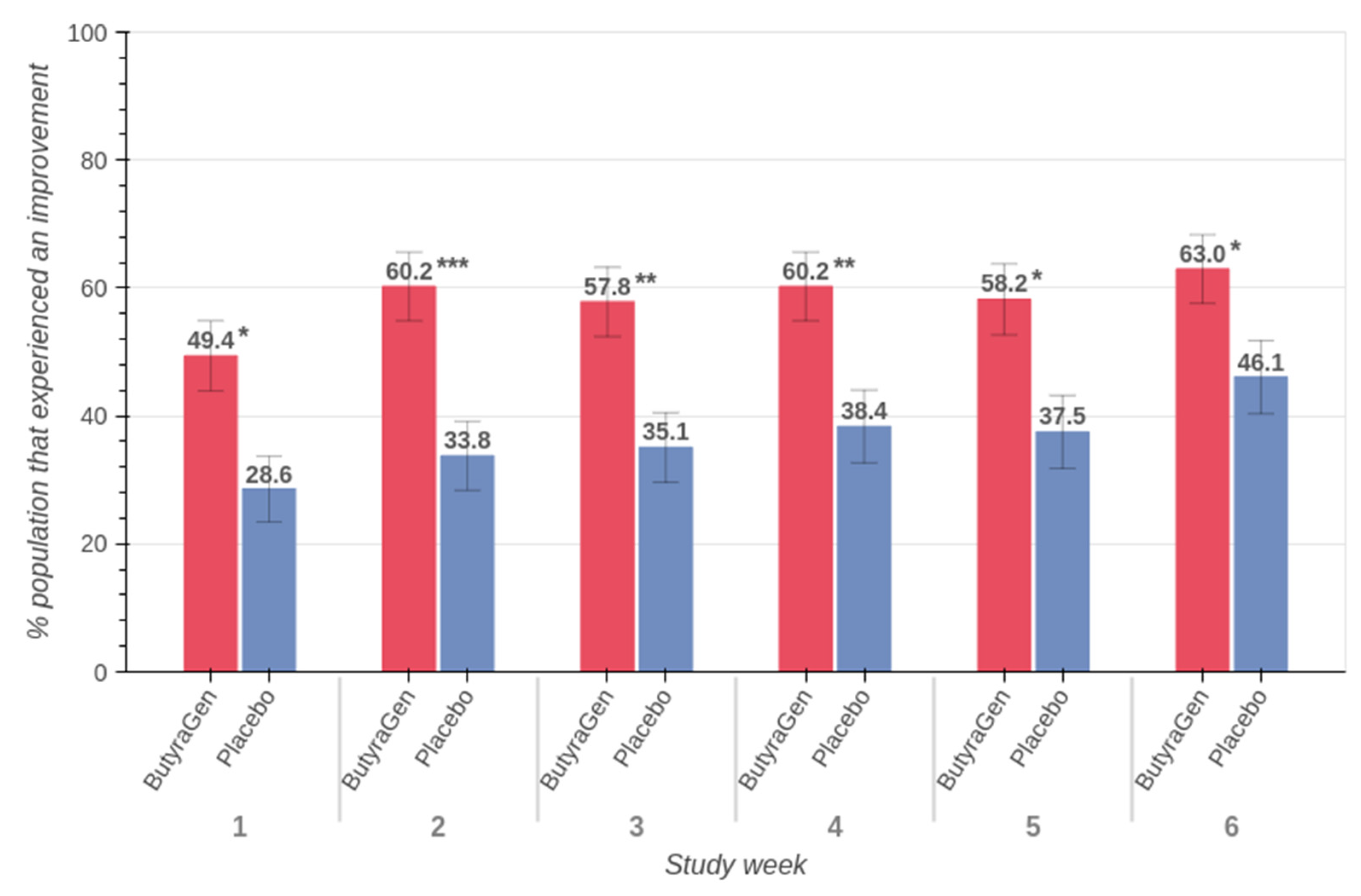
3.4. Belly Pain (PROMIS™ Belly Pain 5A)
3.5. Upper and Lower GI
3.6. Women
3.7. Other Results
4. Discussion
4.1. Belly Pain Improvements and Clinical Relevance
4.2. Implications for GI Health and Quality of Life
4.3. Potential Benefits in Women
4.4. Insights and Opportunities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCFA | Short-chain fatty acid |
| IBS | Irritable bowel syndrome |
| hs-CRP | High-sensitivity C-reactive protein |
| DQLQ | Digestion-associated Quality of Life Questionnaire |
| MCID | Minimal Clinically Important Difference |
| C.I. | Confidence interval |
| GI | Gastrointestinal |
References
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2022, 42, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277, Erratum in Front. Immunol. 2019, 10, 1486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- U.S. National Library of Medicine. MedlinePlus. Abdominal Pain. Available online: https://medlineplus.gov/ency/article/003120.htm (accessed on 28 January 2025).
- Conley, B.A.; Egorin, M.J.; Tait, N.; Rosen, D.M.; Sausville, E.A.; Dover, G.; Fram, R.J.; Van Echo, D.A. Phase I study of the orally administered butyrate prodrug, tributyrin, in patients with solid tumors. Clin. Cancer Res. 1998, 4, 629–634. [Google Scholar] [PubMed]
- Smith, M.; Lelah, M.; Goggans, M.; Tunio, S.; Naqib, A.; Burton-Freeman, B.; Edirisinghe, I. Investigation of the tolerability and potential health benefits of a novel butyrate generating supplement in a pilot human study. Nutr. Health Aging 2024, 9, 133–144. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. ClinicalTrials.gov. A Study to Evaluate RADX-P-2407 in Healthy Participants. Available online: https://clinicaltrials.gov/study/NCT06376695?id=RADX-P-2407&rank=1 (accessed on 28 January 2025).
- Spiegel, B.M.R.; Hays, R.D.; Bolus, R.; Melmed, G.Y.; Chang, L.; Whitman, C.; Khanna, P.P.; Paz, S.H.; Hays, T.; Reise, S.; et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) Gastrointestinal Symptom Scales. Am. J. Gastroenterol. 2014, 109, 1804–1814, Erratum in Am. J. Gastroenterol. 2015, 110, 608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cook, C.E. Clinimetrics Corner: The Minimal Clinically Important Change Score (MCID): A Necessary Pretense. J. Man. Manip. Ther. 2008, 16, E82–E83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Python Software Foundation. Statistics—Mathematical Statistics Functions. Python 3 Documentation. Available online: https://docs.python.org/3/library/statistics.html (accessed on 28 January 2025).
- StatsModels Development Team. Statsmodels: Econometric and Statistical Modeling. Available online: https://www.statsmodels.org/stable/index.html (accessed on 28 January 2025).
- The Pandas Development Team. Pandas—Python Data Analysis Library. Available online: https://pandas.pydata.org/ (accessed on 14 February 2025).
- Bokeh Development Team. Bokeh: Interactive Visualization for Modern Web Browsers. Available online: https://bokeh.org/ (accessed on 28 January 2025).
- Lakhoo, K.; Almario, C.V.; Khalil, C.; Spiegel, B.M.R. Prevalence and Characteristics of Abdominal Pain in the United States. Clin. Gastroenterol. Hepatol. 2021, 19, 1864–1872.e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, K.; Kaniewska, M.; Karłowicz, K.; Rosołowski, M.; Rydzewska, G. The effectiveness of microencapsulated sodium butyrate at reducing symptoms in patients with irritable bowel syndrome. Gastroenterol. Rev. 2022, 17, 28–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scarpellini, E.; Lauritano, E.; Lupascu, A.; Petruzzellis, C.; Novi, M.; Roccarina, D.; Gabrielli, M.; Serricchio, M.; Gasbarrini, G.; Gasbarrini, A. Efficacy of butyrate in the treatment of diarrhoea-predominant irritable bowel syndrome. Dig. Liver Dis. Suppl. 2007, 1, 19–22. [Google Scholar] [CrossRef]
- Spiller, R.C. Postinfectious irritable bowel syndrome. Gastroenterology 2003, 124, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional, and national burden of 10 digestive diseases in 204 countries and territories from 1990 to 2019. Front. Public Health 2023, 11, 1061453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Unno, T.; Kim, B.-Y.; Park, M.-S. Sex Differences in Gut Microbiota. World J. Men’s Health 2020, 38, 48–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, N.; Yang, C. Butyrate as a Potential Modulator in Gynecological Disease Progression. Nutrients 2024, 16, 4196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cain, K.C.; Jarrett, M.E.; Burr, R.L.; Rosen, S.; Hertig, V.L.; Heitkemper, M.M. Gender differences in gastrointestinal, psychological, and somatic symptoms in irritable bowel syndrome. Dig. Dis. Sci. 2008, 54, 1542–1549. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dastmalchi, L.N.; Gulati, M. Increasing the Number of Women and Underrepresented Groups in Clinical Trials. American College of Cardiology. 2022. Available online: https://www.acc.org/Latest-in-Cardiology/Articles/2022/12/09/13/06/Increasing-the-Number-of-Women-and-Underrepresented-Groups-in-Clinical-Trials (accessed on 28 January 2025).



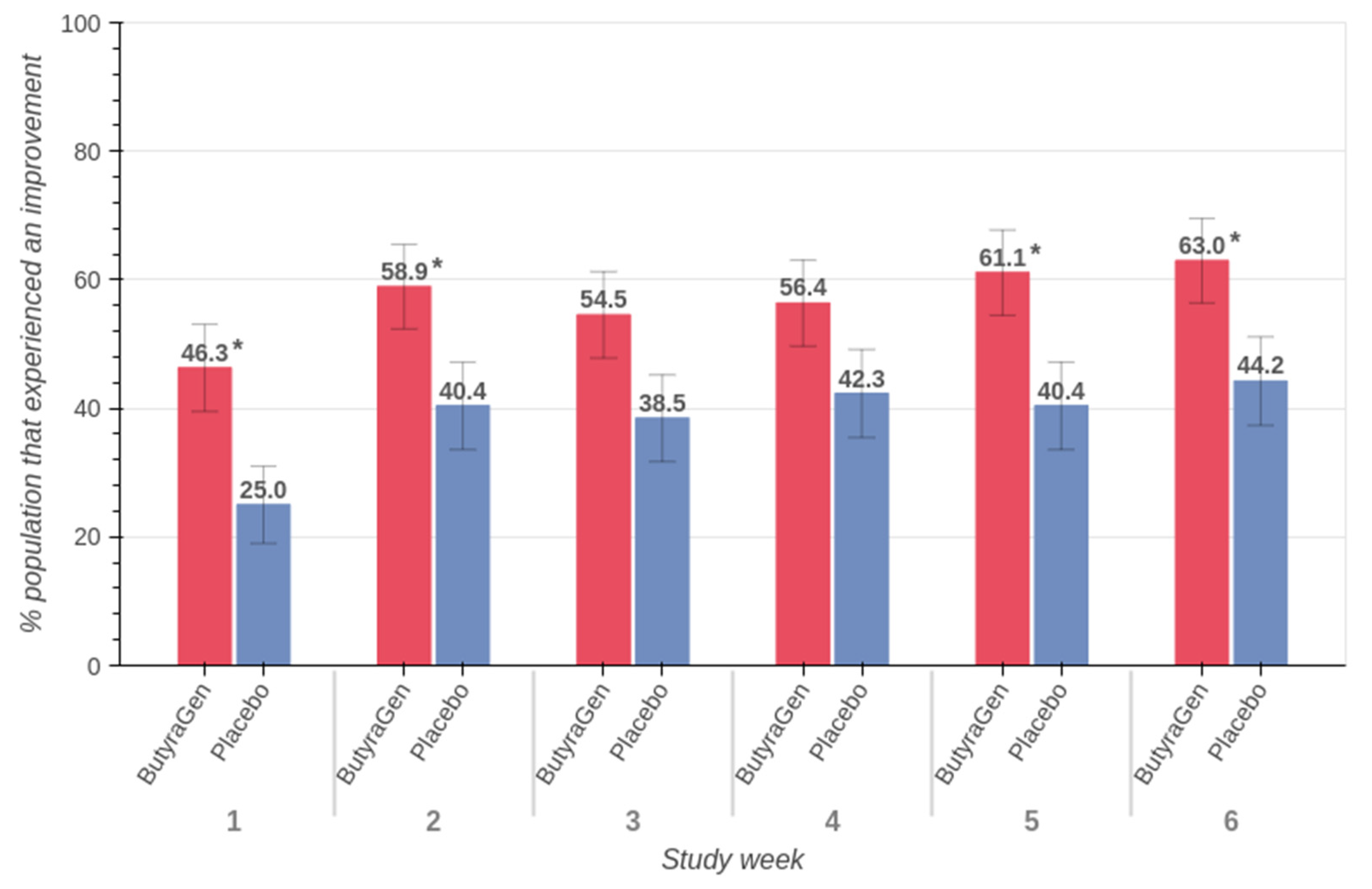

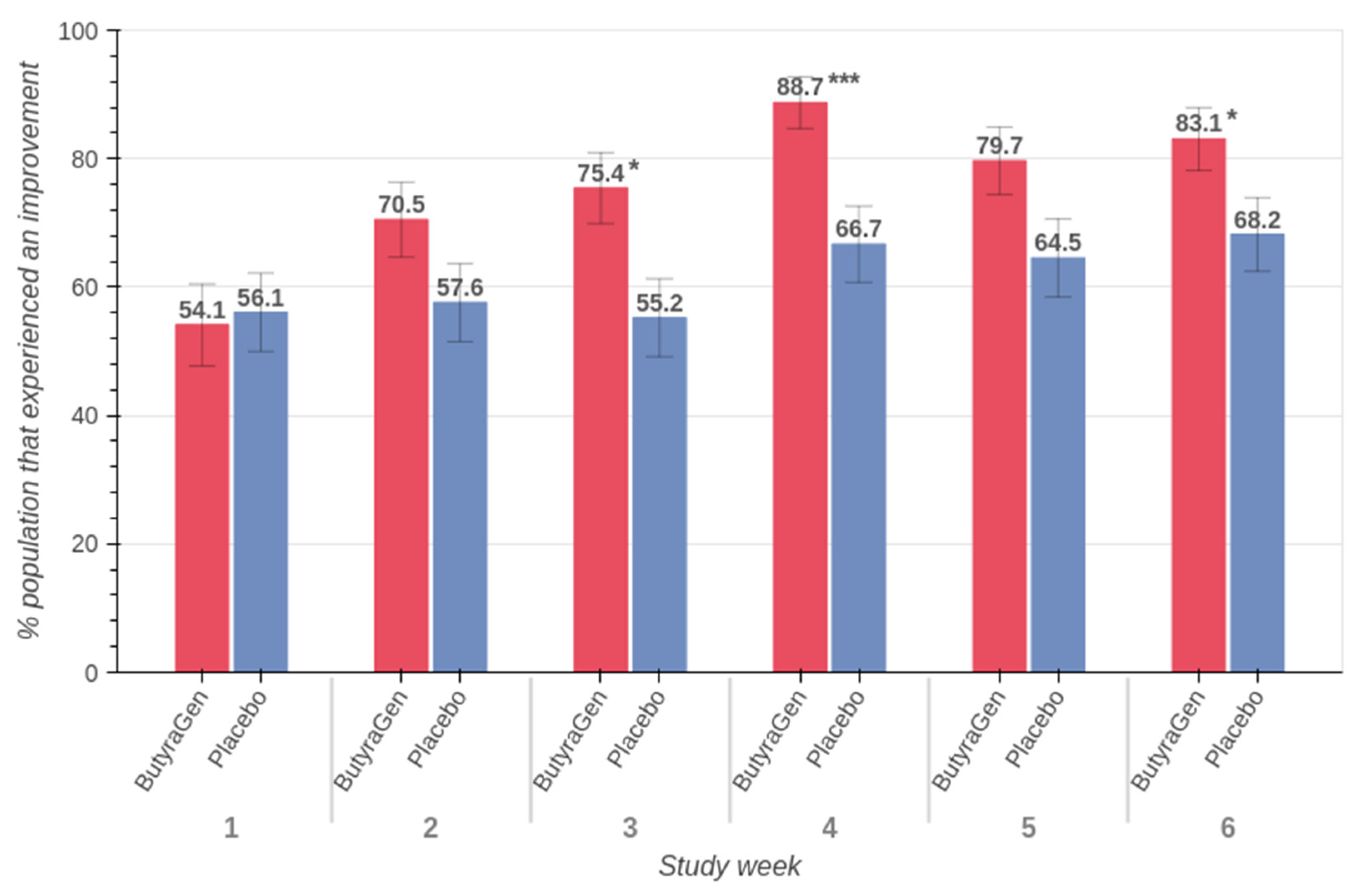

| Study Week | Age | ButyraGen | Placebo | ||
|---|---|---|---|---|---|
| Female | Male | Female | Male | ||
| Baseline | 0–30 | 13 | 9 | 16 | 9 |
| 30–40 | 65 | 31 | 53 | 34 | |
| 40–50 | 76 | 27 | 64 | 29 | |
| 50–60 | 34 | 21 | 46 | 11 | |
| 60+ | 16 | 8 | 21 | 13 | |
| Week 6 | 0–30 | 2 | 0 | 3 | 2 |
| 30–40 | 19 | 8 | 13 | 12 | |
| 40–50 | 29 | 7 | 24 | 11 | |
| 50–60 | 14 | 9 | 20 | 7 | |
| 60+ | 8 | 6 | 13 | 6 | |
| Questionnaire | PROMIS™ Belly Pain 5A |
|---|---|
| Total participants | 213 |
| Active participants | 102 |
| Placebo participants | 111 |
| MCID improvement p-value | 0.0064 |
| MCID risk ratio (+/− 95% C.I) | 1.29 (+/− 0.10) |
| Difference in overall MCID (active–placebo) | 18.0% |
| Question (In the Past 7 Days…) | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| How often did you have belly pain? | never * | one day | 2–6 day | one/day | >1/day |
| At its worst, how would you rate your belly pain? | not bad at all | a little bad | somewhat bad | quite bad | very bad |
| How much did belly pain interfere with your day-to-day activities? | not at all | a little bit | somewhat | quite a bit | very much |
| How much did belly pain bother you? | not at all | a little bit | somewhat | quite a bit | very much |
| How often did you have discomfort in your belly? | never | rarely | sometimes | often | always |
| DQLQ Post Hoc Analysis | DQLQ | DQLQ |
|---|---|---|
| Specific population | Participants starting with normal/mild reflux | Participants starting with normal/mild constipation |
| Region | Upper GI | Lower GI |
| Total participants | 157 | 106 |
| Active participants | 81 | 54 |
| Placebo participants | 76 | 52 |
| MCID improvement p-value | 0.0305 | 0.0440 |
| MCID risk ratio (+/− 95% C.I) | 1.39 (+/− 0.15) | 1.45 (+/− 0.19) |
| Difference in overall MCID (active–placebo) | 16.9% | 18.8% |
| Female-Specific Post Hoc Analysis | PROMIS™ Belly Pain 5A | PROMIS™ Belly Pain 5A | PROMIS™ GI Gas and Bloating 13A |
|---|---|---|---|
| Specific subset population | Women | Women self-identified as post-menopausal | Women who self-identified as experiencing GI distress |
| Total participants | 145 | 55 | 125 |
| Active participants | 72 | 26 | 59 |
| Placebo participants | 73 | 29 | 66 |
| MCID improvement p-value | 0.0057 | 0.0230 | 0.0411 |
| MCID risk ratio (+/− 95% C.I) | 1.36 (+/− 0.11) | 1.44 (+/− 0.16) | 1.23 (+/− 0.10) |
| Difference in overall MCID (active–placebo) | 20.3% | 26.4% | 14.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dosz, E.; Conley, D.; Lelah, M. A Novel Direct Butyrate Generator Reduces Belly Pain in a Randomized, Double-Blind, Placebo-Controlled Clinical Study. Nutraceuticals 2025, 5, 14. https://doi.org/10.3390/nutraceuticals5020014
Dosz E, Conley D, Lelah M. A Novel Direct Butyrate Generator Reduces Belly Pain in a Randomized, Double-Blind, Placebo-Controlled Clinical Study. Nutraceuticals. 2025; 5(2):14. https://doi.org/10.3390/nutraceuticals5020014
Chicago/Turabian StyleDosz, Edward, Devin Conley, and Michael Lelah. 2025. "A Novel Direct Butyrate Generator Reduces Belly Pain in a Randomized, Double-Blind, Placebo-Controlled Clinical Study" Nutraceuticals 5, no. 2: 14. https://doi.org/10.3390/nutraceuticals5020014
APA StyleDosz, E., Conley, D., & Lelah, M. (2025). A Novel Direct Butyrate Generator Reduces Belly Pain in a Randomized, Double-Blind, Placebo-Controlled Clinical Study. Nutraceuticals, 5(2), 14. https://doi.org/10.3390/nutraceuticals5020014





